Abstract
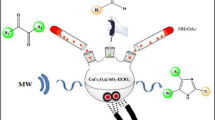
This research paper presents the synthesis and characterization of the magnetic nanoparticle, Fe3O4@Sal@Cu, [Fe3O4@Si–CH2–CH2–CH2–NH–NH–CO–N=CH–(2–HO–C6H4–)@Cu] as a green and retrievable catalyst. This catalyst was characterized by FTIR, XRD, EDX and TGA analyses. In addition, the catalytic activity of this new catalyst was investigated for the synthesis of 2-amino 4H-chromenes by producing good-to-excellent yields under mild reaction conditions. The other advantages of the developed nanocatalyst are its ecofriendliness, being easy to handle, high reusability and being magnetically separable. The synthesis of some new derivatives of 2-amino-4H-chromenes in the presence of this nanocatalyst is also reported.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of magnetic nanocatalysts is an interesting area for the development of sustainable and green procedures due to external magnetic separation and no need for catalyst filtration or centrifugation and providing simple and practical method for the recovering of these catalysts [1]. In addition, multi-component reactions (MCRs) are very powerful weapons in the organic and medicinal chemistry for the preparation of the bulky products in a one-pot and almost one-step from small starting materials [2,3,4,5,6,7]. The MCRs for the synthesis of 2-amino-4H-chromenes derivatives have also gained considerable attention in organic synthesis such as synthesis of 2-amino-4H-chromenes derivatives using nano-ZnO catalyst [8], under solvent-free condition using MOF-5 [9], choline chloride/urea [10], nanocrystalline MgO [11], by Fe(ClO4)3/SiO2 [12], on water CuSO4. 5H2O-catalyzed synthesis of 2-amino-4H-chromenes [13], the synthesis of 2-amino-4H-pyran derivatives using DABCO-CuCl complex [14], preparation of 3-amino-1H-chromenes using ZnO nanoparticles thin-film [15], synthesis of 4, 5-dihydropyrano [c] chromene derivatives over TiO2 nanoparticles [16] and synthesis of aminobenzochromenes using Ag2Cr2O7 nanoparticles [17].
Also, copper-catalyzed synthesis of chromenes has been already extensively reported in the literature, especially which supported copper catalyst on magnetic nanoparticles [18] such as one-pot synthesis of 2-amino-4H-chromene derivatives by MNPs@ Cu [19, 20], sonochemically promoted preparation of silica-anchored cyclodextrin derivatives for efficient copper catalysis [21] and synthesis of benzimidazole derivatives using Cu-Schiff base complexes embedded over MCM-41 [22].
The combination of magnetic nanocatalysts and multi-component reactions will become a worthy protocol for the introducing of green procedure in green synthesis [23,24,25,26,27,28,29,30,31,32]. In addition, due to useful biological activities in the field of medicinal chemistry [33] and to have anti-cancer and anti-coagulant activities of 2-amino-4H-chromenes [34] and to evaluate catalytic activity of prepared catalyst, Fe3O4@Sal@Cu was utilized in the one-pot preparation of 2-amino-4H-chromenes using aryl aldehydes, dimedone and malononitrile, ethyl and methyl 2-cyanoacetate in good-to-high yield in ethanol at room temperature (Scheme 1).
Results and discussion
Characterization of the nanocatalyst
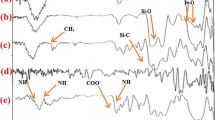
The FTIR spectra of the catalyst are shown in Fig. 1. The broad band at 3436 cm−1 confirms the presence of NH and OH group of amide, amine and phenolic OH, loaded on the surface of Fe3O4@Sal@Cu. The band 1615 cm−1 is related to C=O. The band in 573 cm−1 is related to Fe–O.
In addition, the XRD pattern of the catalyst is shown in Fig. 2. The reflection planes at 14, 30, 36, 44, 55, 59 and 64 which are attributed to the diffraction scattering of Fe3O4 were readily recognized from the XRD pattern. These characteristic peaks adopted with those of standard Fe3O4 (JCPDS file No 04-0755). The observed diffraction peaks were indicated that Fe3O4 mostly exists in a face-centered cubic structure.
The loading of organic compounds on Fe3O4 was determined by EDX analysis, and the content of C, N, O, S, Fe and Cu in Fe3O4@Sal@Cu was proved (Fig. 3).
The SEM images of the synthesized magnetic nanocatalyst are shown in Fig. 4. As can be seen from SEM images, the geometric shape of the nanoparticles is spherical and the nanoparticles have sizes between 15 and 36 nm.
Typical thermal TGA curves are given in Fig. 5. The range of 0–140 °C (region a) is related to release of adsorbed water; the second from 140 to 600 °C (region b) is related to the decomposition of organic matter on the Fe3O4 and region c is represented to Fe3O4. The TGA curve of the synthesized catalyst demonstrates thermal stability, with decomposition starting at around 140 °C under a nitrogen atmosphere.
Catalytic activity evaluation
Firstly, the model reaction was simply carried out by mixing 3-nitro benzaldehyde (1 mmol), malononitrile (1 mmol), dimedone (1 mmol) in ethanol, methanol, n-hexane, chloroform and water as solvent at room temperature in the presence of different amounts of the catalyst (2, 4 and 8 mg). The product was obtained as shown in Table 1. As indicated in Table 1, the best condition reaction is 8 mg of the catalyst in ethanol as solvent at ambient temperature.
However, the scope and generality of this three-component one-pot synthesis of 2-amino-4H-chromenes have been illustrated with different aldehydes and the results are summarized in Table 2. This method has the ability to tolerate a variety of other functional groups such as hydroxyl, methyl, nitro and chloro under the reaction conditions. This protocol is suitable for both electron-rich and electron-deficient aldehydes leading to high yields of products 4a–s.
Also, in a series of reactions, ethyl and methyl cyano acetate was employed instead of malononitrile under reaction condition to give the corresponding ethyl or methyl 2-amino-4H-chromene carboxylate. In these cases, the reactions were evaluated using a variety of structurally diverse aldehydes (entries 13–17, Table 2), respectively. The yields obtained were good-to-excellent. Therefore, the reaction profile is clean and this one-pot three-component procedure presents some improvements and advantages over existing methods. One of the major advantages of this protocol is the isolation and purification of the products, which have been achieved by simple separation (the use of external magnet) and crystallization of the crude products, and there are no by-products were formed in using catalyst. All the products were identified by comparison of analytical data with those of authentic samples. Also, some new compounds were synthesized by this protocol (entries 13–15, Table 2).
A reasonable pathway for the formation of 2-amino-4H-chromenes in the presence of magnetic nanocatalyst is disclosed in Scheme 2.
Also, we study the efficiency of our presented protocol in a comparative with some previously reported methods for the synthesis of 2-amino-7,7-dimethyl-4-(3-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile 4b. Reviewing the collected results as inserted in Table 3 represents higher catalytic performance for our presented catalyst.
Experimental
Chemicals were purchased from Merck Chemical Company. NMR spectra were recorded in CDCl3 and DMSO-d6 on a Bruker Advance DPX-300 instrument using TMS as an internal standard. SEM analysis was determined by using FE-TESCAN, model Mira3-XMU at accelerating voltage of 15 kV. XRD analysis was performed on a Bruker D8-advance X-ray diffractometer or on an X'Pert Pro MPD diffractometer with Cu Kα (λ = 0.154 nm) radiation. TGA analysis was recorded using a Shimadzu Thermogravimetric analyzer (TG-50). FTIR spectra were recorded on a JASCO FT-IR 460 plus spectrophotometer.
Preparation of Fe3O4 NPs
Fifty milliliters of FeCl3.6H2O (0.3 M) was added to 0.5 mL HCl (0.2 M), and the reaction flask was located in the ultrasonic probe and irradiation under 85 kHz at room temperature for 5 min. Then, 20 mL Na2SO3 (0.3 M) was added into 40 mL of above solution, and the color of solution changed from yellow to red. The reaction continues until the yellow color of the solution obtained again. In the following, the resulting solution was poured to 400 mL of water containing 60 mL of ammonium (28%) and followed by sonication for 30 min. Then, the obtained magnetic dispersion was separated by a magnet, washed three times with water and dried under vacuum at 60 °C for 12 h.
Synthesis of 3-Cl-propyl Fe3O4
Fe3O4 NPs was functionalized with (3-chloropropyl) trimethoxysilane according to the literature [56]. Typically, Fe3O4 NPs (2 g) was suspended in toluene (40 mL) and stirred for 15 min by ultrasonic. Then, (3-chloropropyl)trimethoxysilane (2 mL) was introduced and the resulting mixture was refluxed at 111 °C under inert (N2) atmosphere for 24 h. At the end of the reaction, the resulting brown solid was filtered, washed several times with toluene and dried at 90 °C overnight.
Incorporation of semicarbazide with 3-Cl-propyl Fe3O4
Considering the previous reports [51] regarding the reaction of alkyl chloride and semicarbazide, the functionalization of the 3-Cl-propyl Fe3O4 with semicarbazide was carried out as follows: 3-Cl-propyl Fe3O4 (1 g) was suspended in dry toluene (60 mL). Then, semicarbazide (0.5 g) and catalytic amount (1 mL) of trimethylamine as a catalyst were added to the suspension. Subsequently, the resulting mixture was refluxed at 111 °C for 24 h. Upon completion of the reaction, the solid was filtered off and washed with dry toluene for several times. T-Fe3O4- was achieved after drying at 100 °C overnight.
Synthesis of imine functionalized Fe3O4 with salicylaldehyde
Salicylaldehyde (0.5 mL) was dissolved in of methanol (5 mL) and added dropwise to the suspension of semicarbazide-propyl Fe3O4 (1 g) in dried methanol (25 mL). The mixture was subsequently refluxed at 60 °C for 10 h.
Synthesis Fe3O4@Sal@Cu
To incorporate copper, dried salicylaldehyde-Fe3O4 was suspended in absolute ethanol (20 mL). To this suspension, copper(II) acetate (0.2 g) was added and the resulting mixture was kept under refluxing condition for about 8 h at 90 °C. Upon completion of the reaction, the mixture was cooled to room temperature. Subsequently, the precipitate was filtered and purified by washing with ethanol repeatedly. The final catalyst was obtained after drying at 100 °C for 10 h. The schematic of preparation of Fe3O4@Sal@Cu is shown in Fig. 6.
General procedure for the synthesis of 2-amino-4H-chromenes
A mixture of an aromatic aldehyde (1.0 mmol), dimedone (1.0 mmol), malononitrile (1.1 mmol) and nanocatalyst (8 mg) in absolute EtOH (5 ml) was stirred at room temperature. The completion of the reaction was monitored by thin layer chromatography (TLC). After completion of the reaction, the catalyst was separated easily by an external magnet. The pure products were obtained from the reaction mixture by recrystallization from hot EtOH, and no more purification was required. All the product were known compounds which were identified by characterization of their melting points (as indicated in Table 3) by comparison with those authentic literature samples and also in some cases their FT‐IR and 1H NMR.
To disclose the worthy and usable of Fe3O4@Sal@Cu in large scale, we set up reaction with 4-chlorobenzaldehyde (50 mmol, 5.35 g), malononitrile (50 mmol, 3.3 g), dimedone (50 mmol, 7.0 g), Fe3O4@Sal@Cu (0.4 g) and ethanol (250 ml) in a round flask and then stirred for 5 h at room temperature. The reaction was carried out, and the product was obtained in (93% yield, 11.65 g). Therefore, Fe3O4@Sal@Cu could be used for the synthesis 2-amino-4H-chromenes in ethanol at room temperature even in large scale.
Reusability of the catalyst
To evaluate reusability of the catalyst, after completion of the reaction, the catalyst was removed by external magnet and washed by hot ethanol and dried in 60 °C for 3 h. Then, the recovered catalyst was used for the synthesis of 4b for four times. The results in Table 4 depicted that it acts as recovered catalyst as well as fresh catalyst. IR spectra of fresh catalyst and that recovered after five runs are indicated in Fig. 7.
Physical and spectra data for compounds
2-amino-7,7-dimethyl-4-(3-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (4b)
IR (KBr, cm−1): 3430, 3335, 3200, 2985, 2873, 2187, 1681, 1660, 1601, 1530, 1368, 1350, 1212, 1039, 826, 733. 1H NMR (300 MHz, DMSO, ppm) δ 7.17–7.66 (m, 4H Ar), 4.40 (s, 1H), 2.5 (bs, NH), 2.26 (d, J = 16.07 Hz, 2H), 2.10 (d, J = 16.04 Hz, 2H), 1.03 (s, 3H), 0.94 (s, 3H). 13C NMR (75 MHz, DMSO, ppm) δ 195.9, 163.1, 153.1, 150.2, 147.7, 147.0, 134.1, 124.3, 121.3, 119.6, 111.8, 110.1, 100.2, 57.6, 56.2, 44.3, 40.3, 35.6, 28.5, 26.2, 18.4.
Methyl 2-amino-4-(4-hydroxy-3-methoxyphenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (4 m)
IR(KBr, cm−1): 3209, 3028, 2952, 2887,2835, 1641, 1614, 1582, 1484, 1374, 1312, 1252, 1230, 1096, 1008, 758. 1H NMR (300 MHz, DMSO, ppm) δ 6.37–6.82 (m, 3H Ar), 3.64 (s, 1H), 3.44–3.55 (m, 3H), 2,75 (br, OH), 2,14–2,35 (m, 3H), 2.05 (br, NH), 1.46 (s, 2H), 1.30 (s, 2H), 1.05 (s, 3H), 1.01 (s, 3H). 13C NMR (75 MHz, DMSO, ppm) δ 206.1, 204.6, 196.9, 165.1, 164.2, 159.1, 147.7, 146.2, 145.3, 124.3, 118.6, 114.8, 110.1, 100.2, 57.6, 44.3, 38.3, 32.6, 27.5, 26.2.
Methyl 2-amino-4-(4-(dimethylamino)phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (4n)
IR(KBr, cm−1): 3396, 2955, 2888, 1738.13, 1613, 1520, 1377, 1165, 1065, 934, 816. 1H NMR (400 MHz, DMSO, ppm) δ 6.52–7.07 (m, 4H Ar), 3.64 (s, 1H), 3.44–3.55 (m, 3H), 2.80 (s, 6H), 2.29 (s, NH), 1.96 (s, 2H), 1.36–1.46 (dd, 2H), 0.9–1.05 (m, 6H). 13C NMR (75 MHz, DMSO, ppm) δ 196.9, 164.2, 159.1, 147.7, 147.2, 124.3, 118.9, 75.6, 61.7, 51.6, 44.3, 38.3, 30.6, 26.5, 18.2.
Ethyl 2-amino-7,7-dimethyl-5-oxo-4-(pyridin-4-yl)-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate (4o)
IR (KBr): 3402, 2959, 2871, 1742, 1686, 1597, 1533, 1370, 1243, 1203, 861.
1H NMR (300 MHz, DMSO, ppm) δ 7.10–7.66 (m, 4H Ar), 4.46 (s, 1H), 3.90–3.94 (m, 2H), 3.30 (s, 4H), 2.27 (bs, NH), 1.08 (m, 3H), 1.02 (s, 3H), 0.95 (s, 3H). 13C NMR (75 MHz, DMSO, ppm) δ 195.9, 163.2, 158.1, 147.7, 147.0, 124.3, 113.9, 75.6, 61.7, 51.6, 44.3, 38.3, 30.6, 26.5, 18.2.
Conclusions
In summary, the present research has developed an efficient and simple process for the synthesis of 2-amino-4H-chromenes by of Fe3O4@Sal@Cu as a novel, efficient and heterogeneous catalyst via three-component reaction conditions. The simple experimental procedure, short reaction times, easy to handle of the nanocatalyst, high reusability and magnetically separable, and very good yields are the advantages of this method.
References
Karimirad F, Behbahani FK (2020) γ-Fe2O3@ Si-(CH2)3@ mel@(CH2)4SO3H as a magnetically bifunctional and retrievable nanocatalyst for green synthesis of benzo [c] acridine-8 (9 H)-ones and 2-amino-4 H-chromenes. Inorg Nano-Met Chem 51:656–666. https://doi.org/10.1080/24701556.2020.1802751
Gunaganti N, Kharbanda A, Lakkaniga NR, Zhang L, Cooper R, Li HY, Frett B (2018) Catalyst free, C-3 functionalization of imidazo [1, 2-a] pyridines to rapidly access new chemical space for drug discovery efforts. Chem Commun 54:12954–12957. https://doi.org/10.1039/c8cc07063f
Younus HA, Al-Rashida M, Hameed A, Uroos M, Salar U, Rana S, Khan KM (2021) Multicomponent reactions (MCR) in medicinal chemistry: a patent review (2010–2020). Expert Opin Ther Pat 31:267–289. https://doi.org/10.1080/13543776.2021.1858797
Naresh G, Kant R, Narender T (2014) Copper (II) catalyzed expeditious synthesis of furoquinoxalines through a one-pot three-component coupling strategy. Org Lett 16:4528–4531. https://doi.org/10.1021/ol502072k
Slobbe P, Ruijter E, Orru RV (2012) Recent applications of multicomponent reactions in medicinal chemistry. Med Chem Comm 3:1189–1218. https://doi.org/10.1039/c2md20089a
Naresh G, Lakkaniga NR, Kharbanda A, Yan W, Frett B, Li HY (2019) Use of Imidazo [1, 2-a] pyridine as a carbonyl surrogate in a mannich-like, catalyst free, one-pot reaction. Eur J Org Chem 2019:770–777. https://doi.org/10.1002/ejoc.201801430
Weber L (2002) The application of multi-component reactions in drug discovery. Curr Med Chem 9:2085–2093. https://doi.org/10.2174/0929867023368719
Zavar S (2017) A novel three component synthesis of 2-amino-4H-chromenes derivatives using nano ZnO catalyst. Arabian J Chem 10:S67–S70. https://doi.org/10.1016/j.arabjc.2012.07.011
Arzehgar Z, Azizkhani V, Sajjadifar S, Fekri MH (2019) Synthesis of 2-amino-4H-chromene derivatives under solvent-free condition using MOF-5. Chem Method 3:251–260. https://doi.org/10.22034/CHEMM.2018.149048.1089
Maleki B, Tayebee R, Khoshsima A, Ahmadpoor F (2020) Facile Protocol for the Synthesis of 2-amino-4H-chromene derivatives using choline chloride/urea. Org Prep Proc Int 53:34–41. https://doi.org/10.1080/00304948.2020.1833623
Safari J, Zarnegar Z, Heydarian M (2013) Practical, ecofriendly, and highly efficient synthesis of 2-amino-4H-chromenes using nanocrystalline MgO as a reusable heterogeneous catalyst in aqueous media. J Taibah Uni Sci 7:17–25. https://doi.org/10.1016/j.jtusci.2013.03.001
Behbahani FK, Naderi M (2016) One-pot synthesis of 2-amino-4H-chromenes catalyzed by Fe (ClO4)3/SiO2. Russ J Gen Chem 86:2804–2806. https://doi.org/10.1134/s1070363216120434
Behbahani FK, Mehraban S (2015) Synthesis of 2-amino-3-cyano-7-hydroxy-4H-chromenes using l-proline as a biocatalyst. J Korean Chem Soc 59:284–288. https://doi.org/10.5012/jkcs.2015.59.4.284
Behbahani FK, Maryam S (2013) On Water CuSO4. 5H2O-catalyzed synthesis of 2-amino-4H-chromenes. J Korean Chem Soc 57:357–360. https://doi.org/10.5012/jkcs.2013.57.3.357
Baghernejad B, Fiuzat M (2020) A new strategy for the synthesis of 2-amino-4H-pyran derivatives in aqueous media using DABCO-Cucl complex as a novel and efficient catalyst. Eurasian Chem Commun 2:1088–1092. https://doi.org/10.22034/ecc.2020.250740.1078
Abdolmohammadi S, Afsharpour M, Keshavarz-Fatideh S (2014) An efficient green synthesis of 3-Amino-1H-chromenes catalyzed by ZnO Nanoparticles thin-film. South Afr J Chem 67:203–210
Abdolmohammadi S (2013) Solvent-free synthesis of 4, 5-dihydropyrano [c] chromene derivatives over TiO2 nanoparticles as an economical and efficient catalyst. Current Catal 2:116–121. https://doi.org/10.2174/2211544711302020005
Ebrahimi M, Abdolmohammadi S, Kia-Kojoori R (2020) Ultrasonic accelerated efficient synthesis of aminobenzochromenes using Ag2Cr2O7 nanoparticles as a reusable heterogeneous catalyst. J Heterocycl Chem 57:1875–1881. https://doi.org/10.1002/jhet.3915
Ma W, Ebadi AG, Javahershenas R, Jimenez G (2019) One-pot synthesis of 2-amino-4H-chromene derivatives by MNPs@ Cu as an effective and reusable magnetic nanocatalyst. RSC adv 9:12801–12812. https://doi.org/10.1039/C9RA01679A
Salimi M, Nasseri MA, Jazi BN (2019) Cu (II)-immobilized on functionalized magnetic nano-fibrillated cellulose (Fe3O4@ NFC/E-CHDA-Cu II): a novel, efficient and magnetically nanocatalyst for the one-pot synthesis of tetrahydrobenzo [b] pyran derivatives. J Iran Chem Soc 16:2221–2230. https://doi.org/10.1007/s13738-019-01689-0
Martina K, Calsolaro F, Zuliani A, Berlier G, Chávez-Rivas F, Moran MJ, Cravotto G (2019) Sonochemically-promoted preparation of silica-anchored cyclodextrin derivatives for efficient copper catalysis. Molecules 24:2490. https://doi.org/10.3390/molecules24132490
Bharathi M, Indira S, Vinoth G, Mahalakshmi T, Induja E, Shanmuga Bharathi K (2020) Green synthesis of benzimidazole derivatives under ultrasound irradiation using Cu-Schiff base complexes embedded over MCM-41 as efficient and reusable catalysts. J Coord Chem 73:653–670. https://doi.org/10.1080/00958972.2020.1730335
Hasanzadeh F, Behbahani FK (2020) Synthesis of 8-Aryl-7 H-acenaphtho [1, 2-d] imidazoles Using Fe3O4NPs@GO@C4H8SO3H as a Green and Recyclable Magnetic Nanocatalyst. Russ J Org Chem 56:1070–1076. https://doi.org/10.1134/s1070428020060160
Behbahani FK, Rezaee E, Fakhroueian Z (2014) Synthesis of 2-substituted benzimidazoles using 25% Co/Ce-ZrO2 as a heterogeneous and nanocatalyst. Catal Lett 144:2184–2190. https://doi.org/10.1007/s10562-014-1372-8
Yari H, Dehkharghani RA, Bardajee GR, Akbarzadeh-T N (2020) Synthesis, characterization, and applications of novel Co (II)-pyridoxal phosphate-Schiff base/SBA-15 as a nanocatalyst for the green synthesis of benzothiazole heterocycles. J Chin Chem Soc 67:1490–1500. https://doi.org/10.1002/jccs.201900518
Nami N, Zareyee D, Ghasemi M, Asgharzadeh A, Forouzani M, Mirzad S, Hashemi SM (2017) An efficient method for synthesis of some heterocyclic compounds containing 3-iminoisatin and 1, 2, 4-triazole using Fe3O4 magnetic nanoparticles. J Sul Chem 38:279–290. https://doi.org/10.1080/17415993.2017.127876
Rostami Z, Rouhanizadeh M, Nami N, Zareyee D (2018) Fe3O4 magnetic nanoparticles (MNPs) as an effective catalyst for synthesis of indole derivatives. Nanochem Res 3:142–148. https://doi.org/10.22036/NCR.2018.02.003
Fakheri-Vayeghan S, Abdolmohammadi S, Kia-Kojoori R (2018) An expedient synthesis of 6-amino-5-[(4-hydroxy-2-oxo-2H-chromen-3-yl)(aryl) methyl]-1, 3-dimethyl-2, 4, 6 (1H, 3H)-pyrimidinedione derivatives using Fe3O4@TiO2 nanocomposite as an efficient, magnetically separable, and reusable catalyst. Z Naturforsch B 73:545–551. https://doi.org/10.1515/znb-2018-0030
Abdolmohammadi S, Hossaini Z (2019) Fe3O4 MNPs as a green catalyst for syntheses of functionalized [1, 3]-oxazole and 1 H-pyrrolo-[1, 3]-oxazole derivatives and evaluation of their antioxidant activity. Mol Divers 23:885–896. https://doi.org/10.1007/s11030-019-09916-9
Chaghari-Farahani F, Abdolmohammadi S, Kia-Kojoori R (2020) A PANI-Fe3O4@ZnO nanocomposite: a magnetically separable and applicable catalyst for the synthesis of chromeno-pyrido [d] pyrimidine derivatives. RSC Adv 10:15614–15621. https://doi.org/10.1039/d0ra01978j
Abdolmohammadi S, Shariati S, Fard NE, Samani A (2020) Aqueous-Mediated green synthesis of novel spiro [indole-quinazoline] derivatives using kit-6 mesoporous silica coated Fe3O4 nanoparticles as catalyst. J Heterocycl Chem 57:2729–2737. https://doi.org/10.1002/jhet.3981
Abdolmohammadi S, Shariati S, Mirza B (2021) Ultrasound promoted and Kit-6 mesoporous silica-supported Fe3O4 magnetic nanoparticles catalyzed cyclocondensation reaction of 4-hydroxycoumarin, 3, 4-methylenedioxyphenol, and aromatic aldehydes. Appl Organomet Chem 35:e6117. https://doi.org/10.1002/aoc.6117
Kemnitzer W, Kasibhatla S, Jiang S, Zhang H, Wang Y, Zhao J, Jia S, Herich J, Labreque D, Storer R, Meerovitch K, Bouffard D, Rej R, Denis R, Blais C, Lamothe S, Attardo G, Gourdeau H, Tseng B, Drewe J, Cai SX (2004) Discovery of 4-Aryl-4 H-chromenes as a new series of apoptosis inducers using a cell-and caspase-based high-throughput screening assay. 1. structure− activity relationships of the 4-Aryl group. J Med Chem 47:6299–6310. https://doi.org/10.1021/jm049640t
Behbahani F, Alipour F (2015) One-pot synthesis of 2-amino-4H-pyrans and 2-amino-tetrahydro-4H-chromenes using L-proline. Gazi Uni J Sci 28:387–393
Dekamin MG, Eslami M, Maleki A (2013) Potassium phthalimide-N-oxyl: a novel, efficient, and simple organocatalyst for the one-pot three-component synthesis of various 2-amino-4H-chromene derivatives in water. Tetrahedron 69:1074–1085. https://doi.org/10.1016/j.tet.2012.11.068
Mahmoud AF, Abd El-Latif FF, Ahmed AM (2010) Microwave assisted one-pot synthesis of 2-amino-4H-chromenes and spiropyrano [2, 3-d] pyrimidine. Chin J Chem 28:91–96. https://doi.org/10.1002/cjoc.201090041
Azarifar D, Khatami SM, Nejat-Yami R (2014) Nano-titania-supported Preyssler-type heteropolyacid: an efficient and reusable catalyst in ultrasound-promoted synthesis of 4H-chromenes and 4H-pyrano [2, 3-c] pyrazoles. J Chem Sci 126:95–101. https://doi.org/10.1007/s12039-013-0548-x
Jin TS, Wang AQ, Wang X, Zhang JS, Li TS (2004) A clean one-pot synthesis of tetrahydrobenzo [b] pyran derivatives catalyzed by hexadecyltrimethyl ammonium bromide in aqueous media. Synlett 2004:0871–0873. https://doi.org/10.1055/s-2004-820025
Thakur A, Tripathi M, Rajesh UC, Rawat DS (2013) Ethylenediammonium diformate (EDDF) in PEG 600: an efficient ambiphilic novel catalytic system for the one-pot synthesis of 4 H-pyrans via Knoevenagel condensation. RSC Adv 3:18142–18148. https://doi.org/10.1039/C3RA42410C
Banerjee S, Saha A (2013) Free-ZnO nanoparticles: a mild, efficient and reusable catalyst for the one-pot multicomponent synthesis of tetrahydrobenzo [b] pyran and dihydropyrimidone derivatives. New J Chem 37:4170–4175. https://doi.org/10.1039/C3NJ00723E
Maleki B, Baghayeri M, Abadi SAJ, Tayebee R, Khojastehnezhad A (2016) Ultrasound promoted facile one pot synthesis of highly substituted pyran derivatives catalyzed by silica-coated magnetic NiFe2O4 nanoparticle-supported H14[NaP5W30O110] under mild conditions. RSC adv 6:96644–96661. https://doi.org/10.1039/C6RA20895A
Balalaie S, Bararjanian M, Amani AM, Movassagh B (2006) (S)-Proline as a neutral and efficient catalyst for the one-pot synthesis of tetrahydrobenzo [b] pyran derivatives in aqueous media. Synlett 2006:263–266. https://doi.org/10.1055/s-2006-926227
Wang XS, Shi DQ, Tu SJ, Yao CS (2003) A convenient synthesis of 5-Oxo-5, 6, 7, 8-tetrahydro-4 H-benzo-[b]-pyran derivatives catalyzed by KF-Alumina. Synth Commun 33(1):119–126. https://doi.org/10.1081/SCC-120015567
Rostamnia S, Nuri A, Xin H, Pourjavadi A, Hosseini SH (2013) Water dispersed magnetic nanoparticles (H2O-DMNPs) of γ-Fe2O3 for multicomponent coupling reactions: a green, single-pot technique for the synthesis of tetrahydro-4H-chromenes and hexahydroquinoline carboxylates. Tetrahedron Lett 54:3344–3347. https://doi.org/10.1016/j.tetlet.2013.04.048
Tahmassebi D, Bryson JA, Binz SI (2011) 1, 4-Diazabicyclo [2.2. 2] octane as an efficient catalyst for a clean, one-pot synthesis of tetrahydrobenzo [b] pyran derivatives via multicomponent reaction in aqueous media. Synth Commun 41:2701–2711. https://doi.org/10.1080/00397911.2010.515345
Zolfigol MA, Bahrami-Nejad N, Afsharnadery F, Baghery S (2016) 1-Methylimidazolium tricyanomethanide [HMIM]C(CN)3 as a nano structure and reusable molten salt catalyst for the synthesis of tetrahydrobenzo [b] pyrans via tandem Knoevenagel-Michael cyclocondensation and 3, 4-dihydropyrano [c] chromene derivatives. J Mol Liq 221:851–859. https://doi.org/10.1016/j.molliq.2016.06.069
Katkar SS, Lande MK, Arbad BR, Gaikwad ST (2011) A Recyclable and Highly Effective ZnO-beta Zeolite as a Catalyst for One-pot Three-Component Synthesis of Tetrahydrobenzo [b] pyrans. Chin J Chem 29:199–202. https://doi.org/10.1002/cjoc.201190052
Davoodnia A, Allameh S, Fazli S, Tavakoli-Hoseini N (2011) One-pot synthesis of 2-amino-3-cyano-4-arylsubstituted tetrahydrobenzo [b] pyrans catalysed by silica gel-supported polyphosphoric acid (PPA-SiO2) as an efficient and reusable catalyst. Chem Pap 65:714–720. https://doi.org/10.2478/s11696-011-0064-8
Nongrum R, Nongthombam GS, Kharkongor M, Rahman N, Kathing C, Myrboh B, Nongkhlaw R (2016) A nano-organo catalyzed route towards the efficient synthesis of benzo [b] pyran derivatives under ultrasonic irradiation. RSC Adv 6:108384–108392. https://doi.org/10.1039/C6RA24108E
Khoobi M, Ma’mani L, Rezazadeh F, Zareie Z, Foroumadi A, Ramazani A, Shafiee A (2012) One-pot synthesis of 4H-benzo [b] pyrans and dihydropyrano [c] chromenes using inorganic–organic hybrid magnetic nanocatalyst in water. J Mol Catal A Chem 359:74–80. https://doi.org/10.1016/j.molcata.2012.03.023
Metwally MA, Bondock S, El-Azap H, Kandeel EEM (2011) Thiosemicarbazides: synthesis and reactions. J Sul Chem 32:489–519. https://doi.org/10.1080/17415993.2011.601869
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ferdousian, R., Behbahani, F.K. & Mohtat, B. Synthesis and characterization of Fe3O4@Sal@Cu as a novel, efficient and heterogeneous catalyst and its application in the synthesis of 2-amino-4H-chromenes. Mol Divers 26, 3295–3307 (2022). https://doi.org/10.1007/s11030-022-10391-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10391-y