Abstract
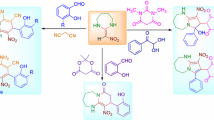
An efficient, eco-friendly protocol has been described for the chemoselective synthesis of tetracyclic pyrido-fused dibenzodiazepines derivatives via catalyst-free, three-component reaction of dimedone, 1,2-diamines, 3-formylchromones, and malononitrile. The significant advantages of this cascade approach are to create two new rings and four new σ bonds containing three C–N and one C–C bond, as well as the breakdown of a C–O bond.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dibenzo-[1,4]diazepines are seven-membered heterocyclic compounds containing two nitrogen atoms. These molecular scaffolds are the essential core of many biological compounds [1]. They display excellent medicinal properties such as HIV protease inhibitors [2], hepatitis C virus (HCV) NS5B, polymerase inhibitors [3], cystathionine β-synthase inhibitors [4], neuromedin B receptor antagonist [5], antimalarial [6], antitumor [7], analgesic [8], and antitrypanosomal [6].
The modification of dibenzo-[1,4]diazepines from 1,4-diazepine fragment has been prominent in the synthesis of tetracyclic systems. Accordingly, various derivatives have been synthesized according to their therapeutic properties, including pyrido[2,3-b]benzo-1,4-diazepines and dipyrido[3,2-b:2,3-e]-1,4-diazepines [9].
Further, fused heterocycle to diazepine nitrogen leads to bioactive properties [10]. For example, pyridooxazepines are progesterone receptor modulators that are used in contraception, hormone replacement therapy (HRT), treatment of gynecological disorders, and cancer [11]. Pyridodiazepines are non-steroidal glucocorticoid receptor with anti-inflammatory activity [12]. Pyrrolo-1,5-benzoxazepines are also used to induce apoptosis in acute lymphoblastic leukemia cells [13]. Altogether, these structures improve the tendency toward the cholecystokinin (CCK2) receptor involved in the pathological situation [14]. Some representative benzodiazepines that fused to heterocycles are shown in Fig. 1.
Accordingly, due to the therapeutic significance of dibenzodiazepines, various synthetic methods have been reported in the literature [1, 15,16,17,18,19,20,21,22,23]. However, the synthesis of the fused-heterocyclic compound containing dibenzodiazepines backbone is underdeveloped [24]. Pyrido-fused dibenzodiazepines were synthesized through a multistep strategy coupling/reduction/N-formylation/ring-closing/hetero-Diels–Alder sequence [12]. But, this procedure employs a multistep method, a toxic reducing reagent, and harsh reaction conditions for the synthesis of tetracyclic compounds [11]. So, developing an efficient approach for rapid access to tetracyclic structures using cascade reactions is remarkable.
There are two conventional approaches for the synthesis of dibenzodiazepines, which involve: 1. enaminones which obtained from the reaction of 1,3-dicarbonyl and o-phenylenediamines were treated with aldehydes in the presence of acetic acid under reflux [21, 25]; 2. o-phenylenediamines were added to Michael adduct intermediate of the reaction of aldehyde and dimedone [26].
Due to the ease and high efficiency of these methods, dibenzodiazepine can be considered as the primary core, and pyrido moiety can be attached to the two remaining positions of the seven-membered ring.
In addition, the Knoevenagel reaction, which is widely used for C=C bond formation, is based on nucleophilic addition in which the active hydrogen compound is added to the carbonyl functional group along with the removal of water. In our laboratory, a broad range of heterocyclic compounds has been synthesized based on Knoevenagel condensation [27,28,29,30,31,32,33,34].
The intermediate obtained from 3-formylchromones and malononitrile has three electrophilic sites: 1. carbon 4 atom, which belongs to the carbonyl group; 2. carbon atom 2, which is considered as a hidden aldehyde; and 3. the Knoevenagel C=C bond. So, in the reaction with enaminone, these different possible pathways may result in various products. Inspired by these facts, we were interested in examining the reaction of Knoevenagel adduct as a suitable substrate with enaminone without using any catalyst or strong acid in relatively short reaction time. So, we commenced our investigation of the catalyst-free multi-component reaction of dimedone, 1,2-diamines, 3-formylchromones, and malononitrile (Scheme 1).
An extensive literature survey revealed that the benzylidene-malononitrile substrates, the double bond of Knoevenagel adduct intermediate, undergo Michael addition reaction to produce benzimidazo[1,2-a]quinoline [35]. Nevertheless, in our approach, the pyrone position is selectively attacked, and the double bond made by Knoevenagel condensation is ultimately involved in the enlarging of the ring underreacting with an amine group and forms a tetracyclic compound. Therefore, this reaction is beneficial due to the formation of a pyrido-fused dibenzodiazepine via Knoevenagel adduct intermediate.
Results and discussion
Inspired by the above results, we became attracted to know the condition, which leads to the reaction of enaminone 4 with Knoevenagel adduct 5. For this purpose, initially, we explored the reaction of 3-formylchromones with malononitrile at room temperature in the mixture of H2O and EtOH to make the Knoevenagel adduct intermediate. Next, the sequential addition of enaminone gave us dibenzo[b,f]pyrido[1,2-d][1,4]diazepine in 35% yield. Later, in order to improve efficiency and to facilitate the formation of enaminone via grinding under solvent-free conditions at 80 °C, the sequential one-pot reaction continued under the same conditions. However, this method was not effective because of low efficiency and difficulties in purification.
The scope of the methodology was optimized under different solvents. Because of the low synthesis efficiency of enaminone in all solvents but ethanol, the reaction was done in ethanol, and the best yield was obtained. Gratifyingly, the new tetracyclic pyrido-fused benzodiazepines 3a–3h were reached in 70–90% yields at room temperature. To optimize the reaction time, we repeated the reaction in ethanol at reflux. We successfully observed that the products were formed in a shorter time of 8 h (instead of 3 days) (Table 1).
Having the optimal conditions in hand, we then examined the scope of the reaction between substituted enaminone substrates and 3-formylchromones derivatives. As shown in Table 2, we synthesized tetracyclic compounds from straightforward and available starting materials. It is worth noting that the electron-withdrawing or -donating moieties on the aromatic rings had no significant effect on the overall efficiency.
Putting the chlorine atom on the chromone ring in the starting material was also assisting the hydrolysis of the nitrile group. It resulted in the formation of amide in the final step. Because phenol is a weak acid, electron-withdrawing substituents on the ring make phenolate ion more stable and phenol more acidic through the delocalization of the negative charge and inductive effects. As a result, due to the presence of chlorine and ketone substituents on the 6-chloro-3-formylchromone moiety, the intramolecular proton transfer is provided, and the amino vicinal electron-donating substituent also accelerates the reaction conditions for the hydrolysis of nitrile group. The structure of representative compound 3d was established from single-crystal X-ray analysis and is depicted in Fig. 2.
The mass spectrum of 3d displayed a molecular ion peak at m/z = 504.16, which was compatible with a 1:1:1:1 adduct of 6-chloro-3-formylchromone, malononitrile, dimedone, and o-phenylenediamine. The 1H NMR spectrum of 3d showed signals in the aliphatic regions related to two methyl groups (δ = 0.96 and 0.99 ppm) and two methylene groups (δ = 1.94, 2.14 ppm). Both the 6-membered heterocyclic hydrogens peak appear as a singlet signal. The aryl moieties give typical signals in the aromatic region. Also, two singlet signals in δ = 9.23 and δ = 9.97 ppm showed the protons of an amide and phenolic O–H, respectively. In the carbon spectrum, 27 particular resonances are consistent with the structure of the dibenzo[b,f]pyrido[1,2-d][1,4]diazepine 3d. Carbonyl carbon and amide groups resonated at δ = 189.36, δ = 193.82, and δ = 171.12.
The plausible mechanism of this chemoselective cascade reactions is proposed, as shown in Scheme 2. At first, the 3-formylchromone 1a undergoes Knoevenagel condensation with malononitrile to give adduct 5. Also, the condensation of dimedone with o-phenylenediamine 2a forms enaminone 4. The selective nucleophilic addition of 4 to the pyrone ring may create an open-chain intermediate 6. This cascade reaction would proceed with nucleophilic substitution of amine to the β-position of the ketone carbonyl. The enlarging the number of rings is continued, possibly with the subsequent addition of the amino group of the diazepine to the C≡N triple bond.
Imine group of intermediate 8 would convert to an amine by a [1, 3]-H shift, and the desired product of pyrido-fused dibenzodiazepine 3 may be formed.
In conclusion, we have investigated a catalyst-free three-component reaction of dimedone, 1,2-diamines, 3-formylchromones, and malononitrile for selective synthesis of dibenzo[b,f]pyrido[1,2-d][1,4]diazepine derivatives. Since most of the fused seven-member heterocyclic compounds are prepared using a catalyst, or in microwave condition, the substrate type can provide the conditions for the catalyst-free reaction. In previous catalytic systems, aldehydes have been used as a substrate. We disclosed that the generated Knoevenagel adduct would act as a soft electrophile and initiate a novel cascade sequence. The pyrone ring can also undergo ring-opening and secondary cyclization reactions. Other advantages of this method are that all starting materials are presented in the product without any metal catalyst, a green reaction medium, ethanol, is used, and the purification is performed without chromatography.
Experimental
Melting points were measured on an Electrothermal 9100 apparatus. Elemental analyses for C, H, and N performed using a Heraeus CHN–O-Rapid analyzer. IR spectra were recorded as KBr pellets on a NICOLET FTIR 100 spectrometer. Mass spectra were recorded on a FINNIGAN-MATT 8430 mass spectrometer operating at an ionization potential of 70 eV. 1H NMR (500 MHz) 1H NMR (300 MHz) and 13C NMR (125 MHz) 13C NMR (75 MHz) spectra were obtained using Bruker DRX-500 AVANCE and Bruker DRX-300 AVANCE spectrometers. All NMR spectra at room temperature were recorded in DMSO-d6. Chemical shifts are reported in parts per million (δ) downfield from an internal tetramethylsilane reference. Coupling constants (J values) are reported in hertz (Hz). The following symbols indicate spin multiplicities: brs (broad singlet), s (singlet), d (doublet), t (triplet), td (triplet of doublets), dd (doublet of doublets), and m (multiplet). All chemicals were purchased from Merck or Aldrich and were used without further purification.
Dibenzo[b,f]pyrido[1,2-d][1,4]diazepine; general procedure
The solution of dimedone (1 mmol) and 1,2-diamines (1 mmol) in EtOH was magnetically stirred at reflux for 1.0 h. Subsequently, the resulting enaminone was reacted with Knoevenagel adduct derived from 3-formylchromone (1 mmol) and malononitrile (1 mmol). Upon completion (50–60 min) as monitored by TLC, the reaction mixture was filtered to give the crude product, which was further washed with EtOH to obtain pure product.
4-Amino-1-(2-hydroxybenzoyl)-12,12-dimethyl-14-oxo-10,11,12,13,14,14b-hexahydrodibenzo[b,f]pyrido[1,2-d][1,4]diazepine-3-carbonitrile (3a, C27H24N4O3)
Yellow powder, mp = 223–226 °C, 0.5 g, yield: 83%. IR (KBr) (νmax, cm−1): 3317 (NH2), 3052 (CH), 2215 (CN), 1713 and 1632 (C=O), 1582 and 1483 (Ar). Anal. Calcd. for C27H24N4O3 (452.18): C, 71.67; H, 5.35; N, 12.38%. Found: C, 71.60; H, 5.26; N, 12.29%. MS (EI, 70 eV): m/z (%) = 452 (12), 437 (26), 384 (18), 355 (21), 302 (21), 282 (100), 254 (32), 191 (29), 132 (29). 1H NMR (400.13 MHz, DMSO-d6): δH 1.01 (6H, s, 2 CH3), 2.00 (1H, d, 2JHH = 16.0 Hz, CH2), 2.20 (1H, d, 2JHH = 16.2 Hz, CH2), 2.51 (2H, ABq, 2JHH = 16.5 Hz, CH2), 5.60 (1H, s, CH14b), 6.63 (2H, bs, NH2), 6.64–7.34 (8H, m, 8 CH of Ar), 7.15 (1H, s, CH2), 9.34 (1H, s, NH), 9.94 (1H, s, OH). 13C NMR (100.00 MHz, DMSO-d6): δC 27.12 (CH3), 27.59 (CH3), 30.95 (C12), 44.66 (CH2), 50.94 (CH2), 56.73 (CH14b), 62.40 (C3), 110.81 (C14a), 116.43 (CH of Ar), 116.78 (C1), 118.70 (CH of Ar), 120.39 (CN), 121.54 (CH6), 123.19 (CH9), 124.89 (Cipso–C=O), 126.71 (CH7), 128.61 (CH8), 129.99 (CH of Ar), 131.12 (CH of Ar), 131.30 (CH2), 137.50 (C9a), 139.41 (C5a), 155.63 (C10a), 157.52 (C4–NH2), 157.52 (C–OH), 190.39 (C=O), 193.56 (C=O).
4-Amino-1-(2-hydroxybenzoyl)-8,12,12-trimethyl-14-oxo-10,11,12,13,14,14b-hexahydrodibenzo[b,f]pyrido[1,2-d][1,4]diazepine-3-carbonitrile (3b, C28H26N4O3)
Yellow powder, mp = 252–254 °C, 0.5 g, yield: 75%. IR (KBr) (νmax, cm−1): 3353 (NH2), 3000, 2931, and 2851 (CH), 2187 (CN), 1752 and 1650 (C=O), 1580 and 1488 (Ar). Anal. Calcd. for C28H26N4O3 (466.54): C, 72.09; H, 5.62; N, 12.01%. Found: C, 71.99; H, 5.59; N, 11.99%. MS (EI, 70 eV): m/z (%) = 465 (66), 450 (71), 399 (66), 315 (100), 243 (35), 221 (66), 186 (68), 145 (91), 121 (67), 57 (67). 1H NMR (400.13 MHz, DMSO-d6): δH 0.96 (3H, s, CH3), 1.01 (3H, s, CH3), 1.95 (1H, d, 2JHH = 16.0 Hz, CH2), 2.17 (1H, d, 2JHH = 16.5 Hz, CH2), 2.24 (3H, s, CH3), 2.26 (1H, d, 2JHH = 16.0 Hz, CH2), 2.49 (1H, d, 2JHH = 16.0 Hz, CH2), 5.55 (1H, s, CH14b), 6.57 (1H, s, CH6), 6.85 (1H, d, 3JHH = 8.2 Hz, CH8), 6.87 (1H, t, 3JHH = 7.9 Hz, CH of Ar), 6.88 (1H, d, 3JHH = 7.9 Hz, CH9), 6.95 (2H, bs, NH2), 7.13 (1H, s, CH2), 7.23 (1H, d, 3JHH = 8.2 Hz, CH of Ar), 7.30 (1H, t, 3JHH = 7.7 Hz, CH of Ar), 7.38 (1H, d, 3JHH = 7.5 Hz, CH of Ar), 9.27 (1H, s, NH), 9.90 (1H, s, OH). 13C NMR (100.00 MHz, DMSO-d6): δC 19.99 (CH3), 27.07 (CH3), 27.62 (CH3), 30.91 (C12), 44.66 (CH2), 50.95 (CH2), 56.72 (CH14b), 62.30 (C3), 110.20 (C14a), 116.43 (CH of Ar), 116.78 (C1), 118.69 (CH of Ar), 120.39 (CN), 121.40 (CH6), 124.90 (Cipso–C=O), 126.75 (CH9), 129.34 (CH8), 129.91 (CH of Ar), 130.98 (Cipso–CH3), 131.24 (CH of Ar), 132.66 (CH2), 136.82 (C9a), 137.40 (C5a), 155.51 (C10a), 157.37 (C4–NH2), 157.51 (C–OH), 190.29 (C=O), 193.39 (C=O).
4-Amino-8-chloro-1-(2-hydroxybenzoyl)-12,12-dimethyl-14-oxo-10,11,12,13,14,14b-hexahydrodibenzo[b,f]pyrido[1,2-d][1,4]diazepine-3-carbonitrile (3c, C27H23ClN4O3)
Yellow powder, mp = 265–267 °C, 0.5 g, yield: 67%. IR (KBr) (νmax, cm−1): 3339 (NH2), 3116, 2958, and 2869 (CH), 2179 (CN), 1604 (C=O), 1577 and 1487 (Ar). Anal. Calcd. for C27H23ClN4O3 (486.15): C, 66.60; H, 4.76; N, 11.51%. Found: C, 66.57; H, 4.73; N, 11.49%. MS (EI, 70 eV): m/z (%) = 486 (91), 471 (100), 429 (29), 389 (58), 336 (19), 313 (26), 249 (29), 222 (26), 193 (45), 166 (58), 121 (68), 92 (39), 65 (35). 1H NMR (400.13 MHz, DMSO-d6): δH 0.96 (3H, s, CH3), 1.01 (3H, s, CH3), 1.96 (1H, d, 2JHH = 16.0 Hz, CH2), 2.19 (1H, d, 2JHH = 16.2 Hz, CH2), 2.49 (2H, ABq, 2JHH = 16.5 Hz, CH2), 5.54 (1H, s, CH14b), 6.58 (1H, s, CH9), 6.86 (1H, d, 3JHH = 8.0 Hz, CH of Ar), 6.87 (1H, t, 3JHH = 8.7 Hz, CH of Ar), 7.08 (2H, bs, NH2), 7.15 (1H, s, CH2), 7.26 (1H, d, 3JHH = 9.0 Hz, CH of Ar), 7.30 (1H, t, 3JHH = 7.8 Hz, CH of Ar), 7.31 (1H, d, 3JHH = 8.5 Hz, CH7), 7.35 (1H, d, 3JHH = 8.5 Hz, CH6), 9.36 (1H, s, NH), 9.86 (1H, s, OH). 13C NMR (100.00 MHz, DMSO-d6): δC 27.21 (CH3), 27.45 (CH3), 30.98 (C12), 44.54 (CH2), 50.91 (CH2), 56.61 (CH14b), 62.57 (C3), 111.18 (C14a), 116.42 (CH of Ar), 116.96 (C1), 118.66 (CH of Ar), 120.24 (CN), 122.80 (CH6), 124.84 (Cipso–C=O), 126.27 (Cipso–Cl), 126.43 (CH9), 128.56 (CH7), 129.91 (CH of Ar), 131.27 (CH of Ar), 132.42 (CH2), 137.52 (C9a), 138.57 (C5a), 155.56 (C10a), 157.19 (C4–NH2), 157.39 (C–OH), 190.32 (C=O), 193.59 (C=O).
4-Amino-1-(5-chloro-2-hydroxybenzoyl)-12,12-dimethyl-14-oxo-10,11,12,13,14,14b-hexahydrodibenzo[b,f]pyrido[1,2-d][1,4]diazepine-3-carboxamide (3d, C27H25ClN4O4)
Yellow powder, mp = 261–263 °C, 0.5 g, yield: 75%. IR (KBr) (νmax, cm−1): 3378, 3310 (NH2), 3125, 2955, and 2869 (CH), 1722, 1668, and 1601 (C=O), 1561 and 1499 (Ar). Anal. Calcd. for C27H25ClN4O4 (504.16): C, 64.22; H, 4.99; N, 11.10%. Found: C, 64.19; H, 4.79; N, 11.01%. MS (EI, 70 eV): m/z (%) = 504 (1), 487 (10), 444 (10), 403 (11), 274 (11), 230 (13), 197 (100), 173 (40), 154 (52), 132 (53), 83 (60), 55 (78). 1H NMR (400.13 MHz, DMSO-d6): δH 0.96 (3H, s, CH3), 0.99 (3H, s, CH3), 1.94 (1H, d, 2JHH = 16.0 Hz, CH2), 2.14 (1H, d, 2JHH = 16.1 Hz, CH2), 2.49 (2H, ABq, 2JHH = 16.4 Hz, CH2), 5.52 (1H, s, CH14b), 6.46 (4H, bs, 2 NH2), 6.85 (1H, d, 3JHH = 8.8 Hz, CH of Ar), 7.04 (1H, t, 3JHH = 7.6 Hz, CH8), 7.09 (1H, s, CH2), 7.14 (1H, dd, 3JHH = 8.5 Hz, 4JHH = 1.5 Hz, CH6), 7.26 (1H, dd, 3JHH = 8.8 Hz, 4JHH = 2.7 Hz, CH of Ar), 7.31 (1H, td, 3JHH = 7.0 Hz, 4JHH = 1.6 Hz, CH7), 7.38 (1H, d, 3JHH = 8.3 Hz, CH9), 7.40 (1H, d, 4JHH = 2.7 Hz, CH of Ar), 9.23 (1H, s, NH), 9.97 (1H, s, OH). 13C NMR (100.00 MHz, DMSO-d6): δC 27.74 (CH3), 28.06 (CH3), 31.42 (C12), 45.10 (CH2), 51.57 (CH2), 60.70 (CH14b), 85.56 (C3), 112.10 (C14a), 115.12 (C1), 118.38 (CH of Ar), 121.86 (CH6), 122.67 (CH9), 123.54 (Cipso–C=O), 127.54 (CH7), 128.42 (Cipso–Cl), 128.89 (CH8), 129.55 (CH of Ar), 130.36 (CH of Ar), 131.34 (CH2), 135.89 (C9a), 140.06 (C5a), 154.73 (C4–NH2), 157.25 (C14a), 158.22 (C–OH), 171.12 (CO2NH2), 189.37 (C=O), 193.82 (C=O). Crystal data for 3d C27H27ClN4O5 (CCDC 1970238): MW = 575.55, orthorhombic, P 21 21 21, a = 9.7052(19) Å, b = 13.015(3) Å, c = 19.712(4) Å, α = 90,00, β = 90,00, γ = 90,00, V = 2489.9(9) Å3, Z = 4, Dc = 1.395 mg/m3, F(000) = 1096, crystal dimension 0.25 × 0.20 × 0.15 mm, radiation, Mo Kα (λ = 0.71073 Å), 2.1 ≤ 2θ ≤ 25.0, intensity data were collected at 293.15 K with a Bruker APEX area-detector diffractometer, and employing ω/2θ scanning technique, in the range of − 11 ≤ h ≤ 11, − 15 ≤ k ≤ 15, − 23 ≤ l ≤ 21; the structure was solved by a direct method, all non-hydrogen atoms were positioned, and anisotropic thermal parameters refined from 4325 observed reflections with R (into) = 0.0750 by a full-matrix least-squares technique converged to R1 = 0.0490, and wR2 = 0.1234 [I > 2sigma(I)].
4-Amino-1-(5-chloro-2-hydroxybenzoyl)-8,12,12-trimethyl-14-oxo-10,11,12,13,14,14b-hexahydrodibenzo[b,f]pyrido[1,2-d][1,4]diazepine-3-carboxamide (3e, C28H27ClN4O4)
Yellow powder, mp = 266–267 °C, 0.5 g, yield: 75%. IR (KBr) (νmax, cm−1): 3477, 3438, 3347, and 3313 (NH2), 3123, 2956, and 2867 (CH), 1648 and 1602 (C=O), 1559 and 1481 (Ar). Anal. Calcd. for C28H27ClN4O4 (519.00): C, 64.80; H, 5.24; N, 10.80%. Found: C, 64.73; H, 5.11; N, 10.69%. MS (EI, 70 eV): m/z (%) = 518 (1), 485 (4), 293 (10), 274 (8), 244 (26), 230 (50), 211 (49), 187 (80), 173 (30), 146 (100), 126 (16), 83 (24). 1H NMR (400.13 MHz, DMSO-d6): δH 0.96 (3H, s, CH3), 0.99 (3H, s, CH3), 1.94 (1H, d, 2JHH = 16.2 Hz, CH2), 2.14 (1H, d, 2JHH = 16.0 Hz, CH2), 2.26 (3H, s, CH3), 2.48 (2H, ABq, 2JHH = 16.4 Hz, CH2), 5.53 (1H, s, CH14b), 6.46 (4H, bs, 2 NH2), 6.86 (1H, d, 3JHH = 9.3 Hz, CH of Ar), 6.98 (1H, s, CH6), 7.09 (1H, s, CH2), 7.13 (1H, d, 3JHH = 8.1 Hz, CH9), 7.25 (1H, d, 3JHH = 8.9 Hz, CH of Ar), 7.29 (1H, d, 3JHH = 8.9 Hz, CH8), 7.41 (1H, s, CH of Ar), 9.14 (1H, s, NH), 10.0 (1H, s, OH). 13C NMR (100.00 MHz, DMSO-d6): δC 20.51 (CH3), 27.69 (CH3), 28.12 (CH3), 31.38 (C12), 45.16 (CH2), 51.58 (CH2), 56.35 (CH14b), 85.57 (C3), 111.55 (C14a), 115.16 (C1), 118.43 (CH of Ar), 122.71 (CH6), 124.54 (Cipso–C=O), 127.36 (Cipso-Cl), 127.63 (CH9), 128.38 (Cipso–CH3), 129.53 (CH of Ar), 129.65 (CH8), 130.40 (CH of Ar), 131.25 (C9a), 133.04 (CH2), 137.46 (C5a), 154.73 (C4–NH2), 158.14 (C10a), 158.32 (C–OH), 171.14 (CO2NH2), 189.39 (C=O), 193.72 (C=O).
4-Amino-8-chloro-1-(5-chloro-2-hydroxybenzoyl)-12,12-dimethyl-14-oxo-10,11,12,13,14,14b-hexahydrodibenzo[b,f]pyrido[1,2-d][1,4]diazepine-3-carboxamide (3f, C27H24Cl2N4O4)
Yellow powder, mp = 271–273 °C, 0.5 g, yield: 77%. IR (KBr) (νmax, cm−1): 3481, 3437, 3344, and 3313 (NH2), 3123, 2956, and 2867 (CH), 1719, 1639, and 1603 (C=O), 1561 and 1492 (Ar). Anal. Calcd. for C27H24Cl2N4O4 (538.12): C, 60.12; H, 4.48; N, 10.39%. Found: C, 59.93; H, 4.39; N, 10.21%. MS (EI, 70 eV): m/z (%) = 538 (4), 480 (4), 438 (7), 370 (9), 264 (20), 230 (53), 209 (37), 193 (37), 166 (100), 147 (13), 126 (27), 99 (20), 83 (43), 55 (37). 1H NMR (400.13 MHz, DMSO-d6): δH 0.95 (3H, s, CH3), 0.99 (3H, s, CH3), 1.94 (1H, d, 2JHH = 16.0 Hz, CH2), 2.15 (1H, d, 2JHH = 16.1 Hz, CH2), 2.48 (2H, ABq, 2JHH = 16.8 Hz, CH2), 5.49 (1H, s, CH14b), 6.50 (4H, bs, 2 NH2), 6.84 (1H, d, 3JHH = 8.7 Hz, CH of Ar), 7.09 (1H, s, CH2), 7.21 (1H, s, CH9), 7.26 (1H, dd, 3JHH = 8.6 Hz, 4JHH = 3.0 Hz, CH of Ar), 7.33 (1H, d, 3JHH = 8.6 Hz, CH7), 7.36 (1H, d, 3JHH = 8.7 Hz, CH6), 7.41 (1H, s, CH of Ar), 9.24 (1H, s, NH), 9.93 (1H, s, OH). 13C NMR (100.00 MHz, DMSO-d6): δC 27.83 (CH3), 27.93 (CH3), 31.45 (C12), 45.01 (CH2), 51.53 (CH2), 56.29 (CH14b), 85.66 (C3), 112.53 (C14a), 115.29 (C1), 118.37 (CH of Ar), 122.67 (CH6), 123.14 (Cipso–C=O), 126.61 (Cipso–Cl), 127.18 (CH9), 128.36 (Cipso–Cl), 128.83 (CH7), 129.56 (CH of Ar), 130.40 (CH of Ar), 132.30 (CH2), 137.81 (C9a), 139.24 (C5a), 154.74 (C4–NH2), 156.96 (C10a), 158.07 (C–OH), 171.08 (CO2NH2), 189.40 (C=O), 193.87 (C=O).
1-(2-Hydroxybenzoyl)-4-imino-12,12-dimethyl-14-oxo-4,5a,6,7,8,9,9a,10,11,12,13,14-dodecahydrodibenzo[b,f]pyrido[1,2-d][1,4]diazepine-3-carbonitrile (3 g, C27H28N4O3)
Yellow powder, mp = 255–257 °C, 0.5 g, yield: 70%. IR (KBr) (νmax, cm−1): 3361, 3340 (NH2), 3158, 2949, and 2851 (CH), 2180 (CN), 1661 and 1614 (C=O), 1588 and 1499 (Ar). Anal. Calcd. for C27H28N4O3 (458.56): C, 71.03; H, 6.18; N, 12.27%. Found: C, 70.60; H, 6.16; N, 12.19%. MS (EI, 70 eV): m/z (%) = 452 (12), 437 (26), 384 (18), 355 (21), 302 (21), 282 (100), 254 (32), 191 (29), 132 (29). 1H NMR (400.13 MHz, DMSO-d6): δH 0.92 (6H, s, 2 CH3), 1.35–1.40 (4H, m, CH2), 1.44–1.48 (2H, m, CH2), 1.64–1.72 (2H, m, CH2), 1.92 (2H, ABq, 2JHH = 16.1 Hz, CH2), 2.20 (2H, ABq, 2JHH = 16.9 Hz, CH2), 3.75 (1H, t, 3JHH = 11.3 Hz, CH), 4.08 (1H, t, 3JHH = 10.0 Hz, CH), 6.36 (1H, s, CH14b), 6.40 (1H, s, CH2), 6.80 (1H, d, 3JHH = 8.1 Hz, CH of Ar), 6.84 (1H, t, 3JHH = 7.5 Hz, CH of Ar), 7.11 (2H, bs, NH2), 7.23 (1H, d, 3JHH = 8.2 Hz, CH of Ar), 7.25 (1H, s, NH), 7.26 (1H, t, 3JHH = 7.8 Hz, CH of Ar), 9.80 (1H, s, OH). 13C NMR (100.00 MHz, DMSO-d6): δC 23.99 (CH2), 24.23 (CH2), 25.58 (CH3), 29.85 (CH3), 30.24 (C12), 30.54 (CH2), 30.74 (CH2), 43.94 (CH2), 47.69 (CH5a), 51.67 (CH2), 56.75 (CH14b), 62.50 (CH9a), 63.27 (C3), 106.32 (C14a), 116.43 (C1), 116.91 (CH of Ar), 119.13 (CH of Ar), 121.64 (CN), 125.77 (Cipso–C=O), 130.35 (CH of Ar), 131.45 (CH of Ar), 137.79 (CH2), 155.50 (C10a), 158.01 (C4–NH), 164.82 (C–OH), 190.23 (C=O), 193.79 (C=O).
4-Amino-1-(5-chloro-2-hydroxybenzoyl)-12,12-dimethyl-14-oxo-5a,6,7,8,9,9a,10,11,12,13,14,14b-dodecahydrodibenzo[b,f]pyrido[1,2-d][1,4]diazepine-3-carboxamide (3 h, C27H31ClN4O4)
Yellow powder, mp = 198–199 °C, 0.5 g, yield: 79%. IR (KBr) (νmax, cm−1): 3401 and 3333 (NH2), 3099, 2953, and 2866 (CH), 1676 and 1619 (C=O), 1577 and 1492 (Ar). Anal. Calcd. for C27H31ClN4O4 (511.02): C, 63.46; H, 6.11; N, 10.39%. Found: C, 61.93; H, 6.09; N, 10.31%. MS (EI, 70 eV): m/z (%) = 510 (33), 371 (67), 328 (28), 236 (28), 219 (100), 204 (56), 166 (56), 140 (94), 121 (28), 83 (83), 57 (72). 1H NMR (400.13 MHz, DMSO-d6): δH 0.82 (3H, s, CH3), 0.86 (3H, s, CH3), 1.48–1.84 (8H, m, CH2), 1.93 (2H, ABq, 2JHH = 16.1 Hz, CH2), 2.10 (2H, ABq, 2JHH = 16.9 Hz, CH2), 4.06 (1H, t, 3JHH = 11.3 Hz, CH2), 4.53 (1H, t, 3JHH = 10.0 Hz, CH2), 5.03 (1H, s, CH14b), 6.64 (1H, s, CH2), 7.00 (1H, d, 3JHH = 8.1 Hz, CH of Ar), 7.17 (1H, d, 3JHH = 7.8 Hz, CH of Ar), 7.43 (2H, bs, NH2), 7.74 (2H, bs, NH2), 7.94 (1H, s, CH of Ar), 9.26 (1H, s, NH), 10.41 (1H, s, OH). 13C NMR (100.00 MHz, DMSO-d6): δC 19.21 (CH2), 25.58 (CH2), 28.19 (CH3), 28.47 (CH3), 30.06 (C12), 32.14 (CH2), 32.14 (CH2), 42.50 (CH2), 47.16 (CH5a), 50.43 (CH2), 56.49 (CH14b), 57.29 (CH9a), 93.98 (C3), 112.01 (C14a), 118.17 (C1), 118.90 (CH of Ar), 123.14 (Cipso–C=O), 127.67 (Cipso–Cl), 129.40 (CH of Ar), 132.15 (CH of Ar), 133.21 (CH2), 146.35 (C10a), 154.39 (C4–NH), 163.55 (C–OH), 164.84 (CONH2), 188.81 (C=O), 194.79 (C=O).
References
McGowan D, Nyanguile O, Cummings MD, Vendeville S, Vandyck K, Van den Broeck W, Boutton CW, De Bondt QLH, Amssoms K, Bonfanti JF, Last S, Rombauts K, Tahri A, Hu L, Delouvroy F, Vermeiren K, Vandercruyssen G, Van der Helm L, Cleiren E, Mostmans W, Lory P, Pille G, Van Emelen K, Fanning G, Pauwels F, Lin TI, Simmen K, Raboisson P (2009) 1,5-benzodiazepine inhibitors of HCV NS5B polymerase. Bioorg Med Chem Lett 19:2492. https://doi.org/10.1016/j.bmcl.2009.03.035
Schimer J, Cigler P, Vesely J, Grantz-Saskova K, Lepsik M, Brynda J, Rezacova P, Kozisek M, Cisarova I, Oberwinkler H, Kraeusslich HG, Konvalinka J (2012) Structure-aided design of novel inhibitors of HIV protease based on a benzodiazepine scaffold. J Med Chem 55:10130. https://doi.org/10.1021/jm301249q
Raboisson PJMB, Vendeville SMH, Bonfanti JF, McGowan D, Vandyck K, Doublet FMM, Hu LL, Nyanguile O (2008) 10-sulfonyl-dibenzodiazepinones useful as hepatitis c virus inhibitors. PCT Int Appl WO 2008099022A1 20080821
Charre D, Blehaut H, Bellamy F (2013) Inhibitors of cystathionine beta-synthase to reduce the neurotoxic overproduction of endogenous hydrogen sulfide. PCT Int Appl WO 2013068592A1 20130516
Fu JS, Shuttleworth SJ, Connors RV, Chai A, Coward P (2009) Discovery and optimization of a novel neuromedin B receptor antagonist. Bioorg Med Chem Lett 19:4264. https://doi.org/10.1016/j.bmcl.2009.05.124
Insuasty B, Ramrez J, Becerra D, Echeverry C, Quiroga J, Abonia R, Robledo SM, Velez ID, Upegui Y, Munoz JA, Ospina V, Nogueras M, Cobo J (2015) An efficient synthesis of new caffeine-based chalcones, pyrazoline and pyrazolo[3,4-b][1,4]diazepines as potential antimalarial, antitrypanosomal and antileishmanial agents. Eur J Med Chem 93:401. https://doi.org/10.1016/j.ejmech.2015.02.040
Insuasty B, Orozco F, Quiroga J, Abonia R, Nogueras M, Cobo J (2008) Microwave induced synthesis of novel 8,9-dihydro-7H-pyrimido[4,5-b][1,4]diazepines as potential antitumor agents. Eur J Med Chem 43:1955. https://doi.org/10.1016/j.ejmech.2007.12.005
Carabateas PM, Harris LS (1966) Analgesic antagonists. I. 4-substituted 1-acyl-2,3,4,5-tetrahydro-1H-1,4-benzodiazepines. J. Med. Chem. 9:6. https://doi.org/10.1021/jm00319a002
Tonkikh NN, Strakovs A, Rizhanova KV, Petrova MV (2004) 11-Aryl-3,3-dimethyl-7- and 7,8-substituted 1,2,3,4,10,11-hexahydro-5H-dibenzo[b, e]-1,4-diazepin-1-ones. Chem Heterocycl Compd 40:949. https://doi.org/10.1023/B:COHC.0000044581.23486.59
Dourlat J, Liu WQ, Gresh N, Garbay C (2007) Novel 1,4-benzodiazepine derivatives with antiproliferative properties on tumor cell lines. Bioorg Med Chem Lett 17:2527. https://doi.org/10.1016/j.bmcl.2007.02.016
Organon NV (2008) Pyridooxazepine progesterone receptor modulators. US2008/90804, A1
Akzo Nobel NV (2006) Non-steroidal glucocorticoid receptor modulators. WO2006/84917, A1
Nathwani SM, Greene LM, Butini S, Campiani G, Williams DC, Samali A, Szegezdi E, Zisterer DM (2016) The pyrrolo-1,5-benzoxazepine, PBOX-15, enhances TRAIL-induced apoptosis by upregulation of DR5 and downregulation of core cell survival proteins in acute lymphoblastic leukemia cells. Int J Oncol 49:74. https://doi.org/10.3892/ijo.2016.3518
Parmar NJ, Barad HA, Pansuriya BR, Teraiya ShB, Gupta VK, Kant R (2012) An efficient one-pot synthesis, structure, antimicrobial and antioxidant investigations of some novel quinolyldibenzo[b, e][1,4]diazepinones. Bioorg Med Chem Lett 22:3816. https://doi.org/10.1016/j.bmcl.2012.03.100
Cherfaoui B, Lakhdari H, Bennamane N, Ameraoui R, Talhi O, Almeida Paz FA, Bachari Kh, Kirsch G, Nejar-Bellara K, Silva AMS (2017) Dibenzo[b, e][1,4]diazepin-1-ones and their ring-opened derivatives: revisited synthesis, 2D NMR and crystal structure. Synlett 28:2247. https://doi.org/10.1055/s-0036-1590306
Jiang B, Li QY, Zhang H, Tu ShJ, Pindi S, Li G (2012) Efficient domino approaches to multi functionalized fused pyrroles and dibenzo[b, e][1,4]diazepin-1-ones. Org Lett 14:700. https://doi.org/10.1021/ol203166c
Orlova ZI, Ukhin LY, Suponitskii KY, Shepelenko EN, Belousova LV, Borodkin GS, Popovaa OS (2013) Synthesis, structure, and properties of new spirooxindolodibenzodiazepine derivatives. Russ Chem Bull Int Ed 62:1409. https://doi.org/10.1007/s11172-013-0203-1
Tolpygin IE, Mikhailenko NV, Bumber AA, Shepelenko EN, Revinsky UV, Dubonosov AD, Bren VA, Minkin VI (2012) 11-R-dibenzo[b, e][1,4]diazepin-1-ones, the chemosensors for transition metal cations. Russ J Org Chem 82:1243. https://doi.org/10.1134/S1070363212070109
Nasir Z, Ali A, Shakir M, Wahab R, Lutfullah Sh (2017) Silica-supported NiO nanocomposites prepared via a sol-gel technique and their excellent catalytic performance for the one-pot multi-component synthesis of benzodiazepine derivatives under microwave irradiation. New J Chem 41:5893. https://doi.org/10.1039/C6NJ04013F
De K, Bhanja P, Bhaumik A, Mukhopadhyay Ch (2017) Zeolite-Y-mediated multicomponent reaction of isatins, cyclic 1,3-diketones, and 1,2-phenylenediamine: easy access to spirodibenzo[1,4]diazepines. Chem Cat Chem 10:590. https://doi.org/10.1002/cctc.201701487
Moeini Korbekandi M, Nasr-Esfahani M, Mohammadpoor-Baltork I, Moghadam M, Tangestaninejad S, Mirkhani V (2019) Preparation and application of a new supported nicotine-based organocatalyst for the synthesis of various 1,5-benzodiazepines. Catal Lett 149:1057. https://doi.org/10.1007/s10562-019-02668-z
Wang Y, Shi F, Yao XX, Sun M, Dong L, Tu Sh (2014) Catalytic asymmetric construction of 3,3′-spirooxindoles fused with seven-membered rings by enantioselective tandem reactions. J Chem Eur J 20:1. https://doi.org/10.1002/chem.201403868
Wang Y, Tu MS, Shi F, Tu Sh (2014) Enantioselective construction of the biologically significant dibenzo[1,4]diazepine scaffold via organocatalytic asymmetric three-component reactions. J Adv Synth Catal 356:2009. https://doi.org/10.1002/adsc.201400095
Wang ShL, Cheng Ch, Wu FY, Li J, Jiang B, Tu Sh (2011) An efficient three-component tandem reaction leading to pentacyclic isoindole-fused benzo[b, e][1,4]diazepines in Water. J Chem Lett 40:834. https://doi.org/10.1246/cl.2011.834
Shaabani A, Hooshmand SE, Nazeri MT, Afshari R, Ghasemi Sh (2016) Deep eutectic solvent as a highly efficient reaction media for the one-pot synthesis of benzo-fused seven-membered heterocycles. Tetrahedron Lett 57:3727. https://doi.org/10.1016/j.tetlet.2016.07.005
Kausar N, Mukherjee P, Das RA (2016) Practical carbocatalysis by graphene oxide nanosheets in aqueous medium towards the synthesis of diversified dibenzo[1,4]diazepine scaffolds. RSC Adv 6:88904. https://doi.org/10.1039/C6RA17520A
Alizadeh A, Bagherinejad A (2020) A catalyst-free synthetic route to modified isoflavone via multi-component reaction. ChemistrySelect 5:1547. https://doi.org/10.1002/slct.201904674
Alizadeh A, Rezvanian A, Zhu LG (2012) Synthesis of heterocyclic [3.3.3]propellanes via a sequential four-component reaction. J Org Chem 77:4385. https://doi.org/10.1021/jo300457m
Alizadeh A, Bayat F, Moafi L, Zhu LG (2015) 5-Hydroxybenzo[g]indoles formation from oxa-aza[3.3.3]propellanes. Tetrahedron 71:8150. https://doi.org/10.1016/j.tet.2015.08.035
Alizadeh A, Ghanbaripour R, Zhu LG (2014) Piperidine-iodine a dual system catalyst for synthesis of coumarin bearing pyrrolo[1,2-a]quinoxaline derivatives via a one-pot three-component reaction. Tetrahedron 70:2048. https://doi.org/10.1016/j.tet.2014.01.038
Alizadeh A, Rezvanian A (2012) Powerful approach to synthesis of fused oxa-aza[3.3.3]propellanes via chemoselective sequential MCR in a single pot. Tetrahedron 68:10164. https://doi.org/10.1016/j.tet.2012.09.101
Alizadeh A, Basiri S, Bayat F, Halvagar MR, Zhu LG (2017) Application of oxa-aza[3.3.3]propellanes in the diastereoselective synthesis of indeno[1,2-b]pyrroles bearing bistriazole unit. Tetrahedron 73:5800. https://doi.org/10.1016/j.tet.2017.08.029
Alizadeh A, Rezvanian A, Zhu LG (2012) Chemo- and regioselective 4CR synthesis of oxathiaaza[3.3.3]propellanes via sequential C–S, C–N and C–O bond formation in a single pot. Synlett 23:2526. https://doi.org/10.1021/jo300457m
Alizadeh A, Bayat F, Zhu LG (2014) Regioselective multicomponent sequential synthesis of oxa-aza[3.3.3]propellanes. Aust J Chem 67:949. https://doi.org/10.1071/CH13654
Adib M, Zainali M, Kim I (2016) An efficient three-component synthesis of benzimidazo[1,2-a]-quinoline-6-carbonitriles. Synlett 27:1844. https://doi.org/10.1055/s-0035-1561939
Acknowledgements
The author would like to thank Esmat Sodagar, a postdoctoral fellow at the University of Southern California.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alizadeh, A., Bagherinejad, A. Synthesis of tetracyclic pyrido-fused dibenzodiazepines via a catalyst-free cascade reaction. Mol Divers 25, 2237–2246 (2021). https://doi.org/10.1007/s11030-020-10114-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10114-1