Abstract
A series of novel 1,2,4-triazolo[1,5-a]pyrimidine-containing quinazolin-4(3H)-one derivatives (8a–8o) were designed, synthesized and assessed for their in vitro antibacterial and antifungal activities in agriculture. All the title compounds were completely characterized via 1H NMR, 13C NMR, HRMS and IR spectroscopic data. In particular, the molecular structure of compound 8f was further corroborated through a single-crystal X-ray diffraction measurement. The turbidimetric method revealed that some of the compounds displayed noticeable bactericidal potencies against the tested plant pathogenic bacteria. For example, compounds 8m, 8n and 8o possessed higher antibacterial efficacies in vitro against Xanthomonas oryzae pv. oryzae with EC50 values of 69.0, 53.3 and 58.9 μg/mL, respectively, as compared with commercialized agrobactericide bismerthiazol (EC50 = 91.4 μg/mL). Additionally, compound 8m displayed an EC50 value of 71.5 μg/mL toward Xanthomonas axonopodis pv. citri, comparable to control bismerthiazol (EC50 = 60.5 μg/mL). A preliminary structure–activity relationship (SAR) analysis was also conducted, based on the antibacterial results. Finally, some compounds were also found to have a certain antifungal efficacy in vitro at the concentration of 50 μg/mL.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant pathogenic bacteria of Xanthomonas oryzae pv. oryzae (Xoo) and Xanthomonas axonopodis pv. citri (Xac) are responsible for rice bacterial blight and citrus bacterial canker, respectively, which lead to enormous economic loss to the farmers around the world each year [1, 2]. Bacterial blight of rice is one of the most deadly rice diseases, which had been reported to significantly reduce rice yield with an estimated loss of nearly 50% in some Asian countries [3]. Additionally, the phytobacteria of Xoo and Rs (Ralstonia solanacearum) ranked 4th and 2nd on the list of top 10 plant pathogenic bacteria, respectively [4]. On the other hand, plant pathogenic fungi remain the most serious plant disease from the perspective of crop protection [5]. Taking the fungus of Verticillium dahliae as an example, it had been reported that this pathogen could affect hundreds of herbaceous and woody host plants, also including some vegetables like tomatoes, potatoes, peppers and eggplants [6]. Although some agricultural antimicrobial agents are currently available for fighting against these plant diseases, the development of new, more effective and environment-friendly antimicrobial agents remains an extremely pressing task facing agricultural chemists, especially considering an increasing resistance of plant pathogens to the currently utilized antimicrobial agents in agriculture (thus leading to a significant reduction in the control efficiency of these agroantibiotics), high pesticide residues and concomitant environmental pollution [7, 8].
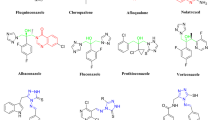
The quinazolin-4-one backbone as a common structural fragment in medicinal and pesticidal chemistry represents an important class of compounds with a wide range of bioactivities [9], such as antibacterial [10,11,12], antifungal [13], anti-viral [14] and anti-tumor [15] effects and so on. Several of quinazolinone-based derivatives have been on the market as drugs or pesticides for several years, including analgesics Diproqualone and Methaqualone (Fig. 1), sedatives Cloroqualone and Afloqualone, fungicides Fluquinconazole and Albaconazole, along with anticancer drugs Thymitaq and Raltitrexed. On the other hand, the 1,2,4-triazolo[1,5-a]pyrimidine derivatives are the medicinally and pesticidally significant family of condensed heterocyclic compounds that were found as a core motif in a number of bioactive molecules, including agrofungicide Ametoctradin, antiplatelet drug Trapidil, and herbicides Metosulam and Pyroxsulam. In addition, some of 1,2,4-triazolo[1,5-a]pyrimidine-based derivatives were also found to demonstrate other biological activities, such as antibacterial [16], anticancer [17] and anti-tubercular effects [18].
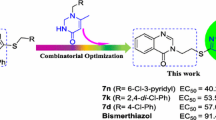
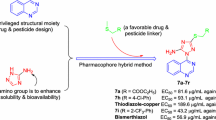
It is well known that the 1,2,4-triazolo[1,5-a]pyrimidine heterocycle can be regarded as the fusion of a pyrimidine ring with a 1,2,4-triazole ring, from the perspective of the molecular structure. Based on the above-mentioned considerations and our long-standing interest in developing quinazolinone-1,2,4-triazole hybrids as agricultural antimicrobial agents [19,20,21,22], we thus synthesized a series of novel quinazolin-4(3H)-one derivatives containing a 1,2,4-triazolo[1,5-a]pyrimidine moiety through a thioether linkage (favorable for improving the drug-likeness [23]) using a pharmacophore hybridization strategy (with the advantages of overcoming the resistance, decreasing the toxicity and optimizing the pharmacokinetic profiles [24]) (Fig. 2), assessed their in vitro antimicrobial activities against several agriculturally important plant pathogenic bacteria and fungi, and conducted a preliminary structure–activity relationship analysis based on the antibacterial results. To our best knowledge, this was the first example of the preparation of quinazolinone-1,2,4-triazolo[1,5-a]pyrimidine hybrids with the aim of acting as antimicrobial agents in agriculture.
Results and discussion
Synthesis
The synthetic route of target compounds 8a–8o was outlined in Scheme 1. Briefly, quinazolinone 2 [19] was firstly reacted with 1,2-dibromoethane in DMF with NaH as a base to furnish the corresponding alkyl bromide 3 [25], which was then subjected to thioetherification to give the intermediate 5 in 78% yield. After a cyclization reaction between 5 and ethyl acetoacetate in refluxing acetic acid, the key intermediate 6 was obtained in 72% yield. Once 6 in hand, it is readily converted into its chloride 7 after treatment with POCl3. Finally, the desired target compounds 8a–8o were readily obtained in 73–85% yield by reaction of intermediate 7 and various arylamines in ethanol at room temperature. All the target compounds were thoroughly characterized via 1H NMR, 13C NMR, HRMS and IR spectroscopic data.
Spectral and crystal structure analysis
Taking compound 8f as a representative example, the intense signals observed at 3270 and 1667 cm−1 in the IR spectroscopy were ascribed to the presence of N–H and C=O groups, respectively. In its 1H NMR spectrum, three singlets observed at 6.22, 8.29 and 10.02 ppm in DMSO-d6 were assigned to the resonances of 6-CH of 1,2,4-triazolo[1,5-a]pyrimidine moiety, 2-CH of quinazolinone ring and NH functionality, respectively. In addition, three diagnostic signals (45.8, 29.7 and 24.7 ppm) were also observed in the far upfield region in the 13C NMR spectrum, owing to the presence of three kinds of aliphatic CH protons within 8f. Finally, high-resolution mass spectrum (HRMS) demonstrated a strong signal at m/z = 448.1343, coming from the chemical species of [M + H]+.
A single crystal of compound 8f suitable for X-ray diffraction analysis was obtained (Fig. 3) by slow evaporation of a CH2Cl2-EtOH (1/3, v/v) solution of 8f at room temperature. Crystallographic data for compound 8f: colorless crystal, C22H19ON7FS, Mr = 447.49, monoclinic, space group P21/c; a = 9.0313(4) Å, b = 16.5322(5) Å, c = 14.3290(5) Å; α = 90°, β = 102.549(4)°, γ = 90°, V = 2088.31(4) Å3, T = 293 K, Z = 4, Dc = 1.423 g/cm3, F(000) = 948.0, Reflections collected/Independent reflections = 3120/2322, Goodness of fit on F2 = 1.033, Fine, R1 = 0.0630, wR2 = 0.1906. Crystallographic data for compound 8f were deposited in the Cambridge Crystallographic Data Center (CCDC 1547339).
In vitro antibacterial activity
The turbidimetric method was employed to assess in vitro antibacterial activities of compounds 8a–8o against three types of plant pathogenic bacteria Xoo, Xac and Rs (based on their particular significance in agricultural production [2, 4, 26]), with commercialized bactericides bismerthiazol (BMT) and thiodiazole-copper (TDC) as the positive control agents. As summarized in Table 1, several of the compounds were found to demonstrate comparable or higher bactericidal activities against the tested bacteria, after comparison with control BMT. For example, compounds 8l, 8n and 8o showed the inhibition rates of 60.5%, 60.6% and 61.9% at 100 μg/mL against the bacterium Xoo, respectively, superior to that of control BMT (50.6%). Under the same concentration, compounds 8l, 8m, 8n and 8o had the inhibition rates of 52.9%, 59.4%, 52.3% and 56.3% against the pathogen Xac, respectively, comparable to control BMT (64.3%). As for the bacterium Rs, only low to moderate bactericidal activities were observed for this series of compounds.
A preliminary structure–activity relationship (SAR) analysis was conducted based on the presented results in Table 1 and some conclusions could be drawn as follows: (a) An improved antibacterial efficiency was observed to different extents for target compounds 8a–8o as compared with intermediate 7, indicating the indispensability of introducing the aniline moiety into final compounds; (b) For the anti-Xoo activity, the presence of halogen-containing substituents within the target compounds was favorable to the bactericidal efficiency (with an inhibition rate > 40% at 100 μg/mL in every case), except for the presence of monofluoro substituent (namely compounds 8d, 8e and 8f); (c) Generally speaking, the presence of doubly substituted phenyl group (that is, compounds 8m, 8n and 8o) was beneficial to the antibacterial activity of target compounds against Xoo and Xac (showing an inhibition rate > 50% at 100 μg/mL), after comparison with the rest of mono-substituted phenyl-containing compounds; (d) Compound 8o with the 2,5-di-OCH3-Ph group was found to exhibit a good broad-spectrum in vitro antibacterial activity against the tested bacteria.
Encouraged by the preliminary antibacterial results, EC50 values (half-maximal effective concentration) of several of the compounds against the bacteria Xoo/Xac were subsequently measured using the serial dilution method. As shown in Table 2, compounds 8m, 8n and 8o had EC50 values of 69.0, 53.3 and 58.9 μg/mL against Xoo, respectively, considerably lower than that of control BMT (91.4 μg/mL). In addition, compound 8l showed an EC50 value of 93.9 μg/mL toward this bacterium, similar to control BMT. Moreover, compound 8m exhibited an EC50 value of 71.5 μg/mL against Xac, slightly less active than that of control BMT (60.5 μg/mL).
In vitro antifungal activity
In vitro fungicidal activities of target compounds 8a–8o against three phytopathogenic fungi (namely Gibberella zeae, Verticillium dahliae and Sclerotinia sclerotiorum) were also evaluated via the mycelial growth rate method. As shown in Table 3, some of the compounds displayed a certain antifungal activity at 50 μg/mL. For example, compounds 8n and 8o had the inhibition rates of 34.0% and 23.4% against V. dahliae, respectively. Overall, antifungal potencies of this class of compounds were far from satisfying.
Conclusions
In summary, a series of novel quinazolin-4(3H)-one derivatives containing a 1,2,4-triazolo[1,5-a]pyrimidine moiety were designed, synthesized and evaluated as antimicrobial agents in agriculture. Among them, molecular structure of compound 8f was clearly confirmed through single-crystal X-ray diffraction analysis. In vitro antibacterial assays indicated that compounds 8n and 8o possessed significantly better bactericidal activity against Xoo, than control bismerthiazol. This study showed the potential of 1,2,4-triazolo[1,5-a]pyrimidine-containing quinazolin-4-one derivatives as promising lead compounds for the development of more efficient agrobactericides.
Experimental
General
All the chemicals were purchased from commercial suppliers and used directly without further purification (unless stated otherwise). Melting points were determined and uncorrected on a XT-4 binocular microscope (Beijing Tech Instrument Co., China). 1H and 13C NMR spectra were determined on a JEOL-ECX 500 NMR spectrometer at room temperature using TMS as an internal standard, and chemical shift (δ) was expressed in parts per million (ppm). The following abbreviations were employed in expressing multiplicity: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet. HRMS-ESI spectra were measured on a Thermo Scientific Q Exactive Hybrid Quadrupole-Orbitrap mass spectrometer. The X-ray crystallographic data were collected using a Bruker Smart Apex CCD area detector diffractometer (Bruker, Germany) with Mo-Kα radiation. The software package SPSS 17.0 is developed by SPSS Inc.
Synthesis of intermediate 5
A mixture of 5-amino-1H-1,2,4-triazole-3-thiol 4 (1.38 g, 11.88 mmol) and 3-(2-bromoethyl)quinazolinone 3 (2.99 g, 11.88 mmol) was dissolved in DMF (20 mL) in the presence of NaOH (1.42 g, 35.6 mmol), stirred at room temperature for 1 h and then heated to 60 °C for 10 h. After completion of the reaction, ice water (15 mL) was added into the reaction mixture and the resultant precipitate was filtered, washed with water and dried under vacuum to give 5 as a white solid. Yield: 78.2%, mp 237–240 °C. 1H NMR (500 MHz, DMSO-d6, ppm) δ: 12.00 (s, 1H), 8.25 (s, 1H), 8.15 (d, J = 8.0 Hz, 1H), 7.83 (t, J = 7.6 Hz, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.55 (t, J = 7.5 Hz, 1H), 6.09 (s, 2H), 4.26 (t, J = 6.3 Hz, 2H), 3.33 (t, J = 6.2 Hz, 2H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 160.2, 157.5, 154.8, 148.1, 148.0, 134.4, 127.2, 127.0, 126.1, 121.6, 45.7, 29.8. HRMS (ESI) calcd for [M + H]+ C12H13N6OS: 289.0866, found: 289.0862.
Synthesis of intermediate 6
A mixture of intermediate 5 (3.00 g, 10.40 mmol) and ethyl acetoacetate (2.62 mL, 20.80 mmol) in acetic acid (20 mL) was heated to reflux and stirred for 8 h. The reaction mixture was then cooled to room temperature, and the resultant precipitate was filtered, washed with AcOH and EtOH, and dried to generate intermediate 6 as a white solid. Yield: 72.3%, mp 244–247 °C. 1H NMR (500 MHz, DMSO-d6, ppm) δ: 13.03 (s, 1H), 8.25 (s, 1H), 8.13 (d, J = 7.9 Hz, 1H), 7.78 (t, J = 7.6 Hz, 1H), 7.57 (d, J = 8.0 Hz, 1H), 7.51 (t, J = 7.5 Hz, 1H), 5.74 (s, 1H), 4.35 (t, J = 6.3 Hz, 2H), 3.57 (t, J = 6.3 Hz, 2H), 2.23 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 161.0, 160.2, 154.7, 151.0, 150.6, 148.0, 147.8, 134.3, 127.0, 126.9, 126.1, 121.4, 98.5, 45.8, 29.6, 18.5. HRMS (ESI) calcd for [M + H]+ C16H15N6O2S: 355.0972, found: 355.0967.
Synthesis of intermediate 7
A mixture of intermediate 6 (3.68 g, 10.40 mmol) and POCl3 (2.62 mL) was heated to reflux and stirred for 2.5 h. Excess POCl3 was removed by distillation under reduced pressure; the residue was dumped into ice water and then neutralized with dilute NaOH solution. The crude product was extracted with CH2Cl2, evaporated and recrystallized with EtOH to afford 7 as a white solid. Yield: 40%, mp 158–160 °C; 1H NMR (500 MHz, DMSO-d6, ppm) δ: 8.25 (s, 1H), 8.12 (d, J = 8.1 Hz, 1H), 7.76 (t, J = 7.5 Hz, 1H), 7.54 (d, J = 3.4 Hz, 1H), 7.51 (t, J = 8.0 Hz, 1H), 7.48 (s, 1H), 4.39 (t, J = 6.3 Hz, 2H), 3.66 (t, J = 5.8 Hz, 2H), 2.54 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 165.6, 165.1, 160.2, 155.4, 147.9, 147.7, 136.9, 134.2, 126.9, 126.8, 126.1, 121.4, 111.0, 45.9, 29.6, 24.3. HRMS (ESI) calcd for [M + H]+ C16H14ON6ClS: 373.0633, found: 373.0627.
General procedure for the synthesis of target compounds 8a–8o
A mixture of intermediate 7 (100 mg, 0.27 mmol) and appropriate arylamine (0.32 mmol) in EtOH (10 mL) was stirred at room temperature for 30 h. The crude product was collected by filtration, washed with EtOH and dried to give compounds 8a–8o.
3-(2-((5-methyl-7-(phenylamino)-[1,2,4] triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (8a)
White solid, mp 204–206 °C, yield: 78.5%. IR (KBr, ν/cm−1): 3274 (N–H), 1668 (C=O); 1H NMR (500 MHz, DMSO-d6, ppm) δ: 10.03 (s, 1H), 8.30 (s, 1H), 8.13 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 6.8 Hz, 1H), 7.60 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.49 (d, J = 7.4 Hz, 2H), 7.44 (d, J = 7.4 Hz, 2H), 7.31 (t, J = 7.5 Hz, 1H), 6.31 (s, 1H), 4.42 (t, J = 6.3 Hz, 2H), 3.65 (t, J = 6.3 Hz, 2H), 2.36 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 163.9, 163.7, 160.2, 155.7, 148.1, 147.8, 144.8, 136.7, 134.2, 129.5, 127.0, 126.9, 126.1, 126.0, 124.3, 121.4, 89.2, 45.7, 29.7, 24.7. HRMS (ESI) calcd for [M + H]+ C22H20ON7S: 430.1445, found: 430.1439.
3-(2-((7-((2-chlorophenyl)amino)-5-methyl-[1,2,4] triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (8b)
White solid, mp 162–165 °C, yield: 81.6%. IR (KBr, ν/cm−1): 3271 (N–H), 1672 (C=O); 1H NMR (500 MHz, DMSO-d6, ppm) δ: 10.06 (s, 1H), 8.32 (s, 1H), 8.15 (d, J = 6.4 Hz, 1H), 7.79 (t, J = 6.9 Hz, 1H), 7.69 (d, J = 7.7 Hz, 1H), 7.60 (t, J = 7.7 Hz, 1H), 7.55 (t, J = 7.8 Hz, 1H), 7.51 (d, J = 6.4 Hz, 1H), 7.49 (d, J = 7.3 Hz, 1H), 7.45 (t, J = 8.7 Hz, 1H), 5.78 (s, 1H), 4.43 (t, J = 6.4 Hz, 2H), 3.65 (t, J = 5.9 Hz, 2H), 2.32 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 164.0, 163.9, 160.2, 155.7, 148.1, 147.9, 145.2, 134.3, 133.5, 131.3, 130.5, 129.7, 129.5, 128.7, 127.1, 126.1, 124.3, 121.5, 89.6, 45.7, 29.6, 24.6. HRMS (ESI) calcd for [M + H]+ C22H19ON7ClS: 464.1055, found: 456.1051.
3-(2-((7-((4-chlorophenyl)amino)-5-methyl-[1,2,4] triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (8c)
White solid, mp 240–243 °C, yield: 80.3%; IR (KBr, ν/cm−1): 3255 (N–H), 1678 (C=O); 1H NMR (500 MHz, DMSO-d6, ppm) δ: 10.09 (s, 1H), 8.29 (s, 1H), 8.13 (d, J = 8.6 Hz, 1H), 7.78 (t, J = 6.9 Hz, 1H), 7.59 (d, J = 7.5 Hz, 1H), 7.54 (d, J = 8.6 Hz, 2H), 7.51 (t, J = 6.9 Hz, 1H), 7.46 (d, J = 8.6 Hz, 2H), 6.36 (s, 1H), 4.41 (t, J = 6.3 Hz, 2H), 3.65 (t, J = 6.3 Hz, 2H), 2.36 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 164.1, 163.7 160.2, 155.7, 148.1, 147.8, 144.6, 135.8, 134.3, 130.0, 129.5, 127.0, 126.9, 126.0, 125.9, 121.4, 89.5, 45.8, 29.7, 24.7. HRMS (ESI) calcd for [M + H]+ C22H19ON7ClS: 464.1055, found: 464.1050.
3-(2-((7-((2-fluorophenyl)amino)-5-methyl-[1,2,4] triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (8d)
White solid, mp 166–169 °C; yield 74.3%; IR (KBr, ν/cm−1): 3395 (N–H), 1660 (C=O); 1H NMR (500 MHz, DMSO-d6, ppm) δ: 10.04 (s, 1H), 8.31 (s, 1H), 8.13 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 6.9 Hz, 1H), 7.60 (d, J = 8.0 Hz, 1H), 7.52 (t, J = 6.8 Hz, 2H), 7.45 (t, J = 8.0 Hz, 2H), 7.34 (t, J = 6.3 Hz, 1H), 5.93 (d, J = 2.0 Hz, 1H), 4.42 (t, J = 6.3 Hz, 2H), 3.65 (t, J = 6.3 Hz, 2H), 2.34 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 164.0, 163.9, 160.2, 158.0, 156.0, 155.6, 148.2, 147.9, 145.2, 134.3, 128.9, 127.1, 126.9, 126.1, 125.5, 125.4, 123.9, 123.8, 121.5, 117.0 116.8, 89.5, 45.7, 29.7, 24.6. HRMS (ESI) calcd for [M + H]+ C22H19ON7FS: 448.1350, found: 448.1353.
3-(2-((7-((3-fluorophenyl)amino)-5-methyl-[1,2,4] triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (8e)
White solid, mp 213–215 °C, yield: 73.6%. IR (KBr, ν/cm−1): 3270 (N–H), 1663 (C=O); 1H NMR (500 MHz, DMSO-d6, ppm) δ: 10.14 (s, 1H), 8.29 (s, 1H), 8.13 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 7.2 Hz, 1H), 7.59 (d, J = 8.0 Hz, 1H), 7.51 (t, J = 8.0 Hz, 2H), 7.31 (d, J = 8.0 Hz, 2H), 7.13 (t, J = 7.4 Hz, 1H), 6.48 (s, 1H), 4.42 (t, J = 6.3 Hz, 2H), 3.66 (t, J = 6.3 Hz, 2H), 2.39 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 164.3, 163.8, 163.4, 161.5, 160.2, 155.7, 148.1, 147.8, 144.3, 138.8, 138.7, 134.3, 131.2, 131.1, 127.1, 126.9, 126.1, 121.5, 119.7, 112.6, 112.4, 111.0, 110.8, 89.9, 45.8, 29.7, 24.7. HRMS (ESI) calcd for [M + H]+ C22H19ON7FS: 448.1350, found: 448.1341.
3-(2-((7-((4-fluorophenyl)amino)-5-methyl-[1,2,4] triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (8f)
White solid, mp 193–196 °C, yield: 74.2%; IR (KBr, ν/cm−1): 3270 (N–H), 1667 (C=O); 1H NMR (500 MHz, DMSO-d6, ppm) δ: 10.02 (s, 1H), 8.29 (s, 1H), 8.13 (d, J = 8.2 Hz, 1H), 7.79 (t, J = 8.2 Hz, 1H), 7.60 (d, J = 7.8 Hz, 1H), 7.51 (t, J = 6.9 Hz, 1H), 7.46 (t, J = 7.4 Hz, 2H), 7.33 (t, J = 8.7 Hz, 2H), 6.22 (s, 1H), 4.41 (t, J = 6.1 Hz, 2H), 3.65 (t, J = 6.0 Hz, 2H), 2.34 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 164.0, 163.7, 161.1, 160.2, 159.1, 155.7, 148.1, 147.9, 145.1, 134.3, 133.0, 127.1, 127.0, 126.9, 126.1, 121.5, 116.5, 116.3, 89.1, 45.8, 29.7, 24.7. HRMS (ESI) calcd for [M + H]+ C22H19ON7FS: 448.1350, found: 448.1343.
3-(2-((5-methyl-7-((4-(trifluoromethyl)phenyl)amino)-[1,2,4] triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (8g)
White solid, mp > 250 °C, yield: 76.9%. IR (KBr, ν/cm−1): 3231 (N–H), 1661 (C=O); 1H NMR (500 MHz, DMSO-d6, ppm) δ: 10.32 (s, 1H), 8.28 (s, 1H), 8.12 (d, J = 8.0 Hz, 1H), 7.83 (d, J = 8.6 Hz, 2H), 7.77 (t, J = 6.9 Hz, 1H), 7.66 (d, J = 8.6 Hz, 2H), 7.58 (d, J = 8.0 Hz, 1H), 7.50 (t, J = 7.4 Hz, 1H), 6.60 (s, 1H), 4.41 (t, J = 6.3 Hz, 2H), 3.66 (t, J = 6.6 Hz, 2H), 2.40 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 164.4, 163.9, 160.2, 155.7, 148.1, 147.8, 143.9, 141.0, 134.3, 127.0, 126.9, 126.7, 126.1, 125.5, 125.3, 125.2, 125.1, 123.5, 121.4, 90.3, 45.8, 29.7, 24.7. HRMS (ESI) calcd for [M + H]+ C23H19ON7F3S: 498.1318, found: 498.1314.
3-(2-((7-((3-bromophenyl)amino)-5-methyl-[1,2,4] triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (8h)
White solid, mp 243–246 °C, yield: 77.8%. IR (KBr, ν/cm−1): 3235 (N–H), 1669 (C=O); 1H NMR (500 MHz, DMSO-d6, ppm) δ: 10.12 (s, 1H), 8.29 (s, 1H), 8.13 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 8.1 Hz, 1H), 7.64 (s, 1H), 7.59 (d, J = 8.0 Hz, 1H), 7.52 (d, J = 8.0 Hz, 1H), 7.49 (t, J = 5.7 Hz, 1H), 7.48 (t, J = 2.9 Hz, 1H), 7.43 (t, J = 8.0 Hz, 1H), 6.41 (s, 1H), 4.42 (t, J = 6.3 Hz, 2H), 3.65 (t, J = 6.3 Hz, 2H), 2.38 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 164.2, 163.8, 160.2, 155.7, 148.1, 147.8, 144.3, 138.6, 134.3, 131.4, 128.6, 127.1, 126.9, 126.7, 126.1, 122.8, 122.1, 121.5, 89.8, 45.8, 29.7, 24.7. HRMS (ESI) calcd for [M + H]+ C22H19ON7BrS: 508.0550, found: 508.0545.
3-(2-((5-methyl-7-(o-tolylamino)-[1,2,4] triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (8i)
White solid, mp 215–217 °C, yield: 82.6%. IR (KBr, ν/cm−1): 3377 (N–H), 1674 (C=O); 1H NMR (125 MHz, DMSO-d6, ppm) δ: 9.53 (s, 1H), 8.33 (s, 1H), 8.14 (d, J = 7.4 Hz, 1H), 7.78 (d, J = 8.6 Hz, 1H), 7.61 (d, J = 7.4 Hz, 1H), 7.50 (t, J = 8.0 Hz, 1H), 7.38 (d, J = 8.0 Hz, 2H), 7.21 (d, J = 8.0 Hz, 1H), 7.06 (t, J = 6.9 Hz, 1H), 5.85 (s, 1H), 4.43 (t, J = 6.3 Hz, 2H), 3.82 (s, 3H), 3.64 (t, J = 6.3 Hz, 2H), 2.33 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 163.8, 163.7, 160.3, 155.6, 154.0, 148.1, 147.9, 145.2, 134.3, 128.6, 127.4, 127.1, 126.9, 126.1, 124.4, 121.5, 120.9, 112.6, 89.5, 55.7, 45.7, 29.6, 24.6. HRMS (ESI) calcd for [M + H]+ C23H22ON7S: 444.1601, found: 444.1605.
3-(2-((5-methyl-7-(m-tolylamino)-[1,2,4] triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (8j)
White solid, mp 246–249 °C, yield: 84.7%. IR (KBr, ν/cm−1): 3248 (N–H), 1670 (C=O); 1H NMR (500 MHz, DMSO-d6, ppm) δ: 9.95 (s, 1H), 8.31 (s, 1H), 8.13 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 8.6 Hz, 1H), 7.60 (d, J = 8.0 Hz, 1H), 7.51 (t, J = 7.4 Hz, 1H), 7.37 (t, J = 8.1 Hz, 1H), 7.23 (d, J = 7.4 Hz, 2H), 7.12 (d, J = 7.4 Hz, 1H), 6.29 (s, 1H), 4.43 (t, J = 6.3 Hz, 2H), 3.65 (t, J = 6.3 Hz, 2H), 2.35 (s, 3H), 2.34 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 163.9, 163.7, 160.2, 155.7, 148.1, 147.9, 144.9, 139.1, 136.6, 134.3, 129.4, 127.1, 126.9, 126.8, 126.1, 124.9, 121.5, 121.4, 89.3, 45.8, 29.7, 24.7, 21.0. HRMS (ESI) calcd for [M + H]+ C23H22ON7S: 444.1601, found: 444.1604.
3-(2-((7-((2-methoxyphenyl)amino)-5-methyl-[1,2,4] triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (8k)
White solid, mp 173–175 °C, yield: 82.4%. IR (KBr, ν/cm−1): 3377 (N–H), 1674 (C=O); 1H NMR (500 MHz, DMSO-d6, ppm) δ: 9.54 (s, 1H), 8.33 (s, 1H), 8.14 (d, J = 8.0 Hz, 1H), 7.79 (t, J = 8.0 Hz, 1H), 7.61 (d, J = 8.5 Hz, 1H), 7.51 (t, J = 7.4 Hz, 1H), 7.40 (t, J = 7.5 Hz, 1H), 7.37 (t, J = 5.7 Hz, 1H), 7.22 (d, J = 8.0 Hz, 1H), 7.07 (t, J = 7.5 Hz, 1H), 5.85 (s, 1H), 4.43 (t, J = 6.3 Hz, 2H), 3.81 (s, 3H), 3.64 (t, J = 6.3 Hz, 2H), 2.33 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 163.8, 163.7, 160.3, 155.6, 154.0, 148.2, 147.9, 145.2, 134.3, 128.6, 127.4, 127.1, 126.9, 126.1, 124.5, 121.5, 120.9, 112.6, 89.5, 55.7, 45.7, 29.6, 24.6. HRMS (ESI) calcd for [M + H]+ C23H22O2N7S: 460.1550, found: 460.1550.
3-(2-((7-((4-methoxyphenyl)amino)-5-methyl-[1,2,4] triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (8l)
White solid, mp 181–183 °C, yield: 72.8%. IR (KBr, ν/cm−1): 3270 (N–H), 1683 (C=O); 1H NMR (500 MHz, DMSO-d6, ppm) δ: 9.89 (s, 1H), 8.30 (s, 1H), 8.13 (d, J = 8.2 Hz, 1H), 7.79 (t, J = 6.9 Hz, 1H), 7.60 (d, J = 7.8 Hz, 1H), 7.51 (t, J = 8.2 Hz, 1H), 7.33 (d, J = 8.7 Hz, 2H), 7.05 (d, J = 9.1 Hz, 2H), 6.09 (s, 1H), 4.42 (t, J = 6.4 Hz, 2H), 3.80 (s, 3H), 3.64 (t, J = 6.4 Hz, 2H), 2.33 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 163.7, 163.6, 160.2, 157.7, 155.7, 148.2, 147.9, 145.6, 134.3, 129.1, 127.1, 126.9, 126.7, 126.1, 121.5, 114.8, 88.9, 55.4, 45.8, 29.6, 24.7. HRMS (ESI) calcd for [M + H]+ C23H22O2N7S: 460.1550, found: 456.1559.
3-(2-((7-((2,4-dichlorophenyl)amino)-5-methyl-[1,2,4] triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (8m)
White solid, mp 210–213 °C, Yield: 75.7%. IR (KBr, ν/cm−1): 3271 (N–H), 1655 (C=O); 1H NMR (500 MHz, DMSO-d6, ppm) δ: 8.81 (s, 2H), 8.16 (d, J = 8.0 Hz, 1H), 7.91 (d, J = 3.4 Hz, 1H), 7.86 (t, J = 8.6 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.63 (t, J = 8.6 Hz, 1H), 7.59 (t, J = 4.6 Hz, 1H), 7.57 (d, J = 5.2 Hz, 1H), 6.05 (s, 1H), 4.48 (t, J = 5.7 Hz, 2H), 3.69 (t, J = 5.7 Hz, 2H), 2.34 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 164.6, 161.0, 159.7, 152.8, 149.0, 146.3, 144.5, 135.0, 133.5, 132.4, 132.3, 130.9, 130.2, 129.0, 127.7, 126.5, 124.8, 120.9, 90.8, 46.3, 29.7, 22.3. HRMS (ESI) calcd for [M + H]+ C22H18ON7Cl2S: 498.0665, found: 498.0655.
3-(2-((7-((2,4-difluorophenyl)amino)-5-methyl-[1,2,4] triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (8n)
White solid, mp 139–142 °C, yield: 84.5%. IR (KBr, ν/cm−1): 3211 (N–H), 1692 (C=O); 1H NMR (500 MHz, DMSO-d6, ppm) δ: 9.98 (s, 1H), 8.31 (s, 1H), 8.13 (d, J = 8.0 Hz, 1H), 7.78 (t, J = 8.6 Hz, 1H), 7.60 (d, J = 8.0 Hz, 1H), 7.57 (t, J = 8.1 Hz, 1H), 7.52 (d, J = 6.9 Hz, 1H), 7.50 (d, J = 5.2 Hz, 1H), 7.24 (t, J = 8.5 Hz, 1H), 5.94 (s, 1H), 4.42 (t, J = 6.3 Hz, 2H), 3.65 (t, J = 6.3 Hz, 2H), 2.34 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 164.1, 164.0, 160.2, 156.3, 155.6, 148.2, 147.9, 145.4, 134.3, 130.6, 130.5, 127.1, 126.9, 126.1, 121.5, 120.6, 120.5, 112.6, 112.4, 105.5, 105.3, 89.5, 45.7, 29.7, 24.6. HRMS (ESI) calcd for [M + H]+ C22H18ON7F2S: 466.1256, found: 466.1260.
3-(2-((7-((2,5-dimethoxyphenyl)amino)-5-methyl-[1,2,4] triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (8o)
Reddish brown solid, mp 202–205 °C, yield: 78.5%. IR (KBr, ν/cm−1): 3367 (N–H), 1672 (C=O); 1H NMR (500 MHz, DMSO-d6, ppm) δ: 9.58 (s, 1H), 8.32 (s, 1H), 8.14 (d, J = 7.4 Hz, 1H), 7.79 (t, J = 8.9 Hz, 1H), 7.61 (d, J = 8.0 Hz, 1H), 7.51 (t, J = 7.4 Hz, 1H), 7.14 (d, J = 9.7 Hz, 1H), 6.96 (d, J = 2.9 Hz, 1H), 6.95 (d, J = 3.4 Hz, 1H), 5.89 (s, 1H), 4.43 (t, J = 6.3 Hz, 2H), 3.76 (s, 3H), 3.74 (s, 3H), 3.64 (t, J = 6.3 Hz, 2H), 2.34 (s, 3H); 13C NMR (125 MHz, DMSO-d6, ppm) δ: 163.8, 163.6, 160.3, 155.5, 153.2, 148.2, 148.1, 147.9, 145.2, 134.3, 127.1, 126.9, 126.1, 125.0, 121.5, 113.5, 113.4 113.0, 89.9, 56.1, 55.6, 45.7, 29.6, 24.6. HRMS (ESI) calcd for [M + H]+ C24H24O3N7S: 490.1656, found: 490.1663.
In vitro antibacterial bioassay
In vitro antibacterial activities of intermediate 7 and target compounds 8a–8o were tested against three types of plant pathogenic bacteria (Xoo, Xac and Rs) using the turbidimetric assay, which is the most frequently utilized method for the measurement of the agricultural bactericidal potency [27,28,29,30,31]. Tested compounds were initially prepared at two concentrations of 200 and 100 μg/mL. Pure DMSO in sterile distilled water was utilized as a blank control, and commercially available bactericides bismerthiazol (BMT) and thiodiazole-copper (TDC) were employed as positive control agents. About 40 μL of solvent NB (3 g of beef extract, 5 g of peptone, 1 g of yeast powder, 10 g of glucose, 1 L of distilled water, pH = 7.0 − 7.2) containing the bacterium Xoo/Xac/Rs was added to the mixed solvent system including 4 mL of solvent NB and 1 mL of 0.1% Tween-20 containing tested compound or BMT/TDC. The above test tube was incubated at 30 ± 1 °C and continuously shaken at 180 rpm for 1–3 days. The bacterial growth was monitored by measuring the optical density at 600 nm (OD600), given by turbiditycorrected values = ODbacterium − ODno bacterium, I = (Ctur − Ttur)/Ctur × 100%. The Ctur represented the corrected turbidity value of bacterial growth of untreated NB (blank control), and Ttur represented the corrected turbidity value of bacterial growth of compound-treated NB. The I represented the inhibition ratio of tested compound against the bacterium.
Finally, antibacterial activities of compounds 8k, 8l, 8m, 8n and 8o against Xoo and compounds 8g, 8l, 8m, 8n and 8o against Xac were further determined under five different concentrations (namely 200, 100, 50, 25 and 12.5 μg/mL) to obtain their EC50 values, which were calculated by Probit analysis using the software package SPSS 17.0.
In vitro antifungal bioassay
In vitro antifungal activities of target compounds 8a–8o were determined against three types of phytopathogenic fungi (namely, G. zeae, V. dahliae and S. sclerotiorum) using the mycelial growth rate method, which is the most common method for the evaluation of the agricultural fungicidal potency [8, 32,33,34]. A DMSO solution containing the tested compound was added into sterilized Petri dishes, which contained about 10 mL molten potato dextrose agar (PDA). Next, a 4 mm diameter of mycelial disk was cut from the fungal colony and placed at the center of PDA plate at 28 ± 1 °C for 4 days. Antifungal assays were conducted in triplicate for every compound. In addition, pure DMSO and commercially available fungicide (Hymexazol) were utilized as negative and positive control agents, respectively.
The inhibition ratio (I) of tested compound was calculated based on the following formula:
In this formula, the C represented the average mycelial diameter of negative control, and T represented the average mycelial diameter of tested compound-treated PDA.
References
Sun X, Cao Y, Yang Z, Xu C, Li X, Wang S, Zhang Q (2004) Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J 37:517–527. https://doi.org/10.1046/j.1365-313X.2003.01976.x
Graham JH, Gottwald TR, Cubero J, Achor DS (2004) Xanthomonas axonopodis pv.citri: factors affecting successful eradication of citrus canker. Mol Plant Pathol 5:1–15. https://doi.org/10.1046/j.1364-3703.2003.00197.X
Huang N, Angeles ER, Domingo J, Magpantay G, Singh S, Zhang G, Kumaravadivel N, Bennett J, Khush GS (1997) Pyramiding of bacterial blight resistance genes in rice: marker-assisted selection using RFLP and PCR. Theor Appl Genet 95:313–320. https://doi.org/10.1007/s001220050565
Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA, Toth I, Salmond G, Foster GD (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13:614–629. https://doi.org/10.1111/j.1364-3703.2012.00804.x
Wang LL, Li C, Zhang YY, Qiao CH, Ye YH (2013) Synthesis and biological evaluation of benzofuroxan derivatives as fungicides against phytopathogenic fungi. J Agric Food Chem 61:8632–8640. https://doi.org/10.1021/jf402388x
Blancard D (2012) Tomato diseases: identification, biology and control: a colour handbook, 2nd edn. Academic Press, Cambridge. https://doi.org/10.1016/C2010-0-66813-1
Yang L, Bao XP (2017) Synthesis of novel 1,2,4-triazole derivatives containing the quinazolinylpiperidinyl moiety and N-(substituted phenyl)acetamide group as efficient bactericides against the phytopathogenic bacterium Xanthomonas oryzae pv. oryzae. RSC Adv 7:34005–34011. https://doi.org/10.1039/C7RA04819J
Zhang M, Dai ZC, Qian SS, Liu JY, Xiao Y, Lu AM, Zhu HL, Wang JX, Ye YH (2014) Design, synthesis, antifungal, and antioxidant activities of (E)-6-((2-phenylhydrazono)methyl)quinoxaline derivatives. J Agric Food Chem 62:9637–9643. https://doi.org/10.1021/jf504359p
Khan I, Ibrar A, Abbas N, Saeed A (2014) Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: synthetic approaches and multifarious applications. Eur J Med Chem 76:193–244. https://doi.org/10.1016/j.ejmech.2014.02.005
Wang X, Li P, Li Z, Yin J, He M, Xue W, Chen Z, Song B (2013) Synthesis and bioactivity evaluation of novel arylimines containing a 3-aminoethyl-2-[(p-trifluoromethoxy)anilino]-4(3H)-quinazolinone moiety. J Agric Food Chem 61:9575–9582. https://doi.org/10.1021/jf403193q
Bouley R, Ding D, Peng Z, Bastian M, Lastochkin E, Song W, Suckow MA, Schroeder VA, Wolter WR, Mobashery S, Chang M (2016) Structure-activity relationship for the 4(3H)-quinazolinone antibacterials. J Med Chem 59:5011–5021. https://doi.org/10.1021/acs.jmedchem.6b00372
Qureshi SI, Chaudhari HK (2019) Design, synthesis, in-silico studies and biological screening of quinazolinone analogues as potential antibacterial agents against MRSA. Bioorg Med Chem 27:2676–2688. https://doi.org/10.1016/j.bmc.2019.05.012
Zhang J, Liu J, Ma Y, Ren D, Cheng P, Zhao J, Zhang F, Yao Y (2016) One-pot synthesis and antifungal activity against plant pathogens of quinazolinone derivatives containing an amide moiety. Bioorg Med Chem Lett 26:2273–2277. https://doi.org/10.1016/j.bmcl.2016.03.052
Chen M, Li P, Hu D, Zeng S, Li T, Jin L, Xue W, Song B (2016) Synthesis, antiviral activity, 3D-QSAR, and interaction mechanisms study of novel malonate derivatives containing quinazolin-4(3H)-one moiety. Bioorg Med Chem Lett 26:168–173. https://doi.org/10.1016/j.bmcl.2015.11.006
Mohamed MA, Ayyad RR, Shawer TZ, Abdel-Aziz AAM, El-Azab AS (2016) Synthesis and antitumor evaluation of trimethoxyanilides based on 4(3H)-quinazolinone scaffolds. Eur J Med Chem 112:106–113. https://doi.org/10.1016/j.ejmech.2016.02.002
Wang H, Lee M, Peng Z, Blázquez B, Lastochkin E, Kumarasiri M, Bouley R, Chang M, Mobashery S (2016) Synthesis and evaluation of 1,2,4-triazolo[1,5-a]pyrimidines as antibacterial agents against Enterococcus faecium. J Med Chem 58:4194–4203. https://doi.org/10.1021/jm501831g
Tao X, Hu Y (2010) Synthesis and antitumor activity of 2-aryl-1, 2, 4-triazolo[1, 5-a]pyridine derivatives. Med Chem 6:65–69. https://doi.org/10.2174/157340610791321505
Bhatt JD, Chudasama CJ, Patel KD (2015) Pyrazole clubbed triazolo[1,5-a] pyrimidine hybrids as an anti-tubercular agents: Synthesis, in vitro screening and molecular docking study. Bioorg Med Chem 23:7711–7716. https://doi.org/10.1016/j.bmc.2015.11.018
Yan BR, Lv XY, Du H, Gao MN, Huang J, Bao XP (2016) Synthesis and biological activities of novel quinazolinone derivatives containing a 1,2,4-triazolylthioether moiety. Chem Pap 70:983–993. https://doi.org/10.1515/chempap-2016-0034
Yan B, Lv X, Du H, Bao X (2016) Design, synthesis and biological activities of novel quinazolinone derivative bearing 4-phenyl-5-thioxo-1,2,4-triazole Mannich bases. Chin J Org Chem 36:207–212. https://doi.org/10.6023/cjoc201506026
Du H, Fan Z, Yang L, Bao X (2018) Synthesis of novel quinazolin-4(3H)-one derivatives containing the 7-oxo-1,2,4-triazolo[1,5-a]pyrimidine moiety as effective agricultural bactericides against the pathogen Xanthomonas oryzae pv. oryzae. Mol Diversity 22:1–10. https://doi.org/10.1007/s11030-017-9782-3
Du H, Fan Z, Yang L, Bao X (2018) Synthesis and antimicrobial activities of novel 1,2,4-triazole-acylhydrazone derivatives containing the quinazolin-4-one moiety. Chin J Org Chem 38:531–538. https://doi.org/10.6023/cjoc201708051
Li YH, Zhang B, Yang HK, Li Q, Diao PC, You WW, Zhao PL (2017) Design, synthesis, and biological evaluation of novel alkylsulfanyl-1,2,4-triazoles as cis-restricted combretastatin A-4 analogues. Eur J Med Chem 125:1098–1106. https://doi.org/10.1016/j.ejmech.2016.10.051
Gao F, Wang T, Xiao J, Huang G (2019) Antibacterial activity study of 1,2,4-triazole derivatives. Eur J Med Chem 173:274–281. https://doi.org/10.1016/j.ejmech.2019.04.043
Yao YP, Dai FY, Dong KK, Mao Q, Wang YL, Chen T (2011) Synthesis and antibacterial activities of pleuromutilin derivatives with quinazolinone and thioether groups. J Chem Res 35:4–7. https://doi.org/10.3184/174751911X556675
Gottwald TR, Hughes G, Graham JH, Sun XA, Riley T (2001) The citrus canker epidemic in Florida: the scientific basis of regulatory eradication policy for an invasive species. Phytopathology 91:30–34. https://doi.org/10.1094/phyto.2001.91.1.30
Xu WM, Han FF, He M, Hu DY, He J, Yang S, Song BA (2012) Inhibition of tobacco bacterial wilt with sulfone derivatives containing an 1,3,4-oxadiazole moiety. J Agric Food Chem 60:1036–1041. https://doi.org/10.1021/jf203772d
Wang X, Yin J, Shi L, Zhang G, Song B (2014) Design, synthesis, and antibacterial activity of novel Schiff base derivatives of quinazolin-4(3H)-one. Eur J Med Chem 77:65–74. https://doi.org/10.1016/j.ejmech.2014.02.053
Fan ZJ, Shi J, Luo N, Ding MH, Bao XP (2019) Synthesis, crystal Structure, and agricultural antimicrobial evaluation of novel quinazoline thioether derivatives incorporating the 1,2,4-triazolo[4,3-a]pyridine moiety. J Agric Food Chem 67:11598–11606. https://doi.org/10.1021/acs.jafc.9b04733
Long QS, Liu LW, Zhao YL, Wang PY, Chen B, Li Z, Yang S (2019) Fabrication of furan-functionalized quinazoline hybrids: their antibacterial evaluation, quantitative proteomics, and induced phytopathogen morphological variation studies. J Agric Food Chem 67:11005–11017. https://doi.org/10.1021/acs.jafc.9b03419
Tao QQ, Liu LW, Wang PY, Long QS, Zhao YL, Jin LH, Xu WM, Chen Y, Li Z, Yang S (2019) Synthesis and in vitro and in vivo biological activity evaluation and quantitative proteome profiling of oxadiazoles bearing flexible heterocyclic patterns. J Agric Food Chem 67:7626–7639. https://doi.org/10.1021/acs.jafc.9b02734
Chen CJ, Song BA, Yang S, Xu GF, Bhadury PS, Jin LH, Hu DY, Li QZ, Liu F, Xue W, Lu P, Chen Z (2007) Synthesis and antifungal activities of 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-thiadiazole 5-(3,4,5-trimethoxyphen- yl)-2-sulfonyl-1,3,4-oxadiazole derivatives. Bioorg Med Chem 15:3981–3989. https://doi.org/10.1016/j.bmc.2007.04.014
Ye YH, Ma L, Dai ZC, Xiao Y, Zhang YY, Li DD, Wang JX, Zhu HL (2014) Synthesis and antifungal activity of nicotinamide derivatives as succinate dehydrogenase inhibitors. J Agric Food Chem 62:4063–4071. https://doi.org/10.1021/jf405437k
Dai ZC, Chen YF, Zhang M, Li SK, Yang TT, Shen L, Wang JX, Qian SS, Zhu HL, Ye YH (2015) Synthesis and antifungal activity of 1,2,3-triazole phenylhydrazone derivatives. Org Biomol Chem 13:477–486. https://doi.org/10.1039/c4ob01758g
Acknowledgements
This work was financially supported by Breeding Program of Guizhou University (No. 20185781), Young Top-Notch Talent Support Program of Guizhou Provincial Education Department (No. 2018038) and Guizhou Provincial High-Level Overseas Talents Innovation and Enterpreneurship Program (No. 201809).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Du, H., Ding, M., Luo, N. et al. Design, synthesis, crystal structure and in vitro antimicrobial activity of novel 1,2,4-triazolo[1,5-a]pyrimidine-containing quinazolinone derivatives. Mol Divers 25, 711–722 (2021). https://doi.org/10.1007/s11030-020-10043-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-020-10043-z