Abstract
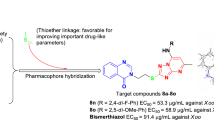
A series of novel quinazolin-4-one derivatives (7a–7n) bearing the 7-oxo-1,2,4-triazolo[1,5-a]pyrimidine moiety were designed, synthesized and evaluated for their inhibition activities against phytopathogenic bacteria and fungi in vitro. All of the target compounds were fully characterized through \(^{1}\hbox {H}\) NMR, \(^{13}\hbox {C}\) NMR, HRMS and IR spectra. Among these compounds, the structure of compound 7e was unambiguously confirmed via single-crystal X-ray diffraction analysis. The turbidimetric assays indicated that compounds 7b, 7d, 7g, 7k and 7n exhibited much more potent inhibition activities against the pathogen Xanthomonas oryzae pv. oryzae (Xoo), relative to control Bismerthiazol. Moreover, antibacterial activities of compounds 7j, 7k and 7n against the pathogen Xanthomonas axonopodis pv. citri (Xac) were comparable to that of control Bismerthiazol. As for the pathogen Ralstonia solanacearum (Rs), only compounds 7g and 7i demonstrated inhibition activities similar to control Thiadiazole-copper. Moreover, this class of compounds did not display inhibition activity against three fungi tested. The above findings indicated that quinazolin-4-one derivatives containing the 7-oxo-1,2,4-triazolo[1,5-a]pyrimidine moiety have a potential as promising candidates for the development of new and more efficient agricultural bactericides.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The phytobacteria of Xoo and Xac are two types of Gram-negative pathogenic bacteria, which cause rice bacterial leaf blight and citrus bacterial canker, respectively, and therefore lead to huge economic losses to global agricultural production every year [1, 2]. Taking the pathogen Xoo as an example, it invades via the vascular system and then colonizes the intercellular spaces of the parenchyma tissue [3], generally bringing about production losses by up to 50% [4]. Moreover, the bacterium Xac is typically spread through windblown rain and enters the host plants via stomata and/or wounds [5]. Although some agricultural bactericides are currently available for fighting against the above pathogenic phytobacteria, new and more efficient antibacterial agents are still extremely demanded, considering agents-associated toxicity and ceaseless evolution of antibiotic-resistant bacteria.
Quinazolin-4(3H)-one, found in more than 200 naturally occurring alkaloids [6], constitutes a significant class of compounds with diverse therapeutic and pharmacological properties such as antibacterial [7, 8], antifungal [9] and antiviral activities [10]. Some commercial medicine/pesticide molecules also contain the quinazolin-4-one backbone, including the analgesic Diproqualone, the sedative Cloroqualone, the agrofungicide Fluquinconazole and the fungicide Albaconazole (Fig. 1). On the other hand, 7-oxo-1,2,4-triazolo[1,5-a]pyrimidine derivatives also display a wide range of bioactivities, such as antiherpetic [11], antileishmanial [12], antiparasital [13], antimalarial [14] and antitumor [15] activities.
From a structural perspective, the fused heterocycle 1,2,4-triazolo[1,5-a]pyrimidin-7-one can be considered as the annulation of pyrimidin-4-one moiety to 1,2,4-triazole ring. Based on all of the above considerations and our continuing interest in searching for efficient 1,2,4-triazole-quinazoline/quinazolinone hybrid derivatives as agricultural antimicrobial agents [16,17,18,19], herein a series of novel quinazolin-4(3H)-one derivatives (7a–7n) containing the 7-oxo-1,2,4-triazolo[1,5-a]- pyrimidine moiety were designed, synthesized based on the “combinatorial optimization” method [20, 21] (Fig. 2) and evaluated for their inhibition activities in vitro against selected common pathogenic phytobacteria and phytofungi.
Materials and methods
All the chemicals were obtained from commercial suppliers and used without further purification (unless stated otherwise). Melting points were determined on a XT-4 binocular microscope (Beijing Tech Instrument Co., China). IR spectra were recorded on a Shimadzu IR Prestige-21 spectrometer using KBr disks. \(^{1}\hbox {H}\) and \(^{13}\hbox {C}\) NMR spectra were recorded on a JEOL-ECX 500 NMR spectrometer at room temperature using TMS as an internal standard and chemical shift (\(\delta \)) was expressed in parts per million (ppm). Multiplicity abbreviations used for the chemical shifts are as follows: s \(=\) singlet, d \(=\) doublet, t \(=\) triplet, q \(=\) quartet, m \(=\) multiplet. HRMS-ESI spectra were recorded on a Thermo Scientific Q Exactive series. X-ray crystallographic data were collected on a Bruker Smart Apex CCD area detector diffractometer (Bruker, Germany) using Mo-\(\hbox {K}\alpha \) radiation. The software package SPSS 17.0 is developed by SPSS Inc., which was downloaded from http://www.126xiazai.com/fileview_715650.html.
Synthesis of intermediate 5
A mixture of 5-amino-1H-1,2,4-triazole-3-thiol 4 (1.38 g, 11.88 mmol) and 3-(2-bromoethyl)quinazolinone 3 [22] (3.01 g, 11.88 mmol) dissolved in DMF (20 mL) in the presence of NaOH (1.42 g, 35.6 mmol) was stirred at room temperature for 1 h and then heated at \(60\,{^\circ }\hbox {C}\) for 10 h. After completion of the reaction indicated by the TLC analysis, ice water (15 mL) was added into the reaction mixture and the resulting precipitate was filtered, washed with water and dried under vacuum to give 5 as a white solid. Yield: 78.2%, mp \(237-240\,{^\circ }\hbox {C}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 12.00 (s, 1H), 8.25 (s, 1H), 8.15 (d, J = 8.0 Hz, 1H), 7.83 (t, J = 7.6 Hz, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.55 (t, J = 7.5 Hz, 1H), 6.09 (s, 2H), 4.26 (t, J = 6.3 Hz, 2H), 3.33 (t, J = 6.2 Hz, 2H); \(^{13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 160.2, 157.5, 154.8, 148.1, 148.0, 134.4, 127.2, 127.0, 126.1, 121.6, 45.7, 29.8. HRMS (ESI) calcd for \([\hbox {M} + \hbox {H}]^{+}\hbox {C}_{12}\hbox {H}_{13}\hbox {N}_{6}\hbox {OS}\): 289.0866, found: 289.0862.
Synthesis of intermediate 6
A mixture of intermediate 5 (3.00 g, 10.40 mmol) and ethyl acetoacetate (2.62 mL, 20.80 mmol) in acetic acid (20 mL) was heated to reflux and stirred for 8 h. The reaction mixture was then cooled to room temperature, and the formed precipitate was filtered, washed with AcOH and EtOH, and then dried to give 6 as a white solid. Yield: 72.3%, mp \(244{-}247\,{^\circ }\hbox {C}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 13.03 (s, 1H), 8.25 (s, 1H), 8.13 (d, J = 7.9 Hz, 1H), 7.78 (t, J = 7.6 Hz, 1H), 7.57 (d, \(J = 8.0\,\hbox {Hz}\), 1H), 7.51 (t, \(J = 7.5\,\hbox {Hz}\), 1H), 5.74 (s, 1H), 4.35 (t, \(J = 6.3\,\hbox {Hz}\), 2H), 3.57 (t, \(J = 6.3\,\hbox {HZ}\), 2H), 2.23 (s, 3H); \(^{13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6}\), ppm) \( \delta \): 161.0, 160.2, 154.7, 151.0, 150.6, 148.0, 147.8, 134.3, 127.0, 126.9, 126.1, 121.4, 98.5, 45.8, 29.6, 18.5. HRMS (ESI) calcd for \([\hbox {M} + \hbox {H}]^{+} \hbox {C}_{16}\hbox {H}_{15}\hbox {N}_{6}\hbox {O}_{2}\hbox {S}\): 355.0972, found: 355.0967.
General procedure for the synthesis of target compounds 7a–7n
A mixture of intermediate 6 (142 mg, 0.40 mmol) and an appropriate chlorinated compound (0.44 mmol) dissolved in \(\hbox {CH}_{3}\hbox {COCH}_{3}\) (45 mL) in the presence of \(\hbox {K}_{2}\hbox {CO}_{3}\) (83 mg, 0.6 mmol) was heated to reflux and stirred for \(12{-}15\) h. After cooling the reaction mixture to room temperature, the pure compounds were separated by flash column chromatography (petroleum ether/ethyl acetate = \(3/1{-}3/2, v/v)\) to afford 7a–7n.
3-(2-((4-Benzyl-5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (7a)
White solid, mp \(205-208\,{^\circ }\hbox {C}\), yield: 53.2%. IR \((\hbox {KBr}, \nu /\hbox {cm}^{-1})\): 1704 (C=O), 1666 (C=O); \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 8.25 (s, 1H), 8.14 (d, \(J = 7.5\,\hbox {Hz}\), 1H), 7.79 (t, \(J = 7.5\,\hbox {Hz}\), 1H), 7.60 (d, \(J = 8.0\,\hbox {Hz}\), 1H), 7.53 (t, \(J = 7.5\,\hbox {Hz}\), 1H), 7.36 (t, \(J = 7.5\,\hbox {Hz}\), 2H), 7.30 (t, \(J = 6.9\,\hbox {Hz}\), 1H), 7.24 (d, \(J = 7.5\,\hbox {Hz}\), 2H), 5.96 (s, 1H), 5.36 (s, 2H), 4.35 (t, \(J = 6.3\,\hbox {Hz}\), 2H), 3.58 (t, \(J = 6.3\,\hbox {Hz}\), 2H), 2.27 (s, 3H); \(^{13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 161.0, 160.2, 153.7, 152.7, 151.6, 148.0, 147.8, 135.3, 134.3, 128.9, 127.8, 127.0, 126.9, 126.4, 126.0, 121.4, 100.9, 50.2, 45.6, 29.7, 18.2; HRMS (ESI) calcd for \([\hbox {M} + \hbox {H}]^{+}\hbox {C}_{23}\hbox {H}_{21}\hbox {N}_{6}\hbox {O}_{2}\hbox {S}\): 445.1441, found: 445.1434.
3-(2-((5-Methyl-4-(4-nitrobenzyl)-7-oxo-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (7b)
White solid, mp \(178-181 \,{^\circ }\hbox {C}\), yield: 41.9%. IR \(({\hbox {KBr}}, \nu /\hbox {cm}^{-1})\): 1713 (C=O), 1674 (C=O); \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 8.21 (s, 1H), 8.20 (d, \(J = 9.2\,\hbox {Hz}\), 2H), 8.13 (d, \(J = 8.1\,\hbox {Hz}\), 1H), 7.79 (t, \(J = 8.0\,\hbox {Hz}\), 1H), 7.59 (d, \(J = 8.6\,\hbox {Hz}\), 1H), 7.52 (t, \(J = 9.2\,\hbox {Hz}\), 3H), 6.00 (s, 1H), 5.51 (s, 2H), 4.33 (t, \(J = 6.3 \hbox {Hz}\), 2H), 3.56 (t, \(J = 6.3\,\hbox {Hz}\), 2H), 2.26 (s, 3H); \(^{13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 161.1, 160.2, 153.9, 152.6, 151.5, 148.1, 147.8, 147.1, 143.0, 134.4, 127.8, 127.1, 127.0, 126.1, 124.1, 121.4, 101.2, 49.8, 45.7, 29.8, 18.3; HRMS (ESI) calcd for \([\hbox {M} + \hbox {H}]^{+}\hbox {C}_{23}\hbox {H}_{20}\hbox {N}_{7}\hbox {O}_4\hbox {S}\): 490.1292, found: 490.1286.
3-(2-((4-(2-Chlorobenzyl)-5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (7c)
White solid, mp \(198-201\,{^\circ }\hbox {C}\), yield: 42.2%. IR \(({\hbox {KBr}},\nu /\hbox {cm}^{-1})\): 1704 (C=O), 1672 (C=O); \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 8.20 (s, 1H), 8.14 (d, \(J = 6.9\,\hbox {Hz}\), 1H), 7.80 (t, \(J = 6.9\,\hbox {Hz}\), 1H), 7.60–7.52 (m, 3H), 7.35 (t, \(J = 6.9 \,{\hbox {Hz}}\), 1H), 7.26 (t, \(J = 7.5 \,{\hbox {Hz}}\), 1H), 6.93 (d, \(J = 7.5 \,{\hbox {Hz}}\), 1H), 6.04 (s, 1H), 5.33 (s, 2H), 4.33 (t, J = 6.3 Hz, 2H), 3.54 (t, \(J = 6.3 \,{\hbox {Hz}}\), 2H), 2.24 (s, 3H); \(^{13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 161.0, 160.1, 153.8, 152.4, 151.4, 147.9, 147.8, 134.3, 132.3, 131.1, 129.7, 129.5, 127.9, 127.0, 126.9, 126.6, 126.0, 121.4, 101.2, 48.1, 45.7, 29.7, 17.9; HRMS (ESI) calcd for \([\hbox {M} +\hbox {H}]^{+} \hbox {C}_{23}\hbox {H}_{20}\hbox {N}_{6}\hbox {O}_{2}\hbox {SCl}\): 479.1052, found: 479.1046.
3-(2-((4-(4-Chlorobenzyl)-5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (7d)
White solid, mp \(204-207\,{^\circ }\hbox {C}\), yield: 35.7%. IR \({(\hbox {KBr}}, \nu /\hbox {cm}^{-1})\): 1714 (C=O), 1674 (C=O); \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 8.25 (s, 1H), 8.13 (d, \(J = 7.5 \,{\hbox {Hz}}\), 1H), 7.79 (t, \(J = 6.9 \,{\hbox {Hz}}\), 1H), 7.59 (d, \(J = 8.0 \,{\hbox {Hz}}\), 1H), 7.52 (t, \(J = 7.5 \,{\hbox {Hz}}\), 1H), 7.41 (d, \(J = 8.1 \,{\hbox {Hz}}\), 2H), 7.29 (d, \(J = 8.0 \,{\hbox {Hz}}\), 2H), 5.96 (s, 1H), 5.35 (s, 2H), 4.35 (t, \(J = 6.3 \,{\hbox {Hz}}\), 2H), 3.57 (t, \(J = 6.3 \,{\hbox {Hz}}\), 2H), 2.26 (s, 3H); \(^{13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 161.1, 160.3, 153.8, 152.7, 151.6, 148.1, 147.8, 134.4, 132.5, 128.9, 128.8, 128.6, 127.1, 127.0, 126.1, 121.4, 101.1, 49.6, 45.7, 29.8, 18.2; HRMS (ESI) calcd for \([\hbox {M} + \hbox {H}]^{+} \hbox {C}_{23}\hbox {H}_{20}\hbox {N}_{6}\hbox {O}_{2}\hbox {SCl}\): 479.1052, found: 479.1047.
3-(2-((4-(2-Fluorobenzyl)-5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (7e)
White solid, mp \(196-199\,{^\circ }\hbox {C}\), yield: 45.8%. IR \({(\hbox {KBr}},\nu /\hbox {cm}^{-1})\): 1710 (C=O), 1674 (C=O); \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 8.22 (s, 1H), 8.13 (d, \(J = 8.1 \,{\hbox {Hz}}\), 1H), 7.79 (t, \(J = 7.5 \,{\hbox {Hz}}\), 1H), 7.59 (d, \(J = 8.0 \,{\hbox {Hz}}\), 1H), 7.53 (t, \(J = 6.9 \,{\hbox {Hz}}\), 1H), 7.38 (d, \(J = 8.8 \,{\hbox {Hz}}\), 1H), 7.27 (t, \(J = 8.6 \,{\hbox {Hz}}\), 1H), 7.16–7.10 (m, 2H), 6.00 (s, 1H), 5.38 (s, 2H), 4.34 (t, \(J = 6.3 \,{\hbox {Hz}}\), 2H), 3.56 (t, \(J = 6.3 \,{\hbox {Hz}}\), 2H), 2.29 (s, 3H); \(^{13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 161.0, 160.4, 160.2, 153.7, 152.5, 151.5, 148.0, 147.8, 134.3, 130.1, 130.0, 128.0, 127.0, 126.9, 126.0, 125.1, 125.0, 122.2, 122.1, 121.4, 115.7, 115.5, 101.0, 45.6, 44.7, 29.7, 18.0; HRMS (ESI) calcd for \([\hbox {M} + \hbox {H}]^{+} \hbox {C}_{23}\hbox {H}_{20}\hbox {FN}_{6}\hbox {O}_{2}\hbox {S}\): 463.1347, found: 463.1343.
3-(2-((4-(3-Fluorobenzyl)-5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (7f)
White solid, mp \(193-195 \,{^\circ }\hbox {C}\), yield: 42.3%. IR \({(\hbox {KBr}}, \nu /\hbox {cm}^{-1})\): 1712 (C=O), 1647 (C=O); \(^{1}\)H NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 8.25 (s, 1H), 8.13 (d, \(J = 7.5 \,{\hbox {Hz}}\), 1H), 7.79 (t, \(J = 7.0 \,{\hbox {Hz}}\), 1H), 7.59 (d, \(J = 8.1 \,{\hbox {Hz}}\), 1H), 7.53 (t, \(J = 6.9 \,{\hbox {Hz}}\), 1H), 7.41 (s, 1H), 7.38-7.37 (m, 2H), 7.19 (t, \(J = 5.8 \,{\hbox {Hz}}\), 1H), 5.97 (s, 1H), 5.37 (s, 2H), 4.35 (t, \(J = 6.3 \,{\hbox {Hz}}\), 2H), 3.57 (t, \(J = 6.3 \,{\hbox {Hz}}\), 2H), 2.26 (s, 3H); \(^{13}\)C NMR (125 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 160.9, 160.2, 153.8, 152.7, 151.4, 148.0, 147.8, 137.9, 134.3, 133.6, 130.8, 127.9, 127.1, 127.0, 126.5, 126.0, 125.1, 121.4, 101.1, 49.6, 45.6, 29.8, 18.2; HRMS (ESI) calcd for \([\hbox {M} + \hbox {H}]^{+} \hbox {C}_{23}\hbox {H}_{20}\hbox {FN}_{6}\hbox {O}_{2}\hbox {S}\): 463.1347, found: 463.1354.
3-(2-((4-(4-Fluorobenzyl)-5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (7g)
White solid, mp \(204-207 \,{^\circ }\hbox {C}\), yield: 51.8%. IR \({(\hbox {KBr}}, \nu /\hbox {cm}^{-1})\): 1703 (C=O), 1678 (C=O); \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 8.26 (s, 1H), 8.13 (d, \(J = 8.1 \,{\hbox {Hz}}\), 1H), 7.79 (t, \(J = 6.9 \,{\hbox {Hz}}\), 1H), 7.59 (d, \(J = 8.0 \,{\hbox {Hz}}\), 1H), 7.53 (t, \(J = 7.5 \,{\hbox {Hz}}\), 1H), 7.32 (d, \(J = 4.1 \,{\hbox {Hz}}\), 2H), 7.18 (t, \(J = 8.6 \,{\hbox {Hz}}\), 2H), 5.95 (s, 1H), 5.34 (s, 2H), 4.36 (t, \(J = 6.3 \,{\hbox {Hz}}\), 2H), 3.58 (t, \(J = 6.3 \,{\hbox {Hz}}\), 2H), 2.27 (s, 3H); \(^{13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 162.6, 161.0, 160.2, 153.7, 152.6, 151.5, 148.0, 147.8, 134.3, 131.6, 131.5, 128.9, 128.8, 127.0, 126.9, 126.0, 121.4, 115.8, 115.6, 101.0, 49.5, 45.7, 29.8, 18.2; HRMS (ESI) calcd for \([\hbox {M} + \hbox {H}]^{+} \hbox {C}_{23}\hbox {H}_{20}\hbox {FN}_{6}\hbox {O}_{2}\hbox {S}\): 463.1347, found: 463.1342.
3-(2-((5-Methyl-4-(3-methylbenzyl)-7-oxo-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (7h)
White solid, mp \(183-186 \,{^\circ }\hbox {C}\), yield: 54.6%. IR \({(\hbox {KBr}}, \nu /\hbox {cm}^{-1})\): 1709 (C=O), 1671 (C=O); \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 8.24 (s, 1H), 8.13 (d, \(J = 8.0 \,{\hbox {Hz}}\), 1H), 7.79 (t, \(J = 6.9 \,{\hbox {Hz}}\), 1H), 7.59 (d, \(J = 8.0 \,{\hbox {Hz}}\), 1H), 7.53 (t, \(J = 7.5 \,{\hbox {Hz}}\), 1H), 7.23 (t, \(J = 7.5 \,{\hbox {Hz}}\), 1H), 7.11 (d, \(J = 7.5 \,{\hbox {Hz}}\), 1H), 7.05 (s, 1H), 6.99 (d, \(J = 7.5 \,{\hbox {Hz}}\), 1H), 5.95 (s, 1H), 5.31 (s, 2H), 4.36 (t, \(J = 6.3 \,{\hbox {Hz}}\), 2H), 3.57 (t, \(J = 6.3 \,{\hbox {Hz}}\), 2H), 2.26 (s, 6H); \(^{13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 161.0, 160.2, 153.8, 152.7, 151.7, 148.0, 147.8, 138.3, 135.2, 134.3, 128.8, 128.5, 127.1, 127.0, 126.8, 126.0, 123.4, 121.4, 100.9, 50.2, 45.7, 29.7, 21.0, 18.2; HRMS (ESI) calcd for \([\hbox {M} + \hbox {H}]^{+} \hbox {C}_{24}\hbox {H}_{23}\hbox {N}_{6}\hbox {O}_{2}\hbox {S}\): 459.1598, found: 459.1592.
3-(2-((5-Methyl-4-(4-methylbenzyl)-7-oxo-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (7i)
White solid, mp \(195-197 \,{^\circ }\hbox {C}\), yield: 56.4%. IR \({(\hbox {KBr}},\nu /\hbox {cm}^{-1})\): 1714 (C=O), 1673 (C=O); \(^{1}\)H NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 8.25 (s, 1H), 8.13 (d, \(J = 8.1 \,{\hbox {Hz}}\), 1H), 7.79 (t, \(J = 7.5 \,{\hbox {Hz}}\), 1H), 7.59 (d, \(J = 8.6 \,{\hbox {Hz}}\), 1H), 7.53 (t, \(J = 7.5 \,{\hbox {Hz}}\), 1H), 7.15 (d, \(J = 8.1 \,{\hbox {Hz}}\), 2H), 7.12 (d, \(J = 8.0 \,{\hbox {Hz}}\), 2H), 5.95 (s, 1H), 5.30 (s, 2H), 4.36 (t, \(J = 6.3 \,{\hbox {Hz}}\), 2H), 3.58 (t, \(J = 6.3 \,{\hbox {Hz}}\), 2H), 2.26 (s, 3H); \(^{13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 161.0, 160.2, 153.7, 152.7, 151.7, 148.0, 147.8, 137.1, 134.3, 132.3, 129.5, 127.0, 126.9, 126.5, 126.0, 121.4, 100.8, 50.0, 45.7, 29.7, 20.7, 18.2; HRMS (ESI) calcd for \([\hbox {M} + \hbox {H}]^{+}\hbox {C}_{24}\hbox {H}_{23}\hbox {N}_{6}\hbox {O}_{2}\hbox {S}\): 459.1598, found: 459.1592.
3-(2-((4-(3-Methoxybenzyl)-5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (7j)
White solid, mp \(171-174 \,{^\circ }\hbox {C}\), yield: 62.8%. IR \({(\hbox {KBr}},\nu /\hbox {cm}^{-1})\): 1705 (C=O), 1677 (C=O); \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 8.24 (s, 1H), 8.13 (d, \(J = 8.0 \,{\hbox {Hz}}\), 1H), 7.79 (t, \(J = 6.9 \,{\hbox {Hz}}\), 1H), 7.59 (d, \(J = 8.0 \,{\hbox {Hz}}\), 1H), 7.53 (t, \(J = 7.5\,\hbox {Hz}\), 1H), 7.26 (t, \(J = 8.0\,{\hbox {Hz}}\), 1H), 6.87 (d, \(J = 8.1\,{\hbox {Hz}}\), 1H), 6.83 (s, 1H), 6.73 (d, \(J = 7.5\,{\hbox {Hz}}\), 1H), 5.96 (s, 1H), 5.32 (s, 2H), 4.36 (t, \(J = 6.3\,{\hbox {Hz}}\), 2H), 3.72 (s, 2H), 3.57 (t, \(J = 6.3\,{\hbox {Hz}}\), 2H), 2.27 (s, 3H); \(^{ 13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 161.1, 160.2, 159.6, 153.8, 152.7, 151.7, 148.1, 147.8, 136.9, 134.4, 130.2, 127.1, 127.0, 126.1, 121.4, 118.3, 113.1, 112.4, 100.9, 55.1, 50.1, 45.7, 29.8, 18.2; HRMS (ESI) calcd for \([\hbox {M} + \hbox {H}]^{+} \hbox {C}_{24}\hbox {H}_{23}\hbox {N}_{6}\hbox {O}_{3}\hbox {S}\): 475.1547, found: 475.1542.
3-(2-((4-(2,4-Dichlorobenzyl)-5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (7k)
White solid, mp \(118{-}121 \,{^\circ }\hbox {C}\), yield: 44.5%. IR \({(\hbox {KBr}},\nu /\hbox {cm}^{-1})\): 1709 (C=O), 1673 (C=O); \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 8.21 (s, 1H), 8.13 (d, \(J = 8.0\,{\hbox {Hz}}\), 1H), 7.81–7.78 (m, 1H), 7.73 (d, \(J = 2.3\,{\hbox {Hz}}\), 1H), 7.59 (d, \(J = 8.1\,{\hbox {Hz}}\), 1H), 7.53 (t, \(J = 8.1\,{\hbox {Hz}}\), 1H), 7.33–7.31 (m, 1H), 7.01 (d, \(J = 8.5\,{\hbox {Hz}}\), 1H), 6.04 (s, 1H), 5.30 (s, 2H), 4.32 (t, \(J = 6.3\,{\hbox {Hz}}\), 2H), 3.54 (t, \(J = 6.3\,{\hbox {Hz}}\), 2H), 2.18 (s, 3H); \(^{ 13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 161.0, 160.1, 153.8, 152.4, 151.3, 147.9, 147.8, 134.3, 133.2, 132.2, 131.6, 129.2, 128.3, 128.0, 127.1, 127.0, 126.0, 121.4, 101.3, 47.8, 45.7, 29.7, 17.9; HRMS (ESI) calcd for \([\hbox {M} + \hbox {H}]^{+} \hbox {C}_{23}\hbox {H}_{19}\hbox {N}_{6}\hbox {O}_{2}\hbox {SCl}_{2}\): 513.0662, found: 513.0658.
3-(2-((4-(2,6-Dichlorobenzyl)-5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (7l)
White solid, mp \(129{-}131 \,{^\circ }\hbox {C}\), yield: 54.5%; IR \({(\hbox {KBr}},\nu /\hbox {cm}^{-1})\): 1711 (C=O), 1670 (C=O); \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 8.15 (s, 1H), 8.13 (s, 1H), 7.80 (t, \(J = 7.5\,{\hbox {Hz}}\), 1H), 7.60 (d, \(J = 8.0\,{\hbox {Hz}}\), 1H), 7.53 (t, \(J = 7.5\,{\hbox {Hz}}\), 1H), 7.49 (d, \(J = 8.1\,{\hbox {Hz}}\), 2H), 7.37 (t, \(J = 8.1\,{\hbox {Hz}}\), 1H), 6.01 (s, 1H), 5.55 (s, 2H), 4.23 (t, \(J = 6.3\,{\hbox {Hz}}\), 2H), 3.48 (t, \(J = 6.3\,{\hbox {Hz}}\), 2H), 2.37 (s, 3H); \(^{ 13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 160.9, 160.2, 153.7, 152.6, 151.9, 148.0, 147.8, 135.0, 134.3, 130.7, 130.1, 129.3, 127.1, 127.0, 126.0, 121.4, 100.9, 47.4, 45.6, 29.9, 19.0; HRMS (ESI) calcd for \([\hbox {M} + \hbox {H}]^{+}\hbox {C}_{23}\hbox {H}_{19}\hbox {N}_{6}\hbox {O}_{2}\hbox {SCl}_{2}\): 513.0662, found: 513.0657.
3-(2-((4-((2-Chlorothiazol-5-yl)methyl)-5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (7m)
White solid, mp \(115-117 \,{^\circ }\hbox {C}\), yield: 39.5%. IR \({(\hbox {KBr}}, \nu /\hbox {cm}^{-1})\): 1697 (C=O), 1640 (C=O); \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 8.28 (s, 1H), 8.13 (d, \(J = 8.0\,{\hbox {Hz}}\), 1H), 7.83 (s, 1H), 7.79 (t, \(J = 6.9\,{\hbox {Hz}}\), 1H), 7.60 (d, \(J = 8.1\,{\hbox {Hz}}\), 1H), 7.52 (t, \(J = 7.5\,{\hbox {Hz}}\), 1H), 5.95 (s, 1H), 5.48 (s, 2H), 4.39 (t, \(J = 6.3\,{\hbox {Hz}}\), 2H), 3.62 (t, \(J = 6.3 \,\hbox {Hz}\), 2H), 2.45 (s, 3H); \(^{13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 161.1, 160.3, 153.7, 151.8, 151.5, 151.1, 148.2, 147.9, 141.8, 134.4, 134.3, 127.1, 127.0, 126.1, 121.4, 101.2, 45.7, 43.2, 30.0, 18.3; HRMS (ESI) calcd for\([\hbox {M} + \hbox {H}]^{+} \hbox {C}_{20}\hbox {H}_{17}\hbox {N}_{7}\hbox {O}_{2}\hbox {S}_{2}\hbox {Cl}\): 486.0568, found: 486.0561.
3-(2-((4-((6-Chloropyridin-3-yl)methyl)-5-methyl-7-oxo-4,7-dihydro-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)thio)ethyl)quinazolin-4(3H)-one (7n)
White solid, mp \(158{-}161 \,{^\circ }\hbox {C}\), yield: 55.1%. IR \({(\hbox {KBr}},\nu /\hbox {cm}^{-1})\): 1711 (C=O), 1675 (C=O);\(^{ 1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 8.42 (s, 1H), 8.25 (s, 1H), 8.13 (d, \(J = 7.5\,{\hbox {Hz}}\), 1H), 7.79 (t, \(J = 7.5\,{\hbox {Hz}}\), 2H), 7.60 (d, \(J = 8.6\,{\hbox {Hz}}\), 1H), 7.54–7.48 (m, 2H), 5.96 (s, 1H), 5.39 (s, 2H), 4.35 (t, \(J = 6.3\,{\hbox {Hz}}\), 2H), 3.57 (t, \(J = 6.3\,{\hbox {Hz}}\), 2H), 2.31 (s, 3H); \(^{ 13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6}\), ppm) \(\delta \): 160.9, 160.2, 153.8, 152.6, 151.3, 149.8, 148.7, 148.1, 147.8, 138.6, 134.3, 130.7, 127.1, 127.0, 126.0, 124.4, 121.4, 101.2, 47.4, 45.6, 29.8, 18.3; HRMS (ESI) calcd for \([\hbox {M} + \hbox {H}]^{+} \hbox {C}_{22}\hbox {H}_{19}\hbox {N}_{7}\hbox {O}_{2}\hbox {SCl}\): 480.1004, found: 480.1000.

Antibacterial bioassay
Antibacterial activities of target compounds 7a–7n were determined against three phytopathogenic bacteria (Xoo, Xac and Rs) based on a turbidimetric method [23,24,25]. Tested compounds were prepared at two concentrations of 200 and 100 \(\upmu \hbox {g}/\hbox {mL}\). Pure DMSO in sterile distilled water was used as blank control, and commercially available bactericide bismerthiazol (BMT) and thiadiazole-copper (TDC) were used as positive controls. About \(40\, \upmu \hbox {L}\) of solvent NB (3 g of beef extract, 5 g of peptone, 1 g of yeast powder, 10 g of glucose, 1 L of distilled water, pH = 7.0–7.2) containing the bacterium Xoo/Xac/Rs was added to the mixed solvent system including 4 mL of solvent NB and 1 mL of 0.1% Tween-20 containing tested compound or BMT/TDC. The above test tube was incubated at \(30\,\pm \,1\,{^\circ }\hbox {C}\) and continuously shaken at 180 rpm for three days. The bacterial growth was monitored by measuring the optical density at 600 nm \((\hbox {OD}_{600})\), given by \(\hbox {turbidity}_{\mathrm{corrected\, value}} = \hbox {OD}_{\mathrm{bacterium}}- \hbox {OD}_{\mathrm{no\, bacterium}}, I = (C_{\mathrm{tur }}-T_{\mathrm{tur}})/C_{\mathrm{tur}} \quad \times 100\%\). The \(C_{\mathrm{tur}}\) represented the corrected turbidity value of bacterial growth of untreated NB (blank control), and \(T_{\mathrm{tur}}\) represented the corrected turbidity value of bacterial growth of tested compound-treated NB. The I represented the inhibition rate of tested compound against the bacterium.
Finally, antibacterial activities of target compounds 7a–7n (against the Xoo) and 7b, 7d, 7j, 7k and 7n (against the Xac) were determined at five different concentrations (200, 100, 50, 25 and \(12.5\, \upmu \hbox {g}/\hbox {mL})\) to obtain their \(\hbox {EC}_{50}\) values, which were determined statistically by Probit analysis with the software package SPSS 17.0 [16, 24].
Antifungal bioassay
Mycelial growth rate method [26, 27] was utilized to assess the antifungal activities of target compounds 7a–7n against three phytopathogenic fungi (G. zeae, V. dahliae and S. sclerotiorum). DMSO solution of the tested compound was added into sterilized Petri dishes, which contained about 10 mL molten potato dextrose agar (PDA). Subsequently, a 4-mm-diameter mycelial plug was cut from the fungal colony and placed at the center of PDA plate at \(28\,\pm \,1\,{^\circ }\hbox {C}\) for 4 days. Antifungal assays were conducted in triplicate for each compound. Additionally, pure DMSO and commercially available fungicide (hymexazol) were utilized as negative and positive control, respectively.
The inhibition rate (I) of tested compound was determined based on the following formula:
In this formula, the C represented the average mycelial diameter of negative control, and T represented the average mycelial diameter of tested compound-treated PDA.
Results and discussion
Synthesis
The synthetic route of target compounds 7a–7n is summarized in Scheme 1. Briefly, quinazolin-4-one 2 [16] was reacted with 1,2-dibromoethane in DMF-NaH to give 3-(2-bromoethyl)quinazolinone 3 [22], which was then subjected to a thioetherification reaction with 5-amino-1H-1,2,4-triazole-3-thiol in DMF-NaOH to generate the intermediate 5 in 78% yield. After a cyclization reaction between 5 and ethyl acetoacetate in refluxing HOAc, the key intermediate 6 was obtained in 72% yield. Finally, intermediate 6 and a substituted benzyl chloride were reacted in \(\hbox {CH}_{3}\hbox {COCH}_{3}{-}\hbox {K}_{2}\hbox {CO}_{3}\) to afford target compounds 7a–7n. All of the target compounds were fully characterized through \(^{1}\hbox {H}\) NMR, \(^{13}\hbox {C}\) NMR, HRMS and IR spectra. It should be noted that the alkylation of 6 occurred at the 4-position nitrogen atom of the 1,2,4-triazolo[1,5-a]pyrimidin-7-one heterocycle (instead of 7-position oxygen atom), which was clearly confirmed by the following crystal structure.
Spectral and single-crystal X-ray diffraction analysis
Taking compound 7e as a representative example, the strong signals at 1710 and 1674 \(\hbox {cm}^{-1}\) in the IR spectrum were due to the presence of two C=O functionalities. In the \(^{1}\hbox {H}\) NMR spectrum, the three singlets at 5.38, 6.00 and 8.22 ppm were assigned to the protons at the benzylic \(\hbox {CH}_{2}\), 6-CH of 1,2,4-triazolo[1,5-a]pyrimidin-7-one and 2-CH of quinazolinone, respectively. Additionally, two signals at 161.0 and 160.4 ppm in the \(^{13}\hbox {C}\) NMR spectrum correspond to two C=O functionalities in 7e. Finally, high-resolution mass spectrum (HRMS) of compound 7e displayed an intense signal at m/z = 463.1343, corresponding to the protonated pseudo-molecular ion of [\(\hbox {M}\,+\, \hbox {H}]^{+}\).
Single crystal of compound 7e \(\cdot \hbox {H}_{2}\hbox {O}\) suitable for X-ray diffraction analysis was obtained (Fig. 3) by slow evaporation of a \(\hbox {CH}_{2}\hbox {Cl}_{2}{-}\hbox {EtOH}\,(1/2, v/v)\) solution of 7e at room temperature. Crystal data for 7e \(\cdot \,\hbox {H}_{2}\hbox {O}\) are as follows: yellow crystal, \(\hbox {C}_{23}\hbox {H}_{21}\hbox {FN}_{6}\hbox {O}_{3}\hbox {S}\), \(M_{r} = 480.52\), triclinic, space group \(P-1\); \(a = 9.511(11)\), \(b = 10.033(11)\), \(c = 11.726(13)\,({\AA })\); \(\alpha = 82.776(12)\), \(\beta = 88.549(12)\), \(\gamma = 86.401(12)\), \(V= 1108(2) {\AA }^{3}\), \(T= 296 K\), \(Z = 2\), \(Dc= 1.441 \hbox {g}/\hbox {cm}^{3}\), \(F(000) = 500\), reflections collected/independent reflections \(=\) 3879/3585, goodness of fit on \(\hbox {F}^{2} = 1.043\), fine, \(R1 = 0.0317\), \(wR2 = 0.0956\). Crystallographic data of compound 7e have been deposited in the Cambridge Crystallographic Data Center (CCDC 1546024).
Antibacterial activity
A turbidimetric method [23,24,25] was conducted to assess the antibacterial activities of compounds 7a–7n against three pathogenic phytobacteria Xoo, Xac and Rs in vitro. Moreover, the commercial bactericides bismerthiazol (BMT) and thiadiazole-copper (TDC) were employed as control agents. As shown in Table 1, more than half of the target compounds were found to have comparable or even better inhibition activities against the pathogen Xoo at 200 and 100 \(\upmu \hbox {g}/\hbox {mL}\), relative to control BMT. Additionally, compounds 7j and 7k showed appreciable antibacterial activities against the bacterium Xac at 100 \(\upmu \hbox {g}/\hbox {mL}\), similar to that of control BMT. In stark contrast to the phytobacteria Xoo and Xac, almost all the target compounds did not demonstrate noticeable inhibitory activity toward the pathogen Rs, except for compounds 7g and 7i. Lastly, compared with intermediate 6, most of the target compounds possessed remarkably improved antibacterial activities against the pathogenic bacteria Xoo and Xac, which proved the necessity of the introduction of substituted benzyl group into the target molecules.
Encouraged by the above experimental results, the \(\hbox {EC}_{50}\) (half-maximal effective concentration) values of compounds 7a–7n (against the Xoo) and 7b, 7d, 7j, 7k and 7n (against the Xac) were further determined using the serial dilution method (200, 100, 50, 25 and 12.5 \(\upmu \hbox {g}/\hbox {mL})\). As displayed in Table 2, a vast majority of target compounds exhibited comparable or much better \(\hbox {EC}_{50}\) values relative to control BMT. On the whole, the presence of electron-withdrawing substitutions helped to enhance their antibacterial activities against the Xoo, such as seen in compounds 7b (4-NO\(_{2}\)-Ph), 7d (4-Cl-Ph), 7g (4-F-Ph) and 7k (2,4-di-Cl-Ph) with \(\hbox {EC}_{50}\) values of 72.1, 57.0, 67.8 and 53.5 \(\upmu \hbox {g}/\hbox {mL}\), respectively, compared to control BMT (91.4 \(\upmu \hbox {g}/\hbox {mL})\). Notably, compound 7n bearing the heterocyclic 6-Cl-3-pyridyl group displayed the strongest inhibition activity \((\hbox {EC}_{50} = 40.2\ \upmu \hbox {g}/\hbox {mL})\) among this class of compounds, which may be due to the extra contribution from hydrogen-bonding interaction between the pyridine nitrogen atom and some specific proteins within the Xoo. Furthermore, the position of electron-withdrawing groups on the benzene ring also produced a remarkable effect on the inhibition activity, such as compounds 7d versus 7c, 7g versus 7f and 7k versus 7l. In other words, the halogen substitution at the para position of the benzene ring exhibited better activity than their ortho- and meta-position counterparts, implying that steric hindrance could result in reduced antibacterial activity. As for the pathogen Xac, compounds 7j, 7k and 7n had \(\hbox {EC}_{50}\) values of 56.9, 54.6 and 67.8 \(\upmu \hbox {g}/\hbox {mL}\), respectively, which were similar to control BMT (60.5 \(\upmu \hbox {g}/\hbox {mL}\)).
Antifungal activity
Finally, antifungal activities of compounds 7a–7n against three pathogenic phytofungi (Gibberella zeae, Verticillium dahliae and Sclerotinia sclerotiorum) were also assessed via the mycelial growth rate method [26, 27]. Unfortunately, all of the target compounds did not show any noticeable inhibition activity against the above fungi at 50 \(\upmu \hbox {g}/\hbox {mL}\) (Table 3).
Conclusion
In summary, a series of novel quinazolin-4-one derivatives containing a 7-oxo-1,2,4-triazolo[1,5-a]pyrimidine moiety were synthesized, and the structure of compound 7e was further determined via single-crystal X-ray crystallography. The obtained results indicate that some of the target compounds possess far more potent antibacterial activities against the pathogenic phytobacterium Xoo, relative to commercial bactericide bismerthiazol. The above findings demonstrate that quinazolin-4(3H)-one derivatives bearing a 7-oxo-1,2,4-triazolo[1,5-a]pyrimidine moiety are promising candidates for the development of new agrobactericides against the bacterium Xoo.
References
Sun X, Cao Y, Yang Z, Xu C, Li X, Wang S, Zhang Q (2004) Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J 37:517–527. doi:10.1046/j.1365-313X.2003.01976.x
Graham JH, Gottwald TR, Cubero J, Achor DS (2004) Xanthomonas axonopodis pv. citri: factors affecting successful eradication of citrus canker. Mol Plant Pathol 5:1–15. doi:10.1046/j.1364-3703.2003.00197.X
Ryan RP, Vorhölter FJ, Potnis N, Jones JB, Van Sluys MA, Bogdanove AJ, Dow JM (2011) Pathogenomics of Xanthomonas: understanding bacterium—plant interactions. Nat Rev Microbiol 9:344–355. doi:10.1038/nrmicro2558
Huang N, Angeles ER, Domingo J, Magpantay G, Singh S, Zhang G, Kumaravadivel N, Bennett J, Khush GS (1997) Pyramiding of bacterial blight resistance genes in rice: marker-assisted selection using RFLP and PCR. Theor Appl Genet 95:313–320. doi:10.1007/s001220050565
Li J, Wang N (2011) Genome-wide mutagenesis of Xanthomonas axonopodis pv. citri reveals novel genetic determinants and regulation mechanisms of biofilm formation. PLos One 6:e21804. doi:10.1371/journal.pone.0021804
Tiwary BK, Pradhan K, Nanda AK, Chakraborty R (2015) Implication of quinazoline-\(4(3H)\)-ones in medicinal chemistry: a brief review. J Chem Biol Ther 1:104–110. doi:10.4172/2572-0406.1000104
Wang X, Li P, Li Z, Yin J, He M, Xue W, Chen Z, Song B (2013) Synthesis and bioactivity evaluation of novel arylimines containing a 3-aminoethyl-2-[(\(p\)-trifluoromethoxy)anilino]-\(4(3H)\)-quinazolinone moiety. J Agric Food Chem 61:9575–9582. doi:10.1021/jf403193q
Bouley R, Ding D, Peng Z, Bastian M, Lastochkin E, Song W, Suckow MA, Schroeder VA, Wolter WR, Mobashery S, Chang M (2016) Structure-activity relationship for the \(4(3H)\)-quinazolinone antibacterials. J Med Chem 59:5011–5021. doi:10.1021/acs.jmedchem.6b00372
Zhang J, Liu J, Ma Y, Ren D, Cheng P, Zhao J, Zhang F, Yao Y (2016) One-pot synthesis and antifungal activity against plant pathogens of quinazolinone derivatives containing an amide moiety. Bioorg Med Chem Lett 26:2273–2277. doi:10.1016/j.bmcl.2016.03.052
Chen M, Li P, Hu D, Zeng S, Li T, Jin L, Xue W, Song B (2016) Synthesis, antiviral activity, 3D-QSAR, and interaction mechanisms study of novel malonate derivatives containing quinazolin-\(4(3H)\)-one moiety. Bioorg Med Chem Lett 26:168–173. doi:10.1016/j.bmcl.2015.11.006
Deev SL, Yasko MV, Karpenko IL, Korovina AN, Khandazhinskaya AL, Andronova VL, Galegov GA, Shestakova TS, Ulomskii EN, Rusinov VL, Chupakhin ON, Kukhanova MK (2010) 1,2,4-Triazoloazine derivatives as a new type of herpes simplex virus inhibitors. Bioorg Chem 38:265–270. doi:10.1016/j.bioorg.2010.09.002
Ramírez-Macías I, Marín C, Salas JM, Caballero A, Rosales MJ, Villegas N, Rodríguez-Dieguez A, Barea E, Sánchez-Moreno M (2011) Biological activity of three novel complexes with the ligand 5-methyl-1,2,4-triazolo[1,5-\(a\)]pyrimidin- \(7(4H)\)-one against leishmania spp. J Antimicrob Chemother 66:813–819. doi:10.1093/jac/dkq537
Caballero AB, Marín C, Rodríguez-Diéguez A, Ramírez-Macías I, Barea E, Sánchez-Moreno M, Salas JM (2011) In vitro and in vivo antiparasital activity against Trypanosoma cruzi of three novel 5-methyl-1,2,4-triazolo[1,5-\(a\)]pyrimidin-\(7(4H)\)-one-based complexes. J Inorg Biochem 105:770–776. doi:10.1016/j.jinorgbio.2011.03.015
Bedingfield PTP, Cowen D, Acklam P, Cunningham F, Parsons MR, Mcconkey GA, Fishwick CWG, Johnson AP (2012) Factors influencing the specificity of inhibitor binding to the human and malaria parasite dihydroorotate dehydrogenases. J Med Chem 55:5841–5850. doi:10.1021/jm300157n
Ruiz J, Villa MD, Cutillas N, López G, de Haro C, Bautista D, Moreno V, Valencla L (2008) Palladium(II) and platinum(II) organometallic complexes with 4,7-dihydro-5-methyl-7-oxo[1,2,4]triazolo[1,5-\(a\)]pyrimidine. Antitumor activity of the platinum compounds. Inorg Chem 47:4490–4505. doi:10.1021/ic701873b
Yan BR, Lv XY, Du H, Gao MN, Huang J, Bao XP (2016) Synthesis and biological activities of novel quinazolinone derivatives containing a 1,2,4-triazolylthioether moiety. Chem Pap 70:983–993. doi:10.1515/chempap-2016-0034
Pan D, Du H, Lü X, Bao X (2016) Synthesis and antibacterial activities of novel quinazoline-2,4-dione derivatives containing the 1,2,4-triazole Schiff-base unit. Chin J Org Chem 36:818–825. doi:10.6023/cjoc201510005
Yan B, Lü X, Du H, Bao X (2016) Design, synthesis and biological activities of novel quinazolinone derivative bearing 4-phenyl-5-thioxo-1,2,4-triazole Mannich bases. Chin J Org Chem 36:207–212. doi:10.6023/cjoc201506026
Liu J, Liu Y, Jian J, Bao X (2013) Synthesis and fungicidal activities of novel quinazoline derivatives containing 1,2,4-triazole Schiff-base unit. Chin J Org Chem 33:370–374. doi:10.6023/cjoc201209023
Xu H, Wang YY (2010) Antifungal agents. Part 5: Synthesis and antifungal activities of aminoguanidine derivatives of \(N\)-arylsulfonyl-3-acylindoles. Bioorg Med Chem Lett 20:7274–7277. doi:10.1016/j.bmcl.2010.10.084
Xu H, Fan LL (2011) Antifungal agents. Part 4: Synthesis and antifungal activities of novel indole[1,2-\(c\)]-1,2,4-benzotriazine derivatives against phytopathogenic fungi in vitro. Eur J Med Chem 46:364–369. doi:10.1016/j.ejmech.2010.10.022
Yao YP, Dai FY, Dong KK, Mao Q, Wang YL, Chen T (2011) Synthesis and antibacterial activities of pleuromutilin derivatives with quinazolinone and thioether groups. J Chem Res 35:4–7. doi:10.3184/174751911X556675
Xu WM, Han FF, He M, Hu DY, He J, Yang S, Song BA (2012) Inhibition of tobacco bacterial wilt with sulfone derivatives containing an 1,3,4-oxadiazole moiety. J Agric Food Chem 60:1036–1041. doi:10.1021/jf203772d
Li P, Shi L, Yang X, Yang L, Chen XW, Wu F, Shi QC, Xu WM, He M, Hu DY, Song BA (2014) Design, synthesis, and antibacterial activity against rice bacterial leaf blight and leaf streak of 2,5-substituted-1,3,4 -oxadiazole/thiadiazole sulfone derivative. Bioorg Med Chem Lett 24:1677–1680. doi:10.1016/j.bmcl.2014.02.060
Wang X, Yin J, Shi L, Zhang G, Song B (2014) Design, synthesis, and antibacterial activity of novel Schiff base derivatives of quinazolin-\(4(3H)\)-one. Eur J Med Chem 77:65–74. doi:10.1016/j.ejmech.2014.02.053
Chen CJ, Song BA, Yang S, Xu GF, Bhadury PS, Jin LH, Hu DY, Li QZ, Liu F, Xue W, Lu P, Chen Z (2007) Synthesis and antifungal activities of 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-thiadiazole 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-oxadiazole derivatives. Bioorg Med Chem 15:3981–3989. doi:10.1016/j.bmc.2007.04.014
Fan Z, Shi Z, Zhang H, Liu X, Bao L, Ma L, Zuo X, Zheng Q, Mi N (2009) Synthesis and biological activity evaluation of 1,2,3-thiadiazole derivatives as potential elicitors with highly systemic acquired resistance. J Agric Food Chem 57:4279–4286. doi:10.1021/jf8031364
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21362003) and the Agricultural Research Projects of Guizhou Province (No. 20093010).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11030_2017_9782_MOESM1_ESM.doc
Supplementary data The original spectral files (including 1H NMR, 13C NMR and HRMS) of intermediates 5 & 6as well as target compounds 7a-7n can be found in this section. 294 bytes
Rights and permissions
About this article
Cite this article
Du, H., Fan, Z., Yang, L. et al. Synthesis of novel quinazolin-4(3H)-one derivatives containing the 7-oxo-1,2,4-triazolo[1,5-a]pyrimidine moiety as effective agricultural bactericides against the pathogen Xanthomonas oryzae pv. oryzae . Mol Divers 22, 1–10 (2018). https://doi.org/10.1007/s11030-017-9782-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-017-9782-3