Abstract
A series of novel quinazolinone derivatives (E1−E31) containing the 1,2,4-triazole Schiff base moiety and an isopropanol linker were designed, synthesized and assessed as antimicrobial agents in agriculture. All the target compounds were fully characterized by 1 H NMR, 13 C NMR, and high-resolution mass spectrometry (HRMS). Among them, the structure of compound E12 was further confirmed via single crystal X-ray diffraction method. The experimental results indicated that many compounds displayed good in vitro antibacterial efficacies against the tested phytopathogenic bacteria including Xanthomonas oryzae pv. oryzae (Xoo), Xanthomonas axonopodis pv. citri (Xac), and Ralstonia solanacearum (Rs). For example, compounds E3, E4, E10, E13, and E22 had EC50 (half-maximal effective concentration) values of 55.4, 39.5, 49.5, 53.5, and 57.4 µg/mL against Xoo, respectively, superior to the commercialized bactericide Bismerthiazol (94.5 µg/mL). In addition, the antibacterial efficacies of compounds E10 and E13 against Xac were about two times more effective than control Bismerthiazol, in terms of their EC50 values. Last, the antifungal assays showed that compounds E22 and E30 had the inhibition rates of 52.7% and 54.6% at 50 µg/mL against Gibberella zeae, respectively, higher than the commercialized fungicide Hymexazol (48.4%).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant pathogenic bacteria are considered as an important factor affecting crop yield and quality, leading to huge economic losses to farmers around the world each year [1,2,3]. For example, the Gram-negative bacteria of Xanthomonas oryzae pv. oryzae (Xoo) and Xanthomonas axonopodis pv. citri (Xac) are the causal agents of bacterial leaf blight (BLB) of rice and citrus canker diseases, respectively. BLB is one of the most destructive rice diseases, since the pathogen Xoo invades through the vascular system and then occupies the intercellular spaces of the parenchyma tissue, triggering annual yield losses of 10 ~ 50% in many rice-planting countries [4, 5]. In addition, the bacterium of Xac can be spreaded by the wind, rain, and touch, this pathogen enters plants via the stomata in leaves, the lenticels, and wounds [6, 7]. Furthermore, tobacco bacterial wilt is caused by the bacterium Ralstonia solanacearum (Rs), which is a soil-borne pathogen that enters the tobacco plant and spreads rapidly through the vascular system and gives rise to the browning of the xylem and lethal wilting [8, 9]. The employment of antibacterial agents to cope with these pathogens was regarded as a cost-effective approach to achieving the crop protection [10]. Bismerthiazol, a thiadiazole-thione derivative, has long been used as an agrobactericide for controlling rice leaf blight and citrus canker diseases since the 1970s. However, the long-term use of Bismerthiazol led to the reduced efficacies and ever-growing resistance of the pathogenic bacteria [11]. Taking these into consideration, the development of new agrobactericides with novel structure, high efficacy, and good environmental compatiblitiy has become an urgent task in the field of crop protection.
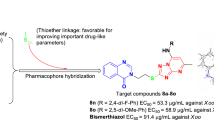
The quinazolinone skeleton is one of the most important nitrogen-containing heterocycles, which is discovered in more than 200 natural compounds [12]. Many quinazolinone derivatives exhibited a range of bioactivities, including antibacterial [13], antifungal [14], antiviral [15], antitumor [16], antimalarial [17], and anti-inflammatory effects [18] et al. Additionally, some of them have been successfully launched into the market as the commercialized pesticides and drugs, such as agrofungicide Fluquinconazole (Fig. 1), soporific drug Cloroqualone, muscle relaxant Afloqualone, anticancer drug Nolatrexed, antimalarial agent Febrifugine, and antiprotozoal agent Halofuginone. On the other hand, the isopropanol linkage is often utilized for constructing bioactive molecules, which can improve the biocompatibility of small molecules and easily establish extra hydrogen-bonding interactions with the relevant target proteins [19]. A few commercialized agents contain this linkage, including antifungal drugs Albaconazole/Fluconazole/Voriconazole and agrofungicide Prothioconazole. Furthermore, some compounds incorporating the linkage also demonstrated other biological activities, like antibacterial [3, 20, 21] and antiviral efficacies [22].
Compared with the 1,3,4-oxadiazole and 1,3,4-thiadiazole rings, the 1,2,4-triazole heterocycle has an unique advantage in the number of modifiable positions, not only in the 3- and 5-positions, but also in the 4-position. The introduction of substituents into 4-position of the 1,2,4-triazole ring can tune the bioactivity and physiochemical properties of the formed molecules [23]. For example, the 3,5-disubstituted-4-amino-1,2,4-triazole could be tranformed into the corresponding Schiff-base derivatives, displaying favorable antibacterial & antifungal effects [24,25,26]. Based on the above-mentioned considerations, we synthesized a series of new quinazolinone derivatives containing both 1,2,4-triazole Schiff base moiety and an isopropanol linkage (Fig. 2) using the molecular hybridization approach, and assessed their inhibitory activites in vitro against some important phytopathogenic bacteria and fungi in agriculture.
Experimental
Instruments
All the chemicals were purchased from commercial suppliers and used without further purification (unless stated otherwise). Melting points were uncorrected and determined on a XT−4 binocular microscope (Beijing Tech Instrument Co., China). 1 H and 13 C NMR spectra were recorded on a JEOL-ECX 500 NMR spectrometer in DMSO-d6 at room temperature using TMS as an internal standard (s = singlet; d = doublet; t = triplet; m = multiple), chemical shift (δ) was reported in parts per million (ppm), and coupling constants were expressed in Hertz (Hz). HRMS-ESI spectra were measured by a Thermo Scientific Q Exactive series. Scanning electron microscopy (SEM) images were visualized and obtained using a Nova NanoSEM 450. The X-ray crystallographic data were collected based on an Agilent SuperNova area detector diffractometer with Mo-Ka radiation. All the strains of bacteria and fungi were provided by the Laboratory of Plant Disease Control at Guizhou University.
Synthesis of intermediate B
To an acetone solution (80 mL) containing quinazolin-4-one A [27] (1.0 g, 6.8 mmol) and anhydrous K2CO3 (1.9 g, 13.7 mmol), epichlorohydrin (4.1 mL, 51.3 mmol) was added dropwise. Next, the above mixture was heated under reflux for 24 h. After the removal of acetone under reduced pressure, the resultant residues were dissolved in ethyl acetate, washed with water, dried over anhydrous Na2SO4, and then evaporated to afford the white intermediate B. Yield: 61%, m.p. 99 − 100 °C. 1 H NMR (500 MHz, DMSO-d6) δ: 8.24 (s, 1 H), 8.14 (d, J = 10.0 Hz, 1 H), 7.80 (t, J = 5.0 Hz, 1 H), 7.65 (d, J = 10.0 Hz, 1 H), 7.53 (t, J = 5.0 Hz, 1 H), 4.28 (d, J = 10.0 Hz, 1 H), 4.05 (d, J = 10.0 Hz, 1 H), 2.78–2.76 (m, 1 H), 2.55 (s, 1 H), 2.47 (s, 1 H). 13 C NMR (125 MHz, DMSO-d6) δ: 160.8, 148.5, 148.4, 135.0, 127.8, 127.7, 126.7, 121.9, 49.7, 47.4, 45.7. ESI-HRMS m/z: [M + H]+ calcd for C11H11N2O2: 203.0815; found: 203.0810.
General procedures for the synthesis of target compounds E1−E31
Intermediate B (0.28 g, 1.4 mmol) and anhydrous NaHCO3 (0.14 g, 1.7 mmol) were added into an ethanol solution (10 mL) and stirred for 10 min, and then the appropriate 1,2,4-triazole Schiff base D [28,29,30] (1.0 mmol) was introduced. The above reaction mixture was continuously stirred at room temperature for 12 h. Next, the mixture was poured into cold water, and the formed precipitate was filtered, washed with water and dried to generate target compounds E1 − E31.
3-(3-((4-((3-bromobenzylidene)amino)-5-methyl-4H-1,2,4-triazol-3-yl)thio)-2-hydroxypropyl)quinazolin-4(3H)-one (E12). Yield: 95%, light-yellow solid, m.p. 155–157 °C. 1 H NMR (500 MHz, DMSO-d6) δ: 8.84 (s, 1 H), 8.19 (s, 1 H), 8.12–8.08 (m, 2 H), 7.91 (d, J = 10.0 Hz, 1 H), 7.81–7.77 (m, 2 H), 7.63 (d, J = 10.0 Hz, 1 H), 7.52–7.49 (m, 2 H), 5.65 (d, J = 5.0 Hz, 1 H), 4.27–4.24 (m, 1 H), 4.08 (s, 1 H), 3.84–3.79 (m, 1 H), 3.36–3.33 (m, 1 H), 3.28–3.23 (m, 1 H), 2.44 (s, 3 H). 13 C NMR (125 MHz, DMSO-d6) δ: 163.0, 161.0, 149.6, 149.2, 148.5, 147.8, 135.9, 134.8, 131.9, 131.8, 130.9, 128.2, 127.6, 127.4, 126.6, 122.9, 122.1, 67.3, 51.1, 37.2, 11.8. ESI-HRMS m/z: [M + H]+ calcd for C21H20N6O2BrS: 499.0546; found: 499.0551.
Results and discussion
Synthesis and spectral analysis
Synthetic routes of target compounds E1−E31 were depicted in Scheme 1. In brief, quinazolinone A was firstly treated with epichlorohydrin in acetone with anhydrous K2CO3 as a catalyst to afford the oxirane-appended quinazolinone B in 61% yield. Once intermediate B in hand, it was then reacted with the appropriate 1,2,4-triazole Schiff base D in the EtOH-NaHCO3 system to give the desired compounds E1−E31 in 52−95% yields. All the target compounds were fully characterized by 1 H NMR, 13 C NMR, and HRMS. Taking compound E12 as an example (dissolved in DMSO-d6), a singlet at 8.84 ppm in its 1 H NMR spectrum was assigned to the imine CH proton signal of the Schiff base moiety. Additionally, 2-position CH signal of the quinazolinone backbone was observed at 8.19 ppm as a well-defined singlet. The resonance at δ = 5.65 ppm was attributed to the hydroxyl proton from the isopropanol linkage. Furthermore, the signal of the methyl group on the 1,2,4-triazole ring appeared at 2.44 ppm. In its 13 C NMR spectrum, four of the diagnostic aliphatic carbon signals were found at 67.3, 51.1, 37.2, and 11.8 ppm, respectively. Finally, compound E12 exhibited an intense peak at m/z = 499.0551 in its mass spectrometry, assigned to the [M + H]+ species.
Crystal structure analysis
Fortunately, single crystals of compound E12 suitable for X-ray diffraction analysis (Fig. 3) were grown by slow evaporation of its methanolic solution at room temperature. Clearly, the imine bond adopted a trans-conformation in the solid state. Crystallographic parameters for this compound: colorless crystal, C21H19BrN6O2S, Mr = 499.4, monoclinic, space group C1c1; a = 5.0265 (6) Å, b = 15.7022 (17) Å, c = 26.915 (3) Å, α = 90º, β = 90.916 (10)º, γ = 90º, V = 2124.0 (4) Å3, T = 100 K, Z = 4, Dc = 1.562 g/cm3, F (000) = 1016.0, reflections collected/independent reflections = 8308/3497, goodness of fit on F2 = 1.076, R1 = 0.0550, wR2 = 0.1002.
In vitro antibacterial activity
In vitro antibacterial effects of compounds E1 − E31 were evaluated against three bacteria Xac, Xoo, and Rs using the classical turbidimetric method [31,32,33]. Meanwhile, the commercialized bactericides Bismerthiazol (BMT) and Thiodiazole copper (TDC) were used as the positive control agents. As listed in Table 1, compounds E10, E13, E20, and E23 exhibited similar anti-Xac efficacies to BMT at 200 µg/mL, having the inhibition rates of 94.3%, 100%, 100%, and 100%, respectively. In addition, some compounds were found to possess higher antibacterial effects against Xoo, relative to BMT. For example, compounds E4, E10, and E13 demonstrated the inhibition rates exceeding 90% towards this pathogen at 200 µg/mL, much better than BMT (69.2%).
Inspired by the preliminary antibacterial results, EC50 (half-maximal effective concentration) values of some of the compounds were further measured according to the serial dilution method (namely 200, 100, 50, 25, and 12.5 µg/mL). As listed in Table 2, compounds E2, E10, E13, E20, E22, and E23 had EC50 values of 61.1, 31.9, 31.5, 47.7, 44.8, and 45.5 µg/mL towards Xac, respectively, better than control BMT (62.8 µg/mL). Moreover, compounds E3, E4, E10, E13, and E22 displayed EC50 values of 55.4, 39.5, 49.5, 53.5, and 57.4 µg/mL against Xoo (Table 3), respectively, superior to control BMT (94.5 µg/mL). Notably, compound E4 was 2.4-fold more effective than BMT in inhibiting the bacterium Xoo in terms of their EC50 values. As far as the pathogen Rs was concerned, compounds E2, E4, E7, E8, and E20 showed better inhibition effects than control TDC (217.4 µg/mL), having EC50 values of 81.5, 83.8, 91.6, 75.4, and 94.2 µg/mL (Table 4), respectively.
SEM Observation of the cell morphology
To examinethe effects of compound E4 on the morphology of Xoo cells, scanning electron microscopy (SEM) experiments were carried out. After comparison with the blank control group (Fig. 4a), the Xoo cells changed from the plump and regular appearances into the contracted and corrugated surfaces after treatment with 50 µg/mL of compound E4 (Fig. 4b). Whilst the tested concentration was further increased to 100 µg/mL, the significant cell surface deformation even cell rupture were observed (Fig. 4c). These findings showed that this compound probably exerted its anti-Xoo effects via causing the cell membrane damage.
In vitro antifungal activity
In vitro antifungal activities of compounds E1−E31 were also assessed against six phytopathogenic fungi using the mycelial growth rate method [34, 35], including Gibberella zeae, Pellicularia sasakii, Phytophthora infestans, Verticillium dahliae, Fusarium oxysporum, and Sclerotinia sclerotiorum. As summarized in Table 5, some compounds exhibited moderate fungicidal effects against certain fungi at 50 µg/mL. For instance, compounds E22 and E30 possessed the inhibition rates of 52.7% and 54.6% against G. zeae, respectively, higher than control agent Hymexazol (48.4%). Moreover, the inhibition rates of compounds E8, E22, and E30 against S. sclerotiorum were all higher than 50%. Last, compounds E22 and E30 showed an inhibitory rate greater than 55% towards the fungus P. sasakii.
Structure-activity relationships (SAR) analysis of target compounds against the pathogenic bacteria
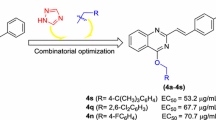
Based on the antibacterial results of target compounds listed in Tables 1, 2, 3 and 4, substitution patterns (including the type, position, and numbers of the substituents) of the terminal phenyl ring had a profound effect on their antibacterial effects. Some preliminary conclusions could be drawn as follow: (a) For the inhibition of the pathogen Xoo, the substitution at the 4-position of the phenyl group was conducive to the activity (relative to their 2-/3-position isomers), as exemplified by several best-performing compounds E4 (4-CH3, EC50 = 39.5 µg/mL), E10 (4-Cl, EC50 = 49.5 µg/mL), and E13 (4-Br, EC50 = 53.5 µg/mL). The same rules also held true in the case of inhibiting the pathogen Xac, like compounds E10 (4-Cl, EC50 = 31.9 µg/mL) and E13 (4-Br, EC50 = 31.5 µg/mL); (b) Among all the substituents, compound E4 bearing a weakly electron-donating 4-CH3 group was identified as the optimal compound for inhibiting the bacterium Xoo, being 2.4-fold more effective than control BMT; (c) Moreover, mono-substituted compounds exhibited higher antibacterial effects than di- and tri-substituted compounds. Three of the most active compounds towards the tested three bacteria (namely compounds E13, E4, and E8 from Tables 2, 3 and 4) all conformed to this rule.
Conclusions
To summarize, a class of new quinazolinone derivatives bearing the 1,2,4-triazole Schiff base moiety and an isopropanol linker were prepared and assessed for their agricultural antimicrobial activities. The bioassay results indicated that many compounds displayed good antibacterial activities in vitro against the tested phytopathogenic bacteria. In particular, compounds E4 and E10 could significantly inhibit the bacterium Xoo (with EC50 values of 39.5 and 49.5 µg/mL, respectively), far better than the commercialized Bismerthiazol (EC50 = 94.5 µg/mL). Additionally, some compounds also exhibited moderate fungicidal activities in vitro against the fungus G. Zeae, such as compounds E22 and E30. In a word, this class of compounds can be considered as the promising lead compounds for developing more effective agricultural bactericides in the future.
References
Mew TW, Alvarez AM, Leach JE, Swings J (1993) Focus on bacterial blight of rice. Plant Dis 77:5–12. https://doi.org/10.1094/PD-77-0005
Liu W, Liu J, Triplett L, Leach JE, Wang GL (2014) Novel insights into rice innate immunity against bacterial and fungal pathogens. Annu Rev Phytopathol 52:213–241. https://doi.org/10.1146/annurev-phyto-102313-045926
Huang X, Liu HW, Long ZQ, Li ZX, Zhu JJ, Wang PY, Qi PY, Liu LW, Yang S (2021) Rational optimization of 1,2,3-triazole-tailored carbazoles as prospective antibacterial alternatives with significant in vivo control efficiency and unique mode of action. J Agric Food Chem 69:4615–4627. https://doi.org/10.1021/acs.jafc.1c00707
Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA, Toth I, Salmond G, Foster GD (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13:614–629. https://doi.org/10.1111/J.1364-3703.2012.00804.X
Lu W, Pan L, Zhao H, Jia Y, Wang Y, Yu X, Wang X (2014) Molecular detection of Xanthomonas oryzae pv. oryzae, Xanthomonas oryzae pv. oryzicola, and Burkholderia glumae in infected rice seeds and leaves. Crop J 2:398–406. https://doi.org/10.1016/j.cj.2014.06.005
Gottig N, Garavaglia BS, Garofalo CG, Orellano EG, Ottado J (2009) A filamentous hemagglutinin-like protein of Xanthomonas axonopodis pv. citri, the phytopathogen responsible for citrus canker, is involved in bacterial virulence. PLoS One 4:e4358. https://doi.org/10.1371/journal.pone.0004358
Maxwell TJ, Rajasekaran P, Das S, Campos MGN, Young M, Mendis HC, Ozcan A, Gerberich KM, Myers ME, Graham JH, Johnson EG, Santra S (2019) Control of citrus canker in greenhouse and field with a zinc, urea, and peroxide ternary solution. J Agric Food Chem 67:12393–12401. https://doi.org/10.1021/acs.jafc.9b05108
Vasse J, Frey P, Trigalet A (1995) Microscopic studies of intercellular infention and protoxylem invasion of tomato roots by Pseudomonas solanacearum. Mol Plant-Microbe Interact 8:241–251. https://doi.org/10.1094/MPMI-8-0241
Buddenhagen I, Kelman A (1964) Biological and physiological aspects of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol 2:203–230. https://doi.org/10.1146/annurev.py.02.090164.001223
Sharma AK, Sharma D, Chopra AK (2020) An overview of pesticides in the development of agriculture crops. J Appl Nat Sci 12:101–109. https://doi.org/10.31018/jans.vi.2254
Zhu XF, Xu Y, Peng D, Zhang Y, Huang TT, Wang JX, Zhou MG (2013) Detection and characterization of bismerthiazol-resistance of Xanthomonas oryzae pv. oryzae. Crop Prot 47:24–29. https://doi.org/10.1016/j.cropro.2012.12.026
Garad DN, Viveki AB, Mhaske SB (2017) Pd-catalyzed regioselective mono-arylation: Quinazolinone as the inherent directing group for C(sp2)–H activation. J Org Chem 82:6366–6372. https://doi.org/10.1021/acs.joc.7b00948
Wang HX, Liu HY, Li W, Zhang S, Wu Z, Li X, Li CW, Liu YM, Chen BQ (2019) Design, synthesis, antiproliferative and antibacterial evaluation of quinazolinone derivatives. Med Chem Res 28:203–214. https://doi.org/10.1007/s00044-018-2276-8
Zhang J, Liu J, Ma Y, Ren D, Cheng P, Zhao J, Zhang F, Yao Y (2016) One-pot synthesis and antifungal activity against plant pathogens of quinazolinone derivatives containing an amide moiety. Bioorg Med Chem Lett 26:2273–2277. https://doi.org/10.1016/j.bmcl.2016.03.052
Ma J, Li P, Li X, Shi Q, Wan Z, Hu D, Jin L, Song B (2014) Synthesis and antiviral bioactivity of novel 3-((2-((1E,4E)-3-oxo-5-arylpenta-1,4-dien-1-yl) phenoxy)methyl)-4(3H)-quinazolinone. J Agri Food Chem 62:8928–8934. https://doi.org/10.1021/jf502162y
Mohamed MA, Ayyad RR, Shawer TZ, Abdel-Aziz AAM, El-Azab AS (2016) Synthesis and antitumor evaluation of trimethoxyanilides based on 4(3H)-quinazolinone scaffolds. Eur J Med Chem 112:106–113. https://doi.org/10.1016/j.ejmech.2016.02.002
Zhu S, Wang J, Chandrashekar G, Smith E, Liu X, Zhang Y (2010) Synthesis and evaluation of 4-quinazolinone compounds as potential antimalarial agents. Eur J Med Chem 45:3864–3869. https://doi.org/10.1016/j.ejmech.2010.05.040
Alagarsamy V, Solomon VR, Dhanabal K (2007) Synthesis and pharmacological evaluation of some 3-phenyl-2-substituted-3H-quinazo lin-4-one as analgesic, anti-inflammatory agent. Bioorg Med Chem 15:235–241. https://doi.org/10.1016/j.bmc.2006.09.065
Xiang M, Zhou X, Luo TR, Wang PY, Liu LW, Li Z, Wu ZB, Yang S (2019) Design, synthesis, antibacterial evaluation, and induced apoptotic behaviors of epimeric and chiral 18β-glycyrrhetinic acid ester derivatives with an isopropanolamine bridge against phytopathogens. J Agric Food Chem 67:13212–13220. https://doi.org/10.1021/acs.jafc.9b06147
Zhao YL, Huang X, Liu LW, Wang PY, Long QS, Tao QQ, Li Z, Yang S (2019) Identification of racemic and chiral carbazole derivatives containing an isopropanolamine linker as prospective surrogates against plant pathogenic bacteria: in vitro and in vivo assays and quantitative proteomics. J Agric Food Chem 67:7512–7525. https://doi.org/10.1021/acs.jafc.9b02036
Liu HW, Ji QT, Ren GG, Wang F, Su F, Wang PY, Zhou X, Wu ZB, Li Z, Yang S (2020) Antibacterial functions and proposed modes of action of novel 1,2,3,4-tetrahydro-β-carboline derivatives that possess an attractive 1,3-diaminopropan-2-ol pattern against rice bacterial blight, kiwifruit bacterial canker, and citrus bacterial canker. J Agric Food Chem 68:12558–12568. https://doi.org/10.1021/acs.jafc.0c02528
Ji J, Shao WB, Chu PL, Xiang HM, Qi PY, Zhou X, Wang PY, Yang S (2022) 1,3,4-Oxadiazole derivatives as plant activators for controlling plant viral Diseases: Preparation and assessment of the effect of auxiliaries. J Agric Food Chem 70:7929–7940. https://doi.org/10.1021/acs.jafc.2c01988
Karabanovich G, Dušek J, Savková K, Pavliš O, Pávková I, Korábečný J, et al. (2019) Development of 3,5-dinitrophenyl-containing 1,2,4-triazoles and their trifluoromethyl analogues as highly efficient antitubercular agents inhibiting decaprenylphosphoryl-β-D-ribofuranose 2’-oxidase. J Med Chem 62:8115–8139. https://doi.org/10.1021/acs.jmedchem.9b00912
Kaplancikli ZA, Turan-Zitouni G, Özdemir A, Revial G (2008) New triazole and triazolothiadiazine derivatives as possible antimicrobial agents. Eur J Med Chem 43:155–159. https://doi.org/10.1016/j.ejmech.2007.03.019
Wu S, Lu Y, Lei Z, Jiang Y, Zhang W, Qi L, Ma H, Ren Y (2019) Synthesis, biological activity and molecular docking of 4-amino-5-substituted–1,2,4-triazole-3-thione Schiff base. Chin J Org Chem 39:1939–1944. https://doi.org/10.6023/cjoc201811016
Mange YJ, Isloor AM, Malladi S, Isloor S, Fun HK (2013) Synthesis and antimicrobial activities of some novel 1,2,4-triazole derivatives. Arab J Chem 6:177–181. https://doi.org/10.1016/j.arabjc.2011.01.033
Chen JN, Wang XF, Li T, Wu DW, Fu XB, Zhang GJ, Shen XC, Wang HS (2016) Design, synthesis, and biological evaluation of novel quinazolinyl-diaryl urea derivatives as potential anticancer agents. Eur J Med Chem 107:12–25. https://doi.org/10.1016/j.ejmech.2015.10.045
Sun XH, Tao Y, Liu YF, Jia YQ, Chen B (2008) Synthesis and biological activities of 4,5-dihydro-3-methyl-4-amino-1,2,4-triazole-5-thione Schiff bases. Acta Chim Sin 66:234–238. https://doi.org/10.3321/j.issn
Liu J, Liu Y, Jian J, Bao X (2013) Synthesis and fungicidal activities of novel quinazoline derivatives containing 1,2,4-triazole Schiff-base unit. Chin J Org Chem 33:370–374. https://doi.org/10.6023/cjoc201209023
Li B, Zhang Z, Zhang JF, Liu J, Zuo XY, Chen F, Zhang GY, Fang HQ, Jin Z, Tang YZ (2021) Design, synthesis and biological evaluation of pleuromutilin-Schiff base hybrids as potent anti-MRSA agents in vitro and in vivo. Eur J Med Chem 223:113624. https://doi.org/10.1016/j.ejmech.2021.113624
Fan Z, Shi J, Luo N, Ding M, Bao X (2019) Synthesis, crystal structure, and agricultural antimicrobial evaluation of novel quinazoline thioether derivatives incorporating the 1,2,4-triazolo[4,3-a]pyridine moiety. J Agric Food Chem 67:11598–11606. https://doi.org/10.1021/acs.jafc.9b04733
Yang L, Ge S, Huang J, Bao X (2018) Synthesis of novel (E)-2-(4-(1H-1,2,4-triazol-1-yl)styryl)-4-(alkyl/arylmethyleneoxy)quinazoline derivatives as antimicrobial agents. Mol Divers 22:71–82. https://doi.org/10.1007/s11030-017-9792-1
Ding M, Wu N, Lin Q, Yan Y, Yang Y, Tian G, An L, Bao X (2022) Discovery of novel quinazoline-2-aminothiazole hybrids containing a 4–piperidinylamide linker as potential fungicides against the phytopathogenic fungus Rhizoctonia Solani. J Agric Food Chem 70:10100–10110. https://doi.org/10.1021/acs.jafc.1c07706
Ding M, Wan S, Wu N, Yan Y, Li J, Bao X (2021) Synthesis, structural characterization, and antibacterial and antifungal activities of novel 1,2,4-triazole thioether and thiazolo[3,2-b]-1,2,4-triazole derivatives bearing the 6-fluoroquinazolinyl moiety. J Agric Food Chem 69:15084–15096. https://doi.org/10.1021/acs.jafc.1c02144
Yang L, Bao XP (2017) Synthesis of novel 1,2,4-triazole derivatives containing the quinazolinylpiperidinyl moiety and N-(substituted phenyl) acetamide group as efficient bactericides against the phytopathogenic bacterium Xanthomonas oryzae pv. oryzae. RSC Adv 7:34005–34011. https://doi.org/10.1039/c7ra04819j
Acknowledgements
This work was financially supported by the Natural Science Foundation of Guizhou Province (No. 20201Z025) and the National Natural Science Foundation of China (No. 32060626).
Author information
Authors and Affiliations
Contributions
L.Y. and X.B. conceived this project. L.Y., M.D., J.S., N.L., Y.W. and D.L. conducted the related experiments. L.Y. drafted this manuscript, and X.B. revised it.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, L., Ding, M., Shi, J. et al. Design, synthesis, X-ray crystal structure, and antimicrobial evaluation of novel quinazolinone derivatives containing the 1,2,4-triazole Schiff base moiety and an isopropanol linker. Mol Divers (2023). https://doi.org/10.1007/s11030-023-10749-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-023-10749-w