Abstract
Neuroinflammation contributes to the pathogenesis of depression. Inulin-type oligosaccharides of Morinda officinalis (IOMO) exert antidepressant-like effects in rodents and patients with depression, while the underlying mechanisms remain unclear. This study used chronic restraint stress (CRS) and lipopolysaccharide (LPS) to induce depression-like behaviors in mice. Western blotting and ELISA analysis were used to investigate the effects of IOMO on inflammatory cytokine levels. Immunofluorescence analysis was used to investigate the effects of IOMO on hippocampal NLRP3 inflammasome and microglial cells. The results suggested that 6 weeks of CRS induced significant depression-like behaviors based on the sucrose preference test (SPT), tail suspension test (TST), and forced swimming test (FST), which were accompanied by increases in the expression of IL-6 and the activation of hippocampal microglial cells. Chronic treatment with IOMO (25 mg/kg, i.g.) for 28 days significantly reversed these depression-like behaviors and inhibited the activation of microglial cells. Furthermore, LPS (0.5 mg/kg, i.p.) also significantly induced depression-like behaviors in the TST, FST, and novelty-suppressed feeding test (NSFT), as well as increased the expression of IL-1β and caspase-1, and activated the microglial cells and the NLRP3 inflammasome in the hippocampus. Treatment with IOMO for 9 days significantly reversed these depression-like behaviors and normalized the LPS-induced activation of the microglial cells and NLRP3 inflammasome. Taken together, these results suggested that IOMO exerted antidepressant-like effects via hippocampal microglial NLRP3 inflammasome mediation followed by caspase-1 inhibition and the production of IL-1β. These findings provide a basis for developing new antidepressants targeting the microglial NLRP3 inflammasome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is a prevalent and debilitating psychiatric disease affecting approximately 350 million people worldwide and causing a huge socioeconomic burden (Malhi & Mann, 2018). However, current existing first-line antidepressants, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs), require weeks to months to produce a therapeutic response and have a low effective rate (50–70%). Therefore, there is an urgent need to develop effective antidepressants with few adverse effects.
Recently, accumulating evidence has shown that Traditional Chinese Medicine (TCM) is a prospective alternative for treating depression with better compliance and lower side effects. Inulin-type oligosaccharides of Morinda officinalis (IOMO, as illustrated in Fig. 1), extracted from M. officinalis roots and mainly consisting of seven oligosaccharides (Hao et al. 2020), were approved as a prescribed traditional herbal medicine for mild and moderate depressive episodes in 2012 by the Chinese Food and Drug Administration (CFDA) (Du et al. 2021). Furthermore, preclinical studies suggested that IOMO exerted significant antidepressant-like effects in rodent models (Chi et al. 2020; Zhu et al. 2020). Although IOMO has been used as a safe and effective form of TCM for depression treatment in clinical trials, the molecular mechanism underlying its antidepressant effects remains unclear.
Accumulating evidence suggests that neuroinflammation is a critical component underlying the pathogenesis of depression (Beurel et al. 2020; Troubat et al. 2021; Won et al. 2021). Dysregulation of both the innate and adaptive immune systems occurs in depressed patients and hinders favorable prognosis, including antidepressant responses (Beurel et al. 2020). In addition, preclinical studies suggested that pro-inflammatory factors, such as lipopolysaccharide (LPS), could induce depression-like behaviors in rodents (Walker et al. 2019; Zhao et al. 2019), and chronic treatment with SSRIs, fluoxetine, or paroxetine, could alleviate inflammatory responses and depression-like behaviors induced by LPS (Li et al. 2021a, b; Zhang et al. 2021). A previous study suggested that M. officinalis extracts affected colitis by regulating inflammation and cell death (Liang et al. 2017), which provided some hints for the role of neuroinflammation in the antidepressant effects of IOMO.
The hippocampus is relatively vulnerable to acute inflammatory challenges in depression (Zhao et al. 2019). Depression-like behaviors induced by chronic stress are accompanied by the release of inflammatory cytokines in the hippocampus (Yang et al. 2021; Zhao et al. 2019). Microglial cells, which are the primary resident immune cells of the central nervous system and play an important role in the first immune defense line in the brain, greatly contribute to the development and progression of depression (Jia et al. 2021; Li et al. 2022a, b; Wang et al. 2022). Importantly, microglial cells release proinflammatory cytokines and their metabolic products in response to stress-triggered neuroinflammation and regulate depression-like behaviors by modulating the function of neurons and astrocytes.
Nucleotide-binding domain leucine-rich repeat family pyrin domain containing 3 (NLRP3) inflammasome is a multimeric protein complex that triggers innate immune responses regulating the cleavage of interleukin-1β (IL-1β) and IL-18 precursors (Swanson et al. 2019), which are important proinflammatory cytokines that play a role in neuroinflammation. Several studies have shown that the NLRP3 inflammasome is involved in stress-induced depression by regulating the production of IL-1β protein, and inhibition of the NLRP3 inflammasome could significantly ameliorate depression-like behaviors (Kouba et al. 2022; Tang et al. 2021). Clinical studies have shown high serum levels of proinflammatory biomarkers in patients with depression (Osimo et al. 2020; Zou et al. 2018), while preclinical studies have revealed significant increases in the levels of IL-1β, NLRP3 inflammasome, and caspase-1 in the hippocampus (Fang et al. 2022).
Therefore, we hypothesized that a neuroinflammation mechanism might underly the antidepressant effects of IOMO. Thus, we first evaluated the antidepressant-like effects of IOMO in chronic restraint stress (CRS)- and LPS-induced mice models of depression. Moreover, we investigated the role of hippocampal neuroinflammation, such as the release of inflammatory cytokines, the expression of the NLRP3 inflammasome, and the activation of microglial cells, in the antidepressant-like effects of IOMO.
Materials and methods
Animals
Male C57BL/6J mice weighing 25–30 g were purchased from Beijing SPF Laboratory Animal Technology Company (Beijing, China). Animals were housed at a constant room temperature (23 ± 1 °C) and humidity (40–60%) and were group housed in 5 mice per cage during experiments (except for the sucrose preference test). Food and water were available ad libitum. Animals were randomly divided into five groups in the CRS experiment and three groups in the LPS experiment. The experiments were performed in compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH publication No. 86–23, revised 2011). All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Beijing Institute of Pharmacology and Toxicology (animal ethics protocol number: IACUC-DWZX-2021-757). The sample size was determined according to a calculator (http://powerandsamplesize.com/) and reported methods (Festing 2018). All efforts were made to minimize animal suffering and reduce the number of animals used for experiments.
Drug and dose
IOMO was purchased from Beijing Tong Ren Tang Ltd. Co. (Beijing, China), and LPS was purchased from Sigma-Aldrich (L2880; St Louis, MO, USA). IOMO and LPS were dissolved in saline and administered intragastrically (i.g.) or intraperitoneally (i.p.) at a volume of 10 mL/kg.
Chronic restraint stress (CRS) procedure
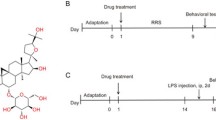
Mice were restrained in a 50-mL centrifuge tube (diameter: 3 cm, length: 10 cm) with some small holes in the periphery for 6 h per day (09:00–15:00) for 42 consecutive days. The mice could freely breathe in the tube, but could not move forward or backward. Each mouse was successively assessed using the sucrose preference test (SPT), open field test (OFT), tail suspension test (TST), and forced swim test (FST). Except for the control group, post-CRS mice were randomly divided into CRS-vehicle and CRS-IOMO groups, which received saline and IOMO (12.5, 25, or 50 mg/kg), respectively. Drugs (including saline) were administered orally once a day at 8:00–9:00 a.m. from 21 days after the CRS regime. An outline of the treatment schedule design and behavioral tests is shown in Fig. 2A.
Sucrose preference test (SPT)
The SPT was performed as previously described (Liu et al. 2018a, b). Briefly, mice were housed individually in a cage with free access to bottles containing a 1% sucrose solution or water for 2 days before testing. After adaptation, the mice were deprived of water and food for 24 h and then placed in the cage with two bottles over a 2 h period. Sucrose preference (%) = [sucrose consumption/(sucrose consumption + water consumption)] ⋅ 100.
Open field test (OFT)
The OFT was performed as previously described (Kraeuter et al. 2019). Mice were placed in the corner of a plastic box (40 × 40 × 20 cm) and their spontaneous locomotor activity was videotaped for 5 min and analyzed using the SMART Video Tracking System V3.0 (Beijing Zhong Shi Di Chuang Technology Development Co., Ltd. labmazev3.0). After each test, the box was cleaned with a 75% ethanol solution to remove odor, urine, or feces.
Forced swimming test (FST)
The FST was performed as previously described with minor modifications (Dang et al. 2022). Briefly, mice were individually forced to swim in an open cylindrical container (height: 25 cm, diameter: 10 cm) containing 18 cm of water maintained at 24 ± 1 °C for 6 min. The immobility duration in the last 4 min of the total 6-min test was recorded. Mice were considered immobile when they ceased struggling and remained floating motionless in the water, making only those movements necessary to keep their head above water.
Tail suspension test (TST)
The TST was performed according to a previously described method (Liu et al. 2022). Briefly, mice were suspended at the top of the apparatus by adhesive tape placed approximately 1 cm from the tip of the tail. The duration of immobility in the last 4 min of a total 6 min suspension was recorded. Immobility was defined as an absence of any limb or body movements, except those caused by respiration. After each test, the box was cleaned with 75% ethanol solution to remove odor, urine, or feces.
LPS-induced depression-like behaviors
LPS was used to induce depression-like behaviors in mice as previously reported (Li et al. 2021a, b; Su et al. 2016; Sun et al. 2021; Zhao et al. 2019) with minor modifications. Briefly, mice received two injections of LPS (0.5 mg/kg, i.p.) on the first and seventh day (08:00–09:00 a.m.), whereas control mice received a vehicle at the same time points. Mice exposed to LPS were randomly divided into LPS-vehicle and LPS-IOMO groups, which received saline and IOMO (25 mg/kg, i.g.), respectively. Drugs (including saline) were administered orally once a day at 8:00–9:00 a.m. from day 1. Each mouse was successively tested using the OFT, TST, FST, and novelty-suppressed feeding test (NSFT).
Novelty-suppressed feeding test (NSFT)
Each mouse was placed in one corner of an open plastic box (40 × 40 × 30 cm) with five food pellets placed in the center after 24 h of food deprivation (Chen et al. 2022) The exploratory activity of each mouse in the plastic box was observed for 5 min, and the latency to feed was recorded. Eating behaviors were defined as chewing and biting. New food pellets were placed in the center after each test.
Enzyme-linked immunosorbent assay (ELISA)
Mice were sacrificed after behavioral testing and the hippocampal tissues were rapidly removed and stored at − 80 °C until assayed. The samples were centrifuged at 10,000 × g for 15 min at 4 °C and the supernatant was added to 96-well microplates. The levels of IL-6, IL-1β, IL-4, and IL-10 were then determined using an ELISA kit according to the manufacturer’s instructions (USCN Life Sciences & Technology Co., Ltd., Wuhan, China). Absorbance was determined using a microplate spectrophotometer at 450 nm (Spectra Max i3x Molecular Devices, Silicon Valley, CA, USA) and sample concentration was calculated using a standard curve.
Western blotting (WB)
After behavioral testing, mice were sacrificed for WB, and hippocampal tissue was extracted by RIPA buffer (Beyotime Biotechnology, Beijing, China) with protease inhibitors and a phosphatase inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA, USA). Samples containing 40 µg of protein were separated using SDS-PAGE before transfer to PVDF membranes, which were then incubated with anti-INOS (1:1000, D6B6S Rabbit; Cell Signaling Technology, Danvers, MA, USA), anti-NLRP3 (1:500, DF7438 Rabbit; Affinity Biologicals, Ancaster, Canada), anti-caspase-1 (1:1000, Rabbit; Proteintech, Rosemont, USA), anti-IL-1β (1:500, Rabbit; ABMART, Shanghai, China), and anti-β-actin (1:1000, Mouse; CWBIO, Beijing, China). Next, the membranes were washed in TBST for three times and incubated for 2 h at room temperature with fluorescent secondary antibodies (1:5000, IRDye800CW Goat anti-Rabbit IgG, IRDye680RD, Goat anti-Mouse IgG, LI-COR Biosciences, Lincoln, NE, USA). Quantification analyses for images were performed using ImageJ software (v 1.45 J; National Institutes of Health, Bethesda, MD, USA). The relative level of each protein was expressed as the density ratio of the target protein band to the β-actin band, and all the protein expression values were normalized to the values obtained in the vehicle groups.
Immunofluorescence staining
After behavioral testing, mice were anesthetized with urethane (1 g/kg, i.p.) and transcardially perfused with phosphate buffered saline (PBS), followed by 4% paraformaldehyde. The brains were then cut into 20-µm sections and thoroughly washed with PBS for 5 min, blocked with 10% goat serum for 2 h, and incubated overnight at 4 °C with primary antibody anti-NLRP3 (Rabbit pAb, 1:200, GB114320, Wuhan Servicebio Technology Co., Ltd., Wuhan, China) or Iba-1 (Rabbit, 1:200, ab178846; Abcam, Cambridge, UK). Secondary antibodies included anti-Rabbit Alexa Flour 594 (# 8889 S; 1: 1000; Cell Signaling Technology), and Alexa Fluor 488 goat anti-mouse IgG (#4408; 1: 1000; Cell Signaling Technology). Coverslips were mounted on glass slides with fluoroshield containing DAPI (F6057-20 mL, Sigma-Aldrich) and incubated at room temperature overnight. Images were acquired at a size of 560 × 900 μm with a 20× NA 0.5 dry objective lens on a fluorescence microscope (Olympus IX73PIF; Olympus Corporation, Tokyo Japan) equipped with a microscope digital camera (Olympus DP74; Olympus Corporation). For analysis, at least 20 images of the hippocampus were used for each group of animals, and at least 4 images from one slice of the brain. The number and staining area of the Iba-1 + cells were determined using ImageJ software (v 1.45 J; NIH, USA), and the number was expressed as the number of Iba-1 + cells per mm2 in a 20-µm thick section. All measurements were performed by an operator blinded to the sections’ identity.
Statistical analysis
All data were analyzed by an observer blinded to the experimental protocol. The normality of data was assessed by the Shapiro-Wilk test and Kolmogorov-Smirnov test, and the variance homogeneity of data was assessed by the F test. Parametric data were presented as mean ± standard deviation (S.D.), and nonparametric data were presented as median and interquartile range. All data were analyzed using GraphPad Prism 8.0 (GraphPad, San Diego, CA, USA). For nonparametric tests, statistical differences between two groups were analyzed using a two-tailed Student’s t-test, and data containing more than two groups were tested using one-way ANOVA followed by Dunnett’s test. For nonparametric tests, statistical differences between two groups were analyzed using the Mann-Whitney U test, and data containing more than two groups were tested using the Kruskal-Wallis H test followed by the Nemenyi test. For all tests, differences were considered statistically significant at a level of p < 0.05.
Results
IOMO reversed the depression-like behaviors induced by CRS
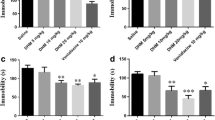
To evaluate the antidepressant-like behavioral effects of IOMO, we first investigated the effects of IOMO on mice exposed to 6 weeks of CRS. CRS significantly decreased the sucrose preference in the SPT (t(18) = 2.288, p = 0.0344 < 0.05; Fig. 2B), decreased the entries into the central zone in the OFT (Mann Whitney U test, U = 14, p = 0.0047 < 0.01), and increased the immobility durations in the TST (Mann Whitney U test, U = 10.5, p = 0.0016 < 0.01) and FST (Mann Whitney U test, U = 14.5, p = 0.0056 < 0.01). These results indicated that 6 weeks of CRS induced significant depression-like behaviors in the mice. Furthermore, IOMO significantly reversed the depression-like behaviors caused by CRS. Specifically, treatment with IOMO for 28 days significantly increased the sucrose preference in the SPT (one-way ANOVA followed by Dunnett’s tests; F(3, 36) = 3.922, p = 0.0161< 0.05; 12.5 vs. CRS, p = 0.9656; 25 vs. CRS, p = 0.0095 < 0.01; 50 vs. CRS, p = 0.6511), and significantly decreased the immobility duration in the TST (Kruskal-Wallis H test, H = 13.14, p = 0.0043 < 0.01; 12.5 vs. CRS, p = 0.0311 < 0.05; 25 vs. CRS, p = 0.0024 < 0.01;50 vs. CRS, p = 0.6516) and in the FST (Kruskal-Wallis H test, H = 10.08, p = 0.0179 < 0.05; 12.5 vs. CRS, p = 0.2349; 25 vs. CRS, p = 0.0077 < 0.01; 50 vs. CRS, p > 0.9999) compared with CRS mice. These results indicated that chronic treatment with IOMO could reverse the depression-like behaviors induced by CRS.
Treatment with IOMO (28 days) reversed the depression-like behaviors induced by CRS. (A) Schematic drawing of the experimental timeline. (B) Effects of IOMO on sucrose preference in the SPT. (C, D) Total distance and entries in the central zone in the OFT, and (E, F) immobility durations in the TST and FST. *p < 0.05 and **p < 0.01, compared with control mice; #p < 0.05 and ##p < 0.01, compared with CRS mice, n = 10 mice/group
IOMO alleviated hippocampal inflammatory cytokines induced by CRS
To investigate the mechanism underlying the antidepressant-like effects of IOMO, we assessed the effects of IOMO on hippocampal inflammatory cytokines and microglial cells. CRS significantly increased the expression of IL-6 in the hippocampus, and treatment with IOMO (25 mg/kg, i.g.) for 28 days significantly reduced the expression of IL-6 (F(2, 12) = 5.495, p = 0.0202 < 0.05; Control vs. CRS, p = 0.0197 < 0.05; IOMO + CRS vs. CRS, p = 0.0351 < 0.05; Fig. 3B), indicating that IOMO decreased the expression of hippocampal pro-inflammatory factors. However, IOMO exerted no effects on IL-1β (Kruskal-Wallis H test, H = 2.340, p = 0.3304), IL-4 (F(2, 12) = 4.363, p = 0.0377 < 0.05; Control vs. CRS, p = 0.0295 < 0.05; IOMO + CRS vs. CRS, p = 0.7947), and IL-10 (F(2,12) = 1.929, p = 0.1877). Notably, CRS significantly increased the number of hippocampal microglial cells compared with that of control mice, and IOMO significantly inhibited the activation of microglial cells (F(2, 10) = 9.355, p = 0.0051 < 0.01; CRS vs. Control, p = 0.0071 < 0.01; IOMO + CRS vs. CRS, p = 0.0091 < 0.01). These results indicated that IOMO could normalize the activity of hippocampal microglial cells.
Treatment with IOMO (25 mg/kg, i.g.) for 28 days alleviated hippocampal inflammatory cytokines and microglial cells induced by CRS. (A-D) Effects of IOMO on the expression of IL-1β, IL-6, IL-4, and IL-10 in the hippocampus. (E) Representative immunofluorescence images showing Iba-1 in the hippocampus of Control, CRS, and CRS + IOMO treated mice. (F) Quantification of the effect of IOMO on the number of hippocampal microglial cells. *p < 0.05 and **p < 0.01, compared with control mice; #p < 0.05 and ##p < 0.01, compared with CRS mice; n = 4–5 mice/group
IOMO reversed the depression-like behaviors induced by LPS
We further evaluated the antidepressant-like effects of IOMO in an LPS mouse model. An outline of the treatment schedule design and behavioral tests is illustrated in Fig. 4A. The results suggested that treatment with IOMO significantly reversed depression-like behaviors induced by LPS. In the OFT, LPS exerted no effect on total distance (F(2, 21) = 0.7230, p = 0.4970; LPS vs. Control, p = 0.9245; IOMO + LPS vs. LPS, p = 0.4136) and entries in the central zone (Kruskal-Wallis H test, H = 4.775, p = 0.0919; LPS vs. Control, p = 0.1823; IOMO + LPS vs. LPS, p = 0.0817). Notably, LPS significantly increased the immobility durations in the TST and FST, and increased the latency to feed in the NSFT, whereas treatment with IOMO (25 mg/kg, i.g.) for 9 days significantly reduced the immobility durations in the TST (F(2, 21) = 4.450, p = 0.0245 < 0.05; LPS vs. Control, p = 0.0482 < 0.05; IOMO + LPS vs. LPS, p = 0.0227 < 0.05) and in the FST (Kruskal-Wallis H test, H = 14.51, p = 0.0007 < 0.001; LPS vs. Control, p = 0.0033 < 0.01; IOMO + LPS vs. LPS, p = 0.0012 < 0.01), and reduced the latency to feed in the NSFT (F(2, 21) = 12.21, p = 0.0003 < 0.001; LPS vs. Control, p = 0.0001 < 0.001; IOMO + LPS vs. LPS, p = 0.0385 < 0.05). These results indicated that IOMO exhibited significant antidepressant-like effects in the LPS model.
Treatment with IOMO for 9 days reversed the depression-like behaviors induced by LPS. (A) Schematic drawing of the experimental timeline. (B, C) Effects of IOMO on total distance and entries in the central zone in the OFT, (D, E) immobility durations in the TST and FST, and (F) the latency to feed in the NFST. *p < 0.05, **p < 0.01, and ***p < 0.001, compared with control mice; #p < 0.05 and ##p < 0.01, compared with LPS mice, n = 8 mice/group
IOMO exerted antidepressant-like effects through microglial cells and NLRP3 inflammasome
To investigate the mechanism of the antidepressant-like effects of IOMO (25 mg/kg, i.g.) in the LPS model, we performed an immunofluorescence assay on hippocampal microglial cells and the NLRP3 inflammasome (Fig. 5). LPS (0.5 mg/kg, i.p.) significantly increased the number of hippocampal microglial cells, whereas IOMO significantly decreased them (one-way ANOVA followed by Dunnett’s tests; F(2, 10) = 17.46, p = 0.0005 < 0.001; LPS vs. Control, p = 0.0004 < 0.001; IOMO + LPS vs. LPS, p = 0.0082 < 0.01). In addition, IOMO significantly inhibited the expression of the NLRP3 inflammasome (Kruskal-Wallis H test, H = 9.099, p = 0.0012 < 0.01; LPS vs. Control, p = 0.0082 < 0.01; IOMO + LPS vs. LPS, p = 0.0705). These findings indicated that microglial cells and the NLRP3 inflammasome might be implicated in the mechanism of antidepressant-like effects of IOMO.
Effects of IOMO (25 mg/kg, i.g.) on hippocampal microglial cells and the NLRP3 inflammasome. (A, B) Representative immunofluorescence images showing Iba-1 and the NLRP3 inflammasome in the hippocampus, respectively. (C, D) Quantitative analysis of the effects of treatment with IOMO on the number of microglial cells and the NLRP3 inflammasome in the hippocampus. **p < 0.01 and ***p < 0.001, compared with control mice; ##p < 0.01, compared with LPS mice. n = 4–5 mice/group
IOMO reduced hippocampal inflammatory-related factors induced by LPS
To further investigate the mechanism underlying the antidepressant-like effects of IOMO, we performed western blotting to quantitate the expression levels of hippocampal inflammatory-related cytokines (Fig. 6). Notably, we found that LPS treatment led to significant increases in the expression of inflammation-related factors in the hippocampus, and treatment with IOMO (25 mg/kg, i.g.) for 9 days significantly decreased the expression of INOS (one-way ANOVA followed by Dunnett’s tests; F(2, 15) = 7.138, p = 0.0066 < 0.01; LPS vs. Control, p = 0.0307 < 0.05; IOMO + LPS vs. LPS, p = 0.0050 < 0.01), caspase-1 (F(2, 15) = 6.515, p = 0.0092 < 0.01; LPS vs. Control, p = 0.0248 < 0.05; LPS vs. IOMO + LPS, p = 0.0078 < 0.01), and IL-β (F(2, 15) = 4.632, p = 0.0271 < 0.05; LPS vs. Control, p = 0.0475 < 0.05; LPS vs. IOMO + LPS, p = 0.0259 < 0.05), induced by LPS. In addition, treatment with IOMO slightly decreased the expression of NLRP3 (Kruskal-Wallis H test, H = 5.930, p = 0.0468 < 0.05; LPS vs. Control, p = 0.0699; LPS vs. IOMO + LPS, p = 0.0699). These results indicated that inflammation-related factors in the hippocampus might be implicated in the mechanism of the antidepressant-like effects of IOMO.
Treatment with IOMO (25 mg/kg, i.g.) for 9 days reduced expression of hippocampal inflammatory-related factors induced by LPS. (A) Western blotting analysis of the expression of INOS, NLRP3, caspase-1, and IL-1β in the hippocampus. (B-E) Quantitative analysis of the effects of IOMO on the expression of INOS, NLRP3, caspase-1, and IL-1β in the hippocampus. *p < 0.05, compared with control mice; #p < 0.05 and ##p < 0.01, compared with LPS mice. n = 6 mice/group
Discussion
In the present study, we confirmed that IOMO exerts significant antidepressant-like effects on CRS- and LPS-induced depression models and we preliminarily uncovered an important role of IL-6 and microglial cells in the antidepressant-like effects of IOMO in CRS mice. Moreover, we revealed that IOMO decreased the expression of the NLRP3 inflammasome, caspase-1, and IL-1β, and inhibited the activation of microglial cells induced by LPS. Collectively, these results indicate that the antidepressant-like action of IOMO may be mediated by the regulation of hippocampal inflammation, which includes inflammatory cytokines, the NLRP3 inflammasome, and microglial activity.
CRS can induce depression-like behaviors, which are accompanied by increases in inflammatory cytokines, such as IL-1β and IL-6 (Yang et al. 2021). Given that CRS is a classical depression model, the present study used this model to evaluate the antidepressant effects of IOMO. Our results showed that 6 weeks of CRS caused significant depression-like behaviors in mice; for example, CRS decreased sucrose preference in the SPT and increased immobility durations in the TST and FST. However, chronic treatment with IOMO significantly reversed these depression-like behaviors induced by CRS, revealing that IOMO exerted significant antidepressant-like behavioral effects on CRS mice. Notably, treatment with IOMO reversed the increase in hippocampal IL-6 induced by CRS, indicating that hippocampal IL-6 might play an important role in the mechanism of the antidepressant effects of IOMO.
Chronic stress leads to the activation of microglia in the hippocampus (Du Preez et al. 2021; Duan et al. 2022). Microglial activation is a key mediator of neuroinflammatory processes (Jia et al. 2021), promoting the release of inflammatory factors in the hippocampus, leading to the disruption of neuroplasticity and cognitive impairment, and contributing to the development of depression (Ruilian et al. 2021; Zhong et al. 2019). Consistent with these studies, the immunofluorescence analysis in the present study showed that CRS induced the activation of microglial cells in the hippocampus, which was accompanied by depression-like behaviors and the release of IL-6. Treatment with IOMO reversed the activation of microglial cells, indicating that microglial cells in the hippocampus might contribute to the mechanism of the antidepressant-like effects of IOMO.
LPS-induced depression is a commonly used animal model of inflammation-induced depression, which causes several depression-like behaviors in rodents (Walker et al. 2019; Zhao et al. 2019). LPS increased the expression of pro-inflammatory cytokine (IL-1β, IL-6) levels (Han et al. 2021; Zhang et al. 2019), and the inhibition of hippocampal inflammation could alleviate LPS-induced depression-like behaviors in mice (Bian et al. 2020; Liu et al. 2018a, b). Consistent with these results, our study also suggested that LPS induced depression-like behaviors. For example, LPS increased immobility durations in the TST and FST, and increased latency to feed in the NSFT. In addition, depression-like behaviors induced by LPS were accompanied by changes in hippocampal inflammation. The subsequent western blotting results suggested that LPS caused significant increases in caspase-1 and IL-1β levels in the hippocampus. Notably, treatment with IOMO for 9 days could reverse these inflammatory responses in the hippocampus and improve depression-like behaviors induced by LPS. Furthermore, LPS has been reported to activate hippocampal microglial cells (Zhao et al. 2019). Consistently, the immunofluorescence analysis suggested that LPS activated the microglial cells and IOMO reversed the activation of hippocampal microglial cells, indicating the important role of microglial cells in the antidepressant-like effects of IOMO.
The NLRP3 inflammasome is mainly located in microglial cells in the hippocampus. It is a key contributor to neuroinflammation and can activate caspase-1 to produce inflammatory factors, such as IL-1β (Dong et al. 2020). Suppression of the NLRP3 inflammasome improved depression-like behaviors in a mouse model of depression (Chai et al. 2022; Xia et al. 2023). Moreover, a recent study suggested that the microglial NLRP3 inflammasome might mediate the molecular mechanism of the development of depression (Li et al. 2022a, b). Consistent with these studies, our results suggested a vital role of the NLRP3 inflammasome in the antidepressant-like effects of IOMO. The immunofluorescence analysis showed that LPS increased the number of microglial NLRP3 inflammasomes, which were normalized by IOMO. Therefore, IOMO induced the secretion of inflammatory factors (IL-1β) by activating the hippocampal microglial NLRP3 inflammasome and caspase-1 to exert antidepressant-like effects. However, considering the statistical validity of the sample size, further studies are needed to investigate the mechanism of the antidepressant-like effects of IOMO with larger sample sizes.
In conclusion, the present study demonstrated that IOMO might exert antidepressant-like effects by inhibiting hippocampal inflammation, providing new insights into the mechanisms underlying the anti-inflammatory effects of IOMO. Importantly, the present study provides new evidence supporting the development of antidepressants that target the microglial NLRP3 inflammasome.
Data Availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Beurel E, Toups M, Nemeroff CB (2020) The bidirectional relationship of depression and inflammation: double trouble. Neuron 107(2):234–256. https://doi.org/10.1016/j.neuron.2020.06.002
Bian H, Wang G, Huang J, Liang L, Zheng Y, Wei Y, Wang H, Xiao L, Wang H (2020) Dihydrolipoic acid protects against lipopolysaccharide-induced behavioral deficits and neuroinflammation via regulation of Nrf2/HO-1/NLRP3 signaling in rat. J Neuroinflammation 17(1):166. https://doi.org/10.1186/s12974-020-01836-y
Chai Y, Cai Y, Fu Y, Wang Y, Zhang Y, Zhang X, Zhu L, Miao M, Yan T (2022) Salidroside ameliorates Depression by suppressing NLRP3-Mediated pyroptosis via P2X7/NF-κB/NLRP3 signaling pathway. Front Pharmacol 13:812362. https://doi.org/10.3389/fphar.2022.812362
Chen Y, Xiong W, Zhang Y, Bai X, Cheng G, Zhang Y, Chen R, Guo Y, Kong H, Zhang Y, Qu H, Zhao Y (2022) Carbon Dots Derived from Os Draconis and their anxiolytic effect. Int J Nanomedicine 17:4975–4988. https://doi.org/10.2147/ijn.S382112
Chi L, Khan I, Lin Z, Zhang J, Lee MYS, Leong W, Hsiao WLW, Zheng Y (2020) Fructo-oligosaccharides from Morinda officinalis remodeled gut microbiota and alleviated depression features in a stress rat model. Phytomedicine 67:153157. https://doi.org/10.1016/j.phymed.2019.153157
Dang R, Wang M, Li X, Wang H, Liu L, Wu Q, Zhao J, Ji P, Zhong L, Licinio J, Xie P (2022) Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J Neuroinflammation 19(1):41. https://doi.org/10.1186/s12974-022-02400-6
Dong Y, Li S, Lu Y, Li X, Liao Y, Peng Z, Li Y, Hou L, Yuan Z, Cheng J (2020) Stress-induced NLRP3 inflammasome activation negatively regulates fear memory in mice. J Neuroinflammation 17(1):205. https://doi.org/10.1186/s12974-020-01842-0
Du Y, Zheng Q, Ou ZH, Cao YJ, Su XP, Li C, Qu M (2021) Efficacy and safety of Morinda officinalis oligosaccharide capsules for depressive disorder: a systematic review and meta-analysis. Braz J Psychiatry 43(3):306–313. https://doi.org/10.1590/1516-4446-2020-0945
Du Preez A, Onorato D, Eiben I, Musaelyan K, Egeland M, Zunszain PA, Fernandes C, Thuret S, Pariante CM (2021) Chronic stress followed by social isolation promotes depressive-like behaviour, alters microglial and astrocyte biology and reduces hippocampal neurogenesis in male mice. Brain Behav Immun 91:24–47. https://doi.org/10.1016/j.bbi.2020.07.015
Duan N, Zhang Y, Tan S, Sun J, Ye M, Gao H, Pu K, Wu M, Wang Q, Zhai Q (2022) Therapeutic targeting of STING-TBK1-IRF3 signalling ameliorates chronic stress induced depression-like behaviours by modulating neuroinflammation and microglia phagocytosis. Neurobiol Dis 169:105739. https://doi.org/10.1016/j.nbd.2022.105739
Fang Y, Guo H, Wang Q, Liu C, Ge S, Yan B (2022) The role and mechanism of NLRP3 inflammasome-mediated astrocyte activation in dehydrocorydaline against CUMS-induced depression. Front Pharmacol 13:1008249. https://doi.org/10.3389/fphar.2022.1008249
Festing MF (2018) On determining sample size in experiments involving laboratory animals. Lab Anim 52(4):341–350. https://doi.org/10.1177/0023677217738268
Han X, Xu T, Fang Q, Zhang H, Yue L, Hu G, Sun L (2021) Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol 44:102010. https://doi.org/10.1016/j.redox.2021.102010
Hao Q, Zhou J, Zhou L, Kang L, Nan T, Yu Y, Guo L (2020) Prediction the contents of fructose, glucose, sucrose, fructo-oligosaccharides and iridoid glycosides in Morinda officinalis radix using near-infrared spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc 234:118275. https://doi.org/10.1016/j.saa.2020.118275
Jia X, Gao Z, Hu H (2021) Microglia in depression: current perspectives. Sci China Life Sci 64(6):911–925. https://doi.org/10.1007/s11427-020-1815-6
Kouba BR, Gil-Mohapel J, AL SR (2022) NLRP3 inflammasome: from pathophysiology to therapeutic target in major depressive disorder. Int J Mol Sci 24(1). https://doi.org/10.3390/ijms24010133
Kraeuter AK, Guest PC, Sarnyai Z (2019) The Open Field Test for measuring locomotor activity and anxiety-like Behavior. Methods Mol Biol 1916:99–103. https://doi.org/10.1007/978-1-4939-8994-2_9
Li W, Ali T, He K, Liu Z, Shah FA, Ren Q, Liu Y, Jiang A, Li S (2021a) Ibrutinib alleviates LPS-induced neuroinflammation and synaptic defects in a mouse model of depression. Brain Behav Immun 92:10–24. 10.1016/j.bbi.2020.11.008
Li W, Ali T, Zheng C, Liu Z, He K, Shah FA, Ren Q, Rahman SU, Li N, Yu ZJ, Li S (2021b) Fluoxetine regulates eEF2 activity (phosphorylation) via HDAC1 inhibitory mechanism in an LPS-induced mouse model of depression. J Neuroinflammation 18(1):38. 10.1186/s12974-021-02091-5
Li B, Yang W, Ge T, Wang Y, Cui R (2022a) Stress induced microglial activation contributes to depression. Pharmacol Res 179:106145. 10.1016/j.phrs.2022a.106145
Li S, Fang Y, Zhang Y, Song M, Zhang X, Ding X, Yao H, Chen M, Sun Y, Ding J, Wang Q, Lu M, Wu G, Hu G (2022b) Microglial NLRP3 inflammasome activates neurotoxic astrocytes in depression-like mice. Cell Rep 41(4):111532. 10.1016/j.celrep.2022b.111532
Liang J, Liang J, Hao H, Lin H, Wang P, Wu Y, Jiang X, Fu C, Li Q, Ding P, Liu H, Xiong Q, Lai X, Zhou L, Chan S, Hou S (2017) The extracts of Morinda officinalis and its hairy roots attenuate Dextran Sodium Sulfate-Induced Chronic Ulcerative Colitis in mice by regulating inflammation and lymphocyte apoptosis. Front Immunol 8:905. https://doi.org/10.3389/fimmu.2017.00905
Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, Chen H, Zhu DY, Zhou QG (2018a) Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc 13(7):1686–1698. 10.1038/s41596-018-0011-z
Liu Y, Zhang Y, Zheng X, Fang T, Yang X, Luo X, Guo A, Newell KA, Huang XF, Yu Y (2018b) Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. J Neuroinflammation 15(1):112. 10.1186/s12974-018-1141-5
Liu S, Fan M, Xu JX, Yang LJ, Qi CC, Xia QR, Ge JF (2022) Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J Neuroinflammation 19(1):35. https://doi.org/10.1186/s12974-022-02393-2
Malhi GS, Mann JJ (2018) Depression. Lancet 392(10161):2299–2312. https://doi.org/10.1016/s0140-6736(18)31948-2
Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD (2020) Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun 87:901–909. https://doi.org/10.1016/j.bbi.2020.02.010
Ruilian L, Honglin Q, Jun X, Jianxin L, Qingyun B, Yilin C, Haifeng M (2021) H(2)S-mediated aerobic exercise antagonizes the hippocampal inflammatory response in CUMS-depressed mice. J Affect Disord 283:410–419. https://doi.org/10.1016/j.jad.2021.02.005
Su Q, Tao W, Huang H, Du Y, Chu X, Chen G (2016) Protective effect of liquiritigenin on depressive-like behavior in mice after lipopolysaccharide administration. Psychiatry Res 240:131–136. https://doi.org/10.1016/j.psychres.2016.04.002
Sun Y, Zhang H, Wu Z, Yu X, Yin Y, Qian S, Wang Z, Huang J, Wang W, Liu T, Xue W, Chen G (2021) Quercitrin rapidly alleviated Depression-like Behaviors in Lipopolysaccharide-Treated mice: the involvement of PI3K/AKT/NF-κB signaling suppression and CREB/BDNF signaling restoration in the Hippocampus. ACS Chem Neurosci 12(18):3387–3396. https://doi.org/10.1021/acschemneuro.1c00371
Swanson KV, Deng M, Ting JP (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol 19(8):477–489. https://doi.org/10.1038/s41577-019-0165-0
Tang M, Liu T, Jiang P, Dang R (2021) The interaction between autophagy and neuroinflammation in major depressive disorder: from pathophysiology to therapeutic implications. Pharmacol Res 168:105586. https://doi.org/10.1016/j.phrs.2021.105586
Troubat R, Barone P, Leman S, Desmidt T, Cressant A, Atanasova B, Brizard B, Hage E, Surget W, Belzung A, C., Camus V (2021) Neuroinflammation and depression: a review. Eur J Neurosci 53(1):151–171. https://doi.org/10.1111/ejn.14720
Walker AK, Wing EE, Banks WA, Dantzer R (2019) Leucine competes with kynurenine for blood-to-brain transport and prevents lipopolysaccharide-induced depression-like behavior in mice. Mol Psychiatry 24(10):1523–1532. https://doi.org/10.1038/s41380-018-0076-7
Wang H, He Y, Sun Z, Ren S, Liu M, Wang G, Yang J (2022) Microglia in depression: an overview of microglia in the pathogenesis and treatment of depression. J Neuroinflammation 19(1):132. https://doi.org/10.1186/s12974-022-02492-0
Won E, Na KS, Kim YK (2021) Associations between Melatonin, Neuroinflammation, and brain alterations in Depression. Int J Mol Sci 23(1). https://doi.org/10.3390/ijms23010305
Xia CY, Guo YX, Lian WW, Yan Y, Ma BZ, Cheng YC, Xu JK, He J, Zhang WK (2023) The NLRP3 inflammasome in depression: potential mechanisms and therapies. Pharmacol Res 187:106625. https://doi.org/10.1016/j.phrs.2022.106625
Yang HL, Li MM, Zhou MF, Xu HS, Huan F, Liu N, Gao R, Wang J, Zhang N, Jiang L (2021) Links between gut dysbiosis and neurotransmitter disturbance in Chronic Restraint Stress-Induced Depressive Behaviours: the role of inflammation. Inflammation 44(6):2448–2462. https://doi.org/10.1007/s10753-021-01514-y
Zhang B, Wang PP, Hu KL, Li LN, Yu X, Lu Y, Chang HS (2019) Antidepressant-like Effect and mechanism of action of Honokiol on the mouse lipopolysaccharide (LPS) Depression Model. Molecules 24(11). https://doi.org/10.3390/molecules24112035
Zhang Y, Anoopkumar-Dukie S, Mallik SB, Davey AK (2021) SIRT1 and SIRT2 modulators reduce LPS-induced inflammation in HAPI microglial cells and protect SH-SY5Y neuronal cells in vitro. J Neural Transm (Vienna) 128(5):631–644. https://doi.org/10.1007/s00702-021-02331-1
Zhao X, Cao F, Liu Q, Li X, Xu G, Liu G, Zhang Y, Yang X, Yi S, Xu F, Fan K, Ma J (2019) Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav Brain Res 364:494–502. https://doi.org/10.1016/j.bbr.2017.05.064
Zhong J, Li G, Xu H, Wang Y, Shi M (2019) Baicalin ameliorates chronic mild stress-induced depression-like behaviors in mice and attenuates inflammatory cytokines and oxidative stress. Braz J Med Biol Res 52(7):e8434. https://doi.org/10.1590/1414-431x20198434
Zhu J, Peng Q, Xu Y, Xu H, Wan Y, Li Z, Qiu Y, Xia W, Guo Z, Li H, Jin H, Hu B (2020) Morinda officinalis oligosaccharides ameliorate depressive-like behaviors in poststroke rats through upregulating GLUT3 to improve synaptic activity. Faseb j 34(10):13376–13395. https://doi.org/10.1096/fj.201902546RR
Zou W, Feng R, Yang Y (2018) Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS ONE 13(6):e0197267. https://doi.org/10.1371/journal.pone.0197267
Funding
This work was supported by STI2030-Major Projects (No. 2021ZD0200900) and the National Natural Science Foundation of China (No. 81773708).
Author information
Authors and Affiliations
Contributions
Zhao-Kai Lai and Yong-Yu Yin performed the research design, behavioral tests, immunofluorescence, ELISA, western blotting experiments, data analysis, and wrote the manuscript. Jiao-Zhao Yan contributed to the behavioral tests and immunofluorescence experiments. Qian-Qian Wei helped with the behavioral tests and writing the manuscript. Bin Wang helped with the immunofluorescence experiments. Yun-Feng Li, Li-Ming Zhang, and Yu-Lu Wang contributed to the research design and revised the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate (include appropriate statements)
Not Applicable.
Consent for publication (include appropriate statements)
Not Applicable.
Competing Interests
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lai, ZK., Yin, YY., Yan, JZ. et al. Inulin-type oligosaccharides of Morinda officinalis exerted antidepressant effects by reducing hippocampal inflammation. Metab Brain Dis 38, 2065–2075 (2023). https://doi.org/10.1007/s11011-023-01223-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-023-01223-5