Abstract
Neuroinflammation is associated with the development of depression. Deacetylases SIRT1 and SIRT2 are reported to exert neuroprotective effects in aging, neurogenesis, neurodegeneration and neuroinflammation. Therefore, this study aimed to investigate the effects of SIRT1 and SIRT2 modulators on LPS-induced neuroinflammation and neurodegeneration in vitro. To achieve this, HAPI rat microglial cells were pre-treated with the SIRT1 activator resveratrol (0.1–20 µM), the selective SIRT1 inhibitor EX527 (0.1; 1 µM), the dual SIRT1/SIRT2 inhibitor sirtinol (0.1–20 µM) and the SIRT2 inhibitor AGK2 (0.1; 1 µM), prior to exposure with LPS (5 ng/mL) for 20 h. The reference antidepressant drug fluoxetine and the nonsteroidal anti-inflammatory drug ibuprofen were also evaluated in the same paradigm, both at 1 μM. Resveratrol and sirtinol inhibited TNF-α production to a greater degree than either fluoxetine or ibuprofen. Resveratrol, sirtinol, EX527 and AGK2 significantly reduced PGE2 production by up to 100% in microglia. Then, the supernatant was transferred to treat SH-SY5Y cells for 24 h. In all cases, SIRT modulator pretreatment significantly protected undifferentiated SH-SY5Y human neuroblastoma cells from the insult of LPS-stimulated HAPI supernatant by up to 40%. Moreover, resveratrol and sirtinol also showed significantly better neuroprotection than fluoxetine or ibuprofen by up to 83 and 69%, respectively. In differentiated SH-SY5Y cells, only sirtinol (20, 10 µM) and AGK2 (0.1 µM) pretreatment protected the cells from LPS-stimulated HAPI supernatant. This study suggests that SIRT1 and SIRT2 modulators are effective in inhibiting LPS-stimulated production of TNF-α and PGE2 in HAPI microglial cells and protecting SH-SY5Y cells from inflammation. Thus, we provide proof of concept for further investigation of the therapeutic effect of SIRT1 and SIRT2 modulators and combination with current antidepressant medication as a treatment option.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is a complex phenomenon with multiple symptoms, such as depressed emotion, suicidal thoughts, insomnia, decreased appetite and fatigue. It is estimated that over 264 million people of all ages suffer from depression worldwide (World Health Organization 2020). Current antidepressant medications aim to increase levels of monoamine neurotransmitters (Penn and Tracy, 2012), but these antidepressants may have multiple side effects and toxicities which can result in poor compliance (Bockting et al. 2008; Penn and Tracy 2012). Furthermore, the NIMH-funded sequenced treatment alternatives to relieve depression study, conducted to determine the effectiveness of antidepressant treatments for major depression, found that overall remission rates were less than 30% with a single treatment, increasing to just 67% even after multiple interventions (Gaynes et al 2009).
Recent evidence has suggested a link between chronic neuroinflammation and the development of depression, with an association between inflammation and a number of depressive symptoms (Jokela et al. 2016; Miller and Raison 2016). Excessive and protracted neuroinflammation can directly result in acute neurological damage or neuronal death and accelerate long-term neurodegeneration (Lyman et al. 2014). Treatment options for neuroinflammation have been investigated, including clinical trials investigating nonsteroidal anti-inflammatory drugs (NSAIDs) and cytokine inhibitors (Zhang et al. 2012; Kostadinov et al. 2015; Köhler et al. 2016). Even so, their efficacy in neuroinflammation has not been established.
In recent years, numerous studies have reported the potential of SIRT1 and SIRT2 modulators in treating neuroinflammation and neurodegeneration (Zhang et al. 2020). The enzymes SIRT1 and SIRT2 belong to the sirtuin family, a unique class of nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases (Landry et al. 2000; Jiang et al. 2017). A series of transcription factors involved in multiple important cellular processes are substrates of SIRT1 and/or SIRT2′ deacetylation activity, such as forkhead transcription factors of class O, tumor suppressor protein p53, PPAR gamma coactivator-1α and nuclear factor-κB (Donmez and Outeiro 2013). SIRT1 activation and SIRT2 inhibition are implicated in a number of processes associated with the onset and development of depression, including aging, oxidative stress, neuronal survival, neurogenesis and neuroinflammation (Donmez and Outeiro 2013; Paraiso et al. 2013). Additionally, variation in SIRT1 and SIRT2 polymorphism or expression can influence the risk of developing depression (Porcelli et al. 2013; Liu et al. 2019; Zhang et al. 2021). This evidence suggests that investigating SIRT1 and SIRT2 may be a useful strategy for identifying potential new therapeutic interventions for depression. However, the reported effects of SIRT1 and SIRT2 activators or inhibitors on neuroinflammation and neurodegeneration are inconsistent, potentially due to the employment of different in vivo depressive-like models and other in vitro disease models (Zhang et al. 2020). Therefore, this study aimed to investigate the effects of the SIRT1 activator resveratrol, selective SIRT1 inhibitor EX527, dual SIRT1/SIRT2 inhibitor sirtinol, and the SIRT2 inhibitor AGK2, on neuroinflammation and neurodegeneration. To achieve this aim, these modulators were assessed in LPS-stimulated HAPI microglial cells and inflammation-conditioned SH-SY5Y neuronal cells.
Materials and methods
Cells and reagents
HAPI rat microglial cells and SH-SY5Y neuroblastoma cells were purchased from Sigma-Aldrich (Merck). Cells were cultured separately in ATCC-formulated DMEM (Gibco by Life Technologies) which contains 1 g/L of d-Glucose, l-glutamine, 110 mg/L of sodium pyruvate and phenol red. The medium was additionally supplemented with 1% penicillin–streptomycin (10,000 U/mL) (Gibco by Life Technologies) and 10% fetal bovine serum (FBS) (Scientifix life). Cells were cultured to 80% confluence in sterile T-75 cm2 flasks and maintained in an incubator at 37 °C with 5% CO2 and high humidity.
Establishment of in vitro model of neuroinflammation and subsequent neurodegeneration
HAPI cells were seeded at 2.5 × 105 cells/mL with 400 µL per well in 24-well plates and incubated at 37 °C with 5% CO2 for 24 h. Then, media control (50 µL) or diluted solutions containing test compounds (50 µL) were added to the corresponding wells of HAPI cells and incubated for 4 h. After the incubation, LPS (E.coli O111: B4) solution (50 µL) was also added to the corresponding wells to induce an inflammatory response, making a final volume of 500 µL, and incubated at 37 °C with 5% CO2 for 20 h. Thus, the final dilution factor for the drug solution and LPS solution was 0.1 and the final concentration of LPS was also 5 ng/mL. After the incubation, the supernatant was collected as samples to run enzyme-linked immunosorbent assays (ELISAs) and treat undifferentiated and differentiated SH-SY5Y neuronal cells as described below.
Undifferentiated neuronal model
SH-SY5Y cells were seeded at 105 cells/mL (100 µL) on 96-well plates and incubated at 37 °C with 5% CO2 for 24 h. To generate the neuroinflammation-induced neurodegeneration, the supernatant above the SH-SY5Y cells was removed and replaced with conditioned supernatant from the HAPI cells for 24 h. The conditioned supernatant was collected using methods described above from HAPI cells in 24-well plates after 20 h LPS treatment with or without 4 h pretreatment of resveratrol, sirtinol, EX527, AGK2, fluoxetine and ibuprofen in a series of concentrations.
Differentiated neuronal model
SH-SY5Y cells were seeded at 105 cells/mL (100 µL) on 96-well plates and incubated at 37 °C with 5% CO2 for 24 h. Then, cells were treated with 10 µM of retinoic acid (RA) every other day for a 5-day period in complete medium containing 10% FBS, followed with a treatment of 50 ng/mL of brain-derived neurotrophic factor (BDNF) for three consecutive days in serum-free medium. After that, the supernatant above SH-SY5Y cells was replaced with conditioned supernatant of HAPI cells for 24 h. The conditioned supernatant was collected using methods described above from HAPI cells in 24-well plates after 20 h LPS treatment with or without 4 h pretreatment of resveratrol, sirtinol, EX527, AGK2, fluoxetine and ibuprofen in a series of concentrations.
Quantitative real-time PCR (rt-PCR)
Following the differentiation of SH-SY5Y cells or the culturing of undifferentiated cells, the mRNA expression of the mature neuronal gene markers was tested using rt-PCR. The total RNA was extracted using TRIzol™ reagent (Invitrogen). The concentration of RNA was measured using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Australia). cDNA was synthesized using Verso cDNA synthesis kit (Thermo Scientific) and ProFlex PCR System (Life technology). Primer sequences (Sigman Aldrich) for rt-PCR are shown in Table 1. Rt-PCR was performed using PowerUpTMSYBR™ Green Master Mix kit and QuantStudioTM Real-Time PCR systems (Thermo Fisher Scientific Australia) according to manufactures’ guidelines. The thermal cycling conditions were 50 °C for 2 min, 95 °C for 2 min followed by 40 cycles of 95 °C for 1 s and 60 °C for 20 s. The dissociation curve conditions were 95 °C for 15 s and 60 °C for 1 min followed by 95 °C for 15 min. The comparative threshold cycles (CT) or 2−ΔΔCT method was used to analyse the CT values of samples of interest and control samples. All CT values were normalized against GAPDH gene.
Resazurin cell viability assay
Resazurin (alamarBlue) assays were run to detect cell viabilities of HAPI cells and SH-SY5Y cells. The oxidized non-fluorescent blue resazurin (Sigma-Aldrich) is reduced to fluorescent dye resorufin by the mitochondrial respiratory chain of living cells (Anoopkumar-Dukie et al. 2005). After the treatments (previously described), the supernatant above the cells was removed and replaced with media containing diluted resazurin (44 µM) and incubated in the dark at 37 °C with 5% CO2 for 3 h. Then, fluorescence intensity (excitation: 530 nm and emission: 590 nm) was determined and recorded using a Tecan Infinite 200 Pro microplate reader (Tecan, Australia).
Measurement of the release of PGE2, TNF-α, IL-1β and IL-10
To investigate the effect of resveratrol, sirtinol, EX527, AGK2 and reference drugs on the production of inflammatory mediators, as previously described, HAPI cells were seeded at 2.5 × 105 cells/well on 24-well plates and incubated at 37 °C with 5% CO2 for 20 h. The test solution was added to HAPI cells and incubated for 4 h. After the incubation, LPS solution was added to the cells (final concentration: 5 ng/mL) and incubated at 37 °C with 5% CO2 for 20 h. For measurement of PGE2 production in HAPI cells after LPS challenge, prostaglandin E2 (PGE2) express ELISA kit (Cayman 500141, Australia) was used to conduct the assay. For measurement of cytokine production of HAPI cells after LPS stimulation, rat TNF-α ELISA kit (Biosensis BEK-2101, Australia), rat IL-1β ELISA kit (Biosensis BEK-2309, Australia) and rat IL-10 ELISA kit (Biosensis BEK-2047, Australia) were used in line with the manufacturer's instructions. Absorbance was measured using a Tecan infinite 200 microplate reader.
Measurement of intracellular oxidative stress production
HAPI cells were seeded at 2 × 104 cells/well on 96-well plates and incubated at 37 °C with 5% CO2 for 20 h. Then, the pretreatment solution was added to the HAPI cells and incubated for 4 h. After the incubation, the LPS solution was added to each well to challenge the HAPI cells and incubated at 37 °C with 5% CO2 for 20 h. After that, reactive oxygen species (ROS) production was measured by conducting a 2,7-dichlorofluorescin diacetate (DCFH-DA) assay, whereby ROS oxidizes DCFH-DA into the highly fluorescent compound dichlorofluorescein (DCF). The supernatant above the HAPI cells was removed and discarded. The diluted DCFH-DA solution (10 µM) was added to respective wells and incubated at 37 °C with 5% CO2 for 1 h and protected from the light. Then, the solution above the cells was replaced with 200 µL of PBS and washed out. Following this, PBS (100 µL) was added into each well. Fluorescence (excitation: 485 nm and emission: 535 nm) was measured and recorded using a Tecan Infinite 200 Pro microplate reader (Tecan, Australia).
Measurement of caspase 3/7 activity
SH-SY5Y cells were seeded at 105 cells/mL (100 µL) on 96-well plates and incubated at 37 °C with 5% CO2 for 24 h. For the undifferentiated cells, the supernatant of HAPI cells after the LPS challenge was transferred onto the cells in the 96-well plates and incubated for 24 h. For the differentiated model, SH-SY5Y cells were induced to differentiate after seeding for an 8-day period, and then underwent the same treatment as for the undifferentiated cells. After that, caspase 3/7 assays (Cayman Chemical, Australia) were conducted to measure the apoptosis level of cells. The protocol was followed according to the manufacturer’s instructions. The fluorescence (excitation: 485 nm and emission: 535 nm) intensity value was read and recorded using a Tecan Infinite 200 Pro microplate reader (Tecan, Australia).
Statistical analysis
All data were analyzed by GraphPad Prism 5 and presented as mean + SD of three independent experiments. One-way ANOVA followed by Turkey’s multiple comparison test was applied. Significance levels were shown as p < 0.05 (*, #), p < 0.01 (**, ##) and p < 0.001 (***, ###).
Results
SIRT1 and SIRT2 modulators induced cytotoxicity in HAPI and SH-SY5Y cells in a dose-dependent way
In HAPI cells, resveratrol and sirtinol had high cytotoxicity at the concentrations of 100 and 50 µM and mild cytotoxicity at 25 µM (Fig. 1a, b). EX527 and fluoxetine had high cytotoxicity at concentrations of 100, 50, and 25 µM and mild cytotoxicity at 10 µM (Fig. 1c, e). AGK2 demonstrated high cytotoxicity at 100, 50, 25, and 10 µM (Fig. 1d). Ibuprofen had only mild cytotoxicity at 100 µM (Fig. 1f). Dimethyl sulfoxide (DMSO) as the co-solvent of drug solution in each well was below < 0.01% and did not induce cytotoxicity in HAPI cells (data not shown). Non-toxic concentrations of the compounds were used in the following cellular assays.
In undifferentiated SH-SY5Y cells, all SIRT1 and SIRT2 modulators were shown to have dose-dependent cytotoxicity (Fig. 2a–d). Fluoxetine and ibuprofen did not exert marked cytotoxicity in undifferentiated SH-SY5Y cells at 10 and 1 µM (Fig. 2e, f). DMSO (< 0.01%) as the solvent of drug solution and LPS (5 ng/mL) did not induce cytotoxicity in SH-SY5Y cells (Data not shown).
SIRT1 and SIRT2 modulators inhibit LPS-induced inflammation in HAPI microglial cells
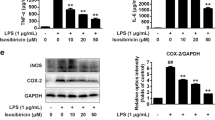
All SIRT modulators, fluoxetine and ibuprofen were shown to significantly reduce PGE2 production by HAPI cells after LPS (5 ng/mL) challenge for 20 h (p < 0.05) (Fig. 3a–d). Resveratrol (20 µM), sirtinol (1–20 µM), EX527 (0.1 µM) and AGK2 (0.1 µM) strongly reduced PGE2 production of HAPI cells after LPS stimulation to different degrees. Among these, the treatment of resveratrol at 20 µM and sirtinol at 1 µM induced 97 and 100% reductions, respectively. The antidepressant fluoxetine (1 µM) and the NSAID ibuprofen (1 µM), used as reference compounds, also were shown to have significant effects on the reduction of PGE2 production of HAPI cells.
SIRT1 and SIRT2 drugs repress LPS-induced PGE2 production in HAPI cells. HAPI cells were treated with resveratrol (a), sirtinol (b), EX527 (c) and AGK2 (d) at a series of concentrations 4 h prior to LPS (5 ng/mL) challenge for 20 h and assessed for PGE2 production using ELISA. # vs untreated media control only, * vs LPS only
Resveratrol (0.1–20 µM) decreased TNF-α production by HAPI cells after LPS (5 ng/mL) challenge for 20 h (p < 0.001) with concentrations of 20 and 10 µM showing 95 and 90% reductions, respectively (Fig. 4a). Compared to fluoxetine (1 µM) and ibuprofen (1 µM), resveratrol (0.1–20 µM) had a more potent inhibiting effect (p < 0.05). Sirtinol with concentrations of 20 and 10 µM also significantly reduced TNF-α production by approximately 93% and 85%, respectively (Fig. 4b). EX527 (Fig. 4c), AGK2 (Fig. 4d), and ibuprofen had no significant effect on TNF-α production of HAPI cells after LPS treatment. However, fluoxetine at 1 µM was shown to enhance TNF-α release after LPS stimulation in HAPI cells by approximately 79% (p < 0.05).
SIRT1 and SIRT2 drugs repress LPS-induced TNF-α production in HAPI cells. HAPI cells were treated with resveratrol (a), sirtinol (b), EX527 (c) and AGK2 (d) at a series of concentrations 4 h prior to LPS (5 ng/mL) challenge for 20 h and assessed for TNF-α production using ELISA. # vs untreated media control only, *vs LPS only
IL-1β and IL-10 were not detectable in the supernatant of LPS (5 ng/mL)-activated HAPI cells. SIRT1 and SIRT2 modulators including resveratrol (0.1–20 µM), sirtinol (0.1–20 µM), EX527 (0.1, 1 µM), AGK2 (0.1, 1 µM) and fluoxetine and ibuprofen (01 µM) also did not stimulate the production of IL-1β or IL-10 in LPS (5 ng/mL)-induced HAPI cells.
SIRT1 and SIRT2 modulators affect the production of LPS-induced reactive oxidative species in HAPI cells
DCF assays were conducted to determine whether SIRT1 and SIRT2 modulators affect the production of reactive oxidative species (ROS) in LPS-challenged (5 ng/mL) HAPI cells. Resveratrol (0.1–20 µM) significantly reduced ROS production of LPS-challenged HAPI cells (p < 0.01), which at 20 µM was more marked than seen with fluoxetine (1 µM) or ibuprofen (1 µM) (p < 0.001) (Fig. 5a). In contrast, sirtinol pretreatment led to significantly higher ROS levels in LPS-challenged HAPI cells at 20 and 10 µM (p < 0.001) (Fig. 5b). EX527 (1 µM) significantly decreased ROS production (p < 0.05) (Fig. 5c), however, AGK2 was not shown to affect the ROS production of LPS-challenged HAPI cells (Fig. 5d). Fluoxetine (1 µM) and ibuprofen (1 µM) significantly reduced the ROS production of LPS-challenged HAPI cells (p < 0.001).
SIRT1 and SIRT2 drugs affect LPS-induced oxidative stress production in HAPI cells. HAPI cells were treated with resveratrol (a), sirtinol (b), EX527 (c) and AGK2 (d) at a series of concentrations 4 h prior to LPS (5 ng/mL) challenge for 20 h and assessed for oxidative stress production using DCF assay. # vs untreated media control only, *vs LPS only
SIRT1 and SIRT2 modulators protected undifferentiated SH-SY5Y cells from death induced by HAPI-conditioned supernatant
To test whether SIRT1 and SIRT2 modulators protect SH-SY5Y neuronal cells from subsequent neurodegeneration, undifferentiated SH-SY5Y cells were incubated for 24 h with supernatant collected from HAPI cells after incubation with LPS (5 ng/mL) and the test compounds as described in the methods. Then, a resazurin assay was conducted to measure cell viability. Resveratrol pretreatment at 0.1–20 µM on HAPI cells protected undifferentiated SH-SY5Y cells against the LPS-challenged of HAPI supernatant, increasing viability by 11–21% (p < 0.05) (Fig. 6a). Sirtinol (0.1–20 µM) also significantly improved the viability of undifferentiated SH-SY5Y cells by 15–40% (p < 0.001) (Fig. 6b), as did EX527 (0.1, 1 µM) and AGK2 (0.1 µM) by 8 to 9% (p < 0.01) and 10% (p < 0.001), respectively (Fig. 6c, d). Ibuprofen (1 µM)-conditioned supernatant (p < 0.01) significantly decreased the toxicity to undifferentiated cells.
SIRT1 and SIRT2 drugs protected undifferentiated SH-SY5Y cells from death induced by supernatant of LPS-challenged HAPI cells. Undifferentiated SH-SY5Y cells were treated with supernatant produced by HAPI cells after treatment of resveratrol (a), sirtinol (b), EX527 (c) and AGK2 (d) at a series of concentrations 4 h prior to LPS (5 ng/mL) challenge for 20 h. # vs untreated media control only, *vs LPS only
Sirtinol and AGK2 protected differentiated SH-SY5Y cells from death induced by HAPI-conditioned supernatant
Differentiated SH-SY5Y cells were treated with HAPI-conditioned supernatant in the same way, and resazurin assay conducted to measure the cell viability. Sirtinol (10, 20 µM) and AGK2 (0.1 µM) pretreatment on HAPI cells protected differentiated SH-SY5Y cells against the toxicity of LPS-conditioned HAPI supernatant challenge, improving cell viability by 19 to 30% (p < 0.01) and 20% (p < 0.05), respectively (Fig. 7b, d). Sirtinol at 20 µM also showed significantly greater viability protection than fluoxetine (1 µM) (p < 0.001) but not ibuprofen (1 µM). Resveratrol and EX527 had no significant effect on the viabilities of SH-SY5Y cells after the conditioned-supernatant challenge (Fig. 7a, c). Fluoxetine was not shown to protect the SH-SY5Y cells from toxicity after the conditioned-supernatant challenge, whereas ibuprofen (1 µM) significantly protected differentiated SH-SY5Y cells after the conditioned-supernatant challenge (p < 0.001).
Effects of SIRT1 and SIRT2 drugs on neuroinflammation-induced neurodegeneration in differentiated SH-SY5Y cells. Differentiated SH-SY5Y cells were treated with supernatant collected from HAPI cells treated with resveratrol (a), sirtinol (b), EX527 (c) and AGK2 (d) combining LPS (5 ng/mL) challenge for 24 h and assessed for cell viability using resazurin assay. #vs untreated media control only, *vs LPS only
Resveratrol pretreatment decreased apoptosis in inflammation-challenged undifferentiated SH-SY5Y cells by attenuating caspase 3/7 activity
In undifferentiated SH-SY5Y cells, LPS (5 ng/mL)-conditioned supernatant collected from HAPI cells significantly increased the level of caspase 3/7 (Fig. 8). Resveratrol (10, 20 µM) pretreatment in HAPI cells significantly decreased the elevation of caspase 3/7 activity induced by the treatment of LPS-conditioned supernatant (p < 0.001) (Fig. 8a). Sirtinol, EX527, AGK2, fluoxetine (1 µM) and ibuprofen (1 µM) had no effect on reducing caspase 3/7 activities compared to LPS stimulation-conditioned supernatant (Fig. 8b–d).
Effects of SIRT1 and SIRT2 drugs on caspase 3/7 activation in neuroinflammation-induced neurodegeneration in undifferentiated SH-SY5Y cells. Undifferentiated SH-SY5Y cells were treated with supernatant collected from HAPI cells treated with resveratrol (a), sirtinol (b), EX527 (c) and AGK2 (d) combining LPS (5 ng/mL) challenge for 24 h and assessed for caspase 3/7 activation. # vs untreated media control only, *vs LPS only
In differentiated SH-SY5Y cells (Fig. 9), none of these modulators were shown to reduce caspase 3/7 activity. Indeed, EX527 (0.1, 1 µM) and AGK2 (0.1, 1 µM) significantly increased caspase 3/7 activities compared to LPS stimulation-conditioned supernatant (p < 0.05) (Fig. 9b, c).
Effects of SIRT1 and SIRT2 drugs on caspase 3/7 activation in neuroinflammation-induced neurodegeneration in differentiated SH-SY5Y cells. Differentiated SH-SY5Y cells were treated with supernatant collected from HAPI cells treated with resveratrol (a), sirtinol (b), EX527 (c) and AGK2 (d) combining LPS (5 ng/mL) challenge for 24 h and assessed for caspase 3/7 activation. # vs untreated media control only, *vs LPS only
Discussion
This study first investigated and compared the effects of SIRT1 and SIRT2 modulators on the release of inflammatory mediators in LPS (5 ng/mL)-treated HAPI microglial cells. The antidepressant fluoxetine and the NSAID ibuprofen were used as controls. Resveratrol, EX527, sirtinol and AGK2 were shown to reduce the expression of PGE2 production. However, for TNF-α production, only resveratrol and sirtinol showed significant inhibiting effects. Resveratrol’s anti-inflammatory effects in HAPI cells are consistent with numerous other studies. Its protective effect against neuroinflammation has been found in different in vitro models, such as murine macrophages, mouse microglial N9 and BV-2 cell lines, working through different mechanisms including down-regulating NF-κB activation or stimulating PGC-1α expression (Bi et al. 2005; de Sá Coutinho et al. 2018).
In previous neurodegeneration studies, sirtinol was often used as a SIRT1 inhibitor and shown to reverse the protective effects of SIRT1 (Albani et al. 2009; Abe-Higuchi et al. 2016). However, in this study, it was found that sirtinol exerts significant anti-inflammatory effects in HAPI microglial cells. EX527 is another frequently used SIRT1 inhibitor that can reverse SIRT1 activator resveratrol’s protection in neurodegeneration studies (Diaz-Ruiz et al. 2015; Guo et al. 2016). However, our results showed that EX527 had a similar inhibiting effect on PGE2 production to resveratrol. AGK2 as a SIRT2 inhibitor did not have a very promising effect on inhibiting LPS-induced inflammation. In contrast, AGK2 was found to be potentially beneficial in antagonizing neuroinflammation and neurodegeneration in previous studies. In these studies, AGK2 was reported to block LPS-induced gene expression of TNF-α and IL-6 in a neuroinflammation mouse model and inhibit astrocyte activation and production of iNOS in Aβ-induced primary astrocytes (Scuderi et al. 2014; Wang et al. 2016). Therefore, different cell lines, experimental systems, types of stimuli and animal experiments can result in different findings.
It has been reported that fluoxetine can inhibit the production of TNF-α, PGE2 and IL-6 in the LPS-stimulated BV-2 microglial cells (Liu et al. 2011), which is in contrast to our results in HAPI cells. In our study, TNF-α production is exaggerated by fluoxetine after LPS-stimulation. It is apparent that fluoxetine can have different effects in different experimental models. For example, fluoxetine reduced TNF-α production in LPS-induced inflammation in rats, and also had an inhibitory effect in carrageenan-induced inflammation following multiple doses, but enhanced TNF-α production in the same model if only given as a single dose (Kostadinov et al. 2015). Hence, it is apparent that the dose, frequency/duration of administration and model of inflammation will lead to contrasting observations in relation to the effects of fluoxetine on TNF-α. There are several hypotheses that have been suggested regarding the influence of fluoxetine on TNF-α in different models of inflammation including acting via the immunosuppressant properties of serotonin, inhibition of the transcription factor NF-κB and inhibition of the phosphorylation of p38 MAPK, or potentially acting as a serotonin antagonist on 5-HT2A receptors (Kostadinov et al. 2015). The present study does not provide data to enable the elucidation of the role of serotonin or the other mentioned pathways’ participation in our model. Besides, the subtypes and numbers of 5-HT receptors expressed on microglia have not been identified (Krabbe et al. 2012). Therefore, the extent of fluoxetine’s interaction with serotonin 5-HT receptors is also unknown in our study. Furthermore, fluoxetine has been shown to increase the expression of pro-inflammatory markers in experimental mice living in an enriched environment, but had an anti-inflammatory effect in mice living in a stressful condition, which further indicates that there are complex mechanisms involved (Alboni et al. 2016). The data provided by limited clinical studies are also highly variable and contradictory. Therefore, it cannot yet be concluded that the antidepressant therapeutic effect of fluoxetine is partially attributed to its anti-inflammatory effect. However, the inhibition of neuroinflammation can help to prevent the imbalanced metabolism of monoamine neurotransmitters (Miller and Raison 2016). Thus, combination treatment with antidepressant and SIRT modulators might be worth investigating in animal models and clinical studies to identify whether there are potential synergistic effects in the treatment of depression.
The subsequent neurodegeneration was assessed by treating both undifferentiated and differentiated SH-SY5Y cells with HAPI supernatant after LPS challenge with/without drug pretreatment. The SH-SY5Y cell line has neuron-like properties and has been used as a neuronal cell model in many other neurological studies (Cheung et al. 2009). The differentiation of SH-SY5Y cells aims to cease progressive proliferation and induce the expression of mature neuronal genes. However, many differentiation methods, including the one used in this study, have limitations. Therefore, we believe that using both undifferentiated and differentiated SH-SY5Y cells, we are able to present a more comprehensive data-set that will inform furture work regardless of which model others are working with. It was found that undifferentiated SH-SY5Y cells are protected against the supernatant from LPS-challenged microglia by resveratrol, sirtinol, EX527 and AGK2 pretreatment. Whereas, in differentiated SH-SY5Y cells, only sirtinol and AGK2-pre-treated supernatant were shown to exert neuroprotection against LPS-induced inflammation. This indicates the importance of the differentiation of SH-SY5Y cells. Even though SH-SY5Y cells have neuronal-like traits, they still maintain the characteristics of neuroblastomas. The reduction of cell viability induced by the treatment of LPS-conditioned supernatant in undifferentiated SH-SY5Y cells may be partially due to the inhibition of aggressive cell proliferation. Therefore, it was appropriate to also test the effects of LPS-conditioned supernatant on neuronally differentiated SH-SY5Y cells. There was a difference in apoptosis activity observed in differentiated vs undifferentiated cells. LPS (5 ng/mL)-conditioned supernatant collected from HAPI cells significantly increased caspase 3/7 activity in undifferentiated SH-SY5Ycells but not the differentiated cells. This work indicates that apoptosis might not be the pathway leading to neuronal death in a microenvironment with a mild level of neuroinflammation.
From these results, resveratrol and sirtinol exert significant inhibitory effects on the production of TNF-α and PGE2 in HAPI cells, which corresponds with the result that SH-SY5Y cells are most protected by resveratrol and sirtinol pre-treated supernatant. TNF-α can induce cell death by binding with TNF receptors (Wajant et al. 2003). Thus, the reduction of TNF-α after the treatment with resveratrol and sirtinol might account for the decrease in cell death of SH-SY5Y cells. Prostaglandins have been demonstrated to play an important role in the development of chronic inflammation (Honda et al. 2006; Narumiya 2009). They also contribute to prolonging acute inflammatory responses by activating chronic gene expression through inhibiting the differentiation of Th2 cells which produce the anti-inflammatory cytokine IL-10 (Leonard 2018). Therefore, SIRT modulators’ inhibiting effects on PGE2 might exert potential benefits via the enhanced production of anti-inflammatory mediators.
Resveratrol, EX527, fluoxetine and ibuprofen were shown to have significant inhibitory effects on oxidative stress in HAPI cells exposed to LPS. In contrast, sirtinol caused a significant increase in ROS production. This result indicates that sirtinol could potentially cause damage to tissues and organ systems. Other compounds including resveratrol, EX527, AGK2, fluoxetine and ibuprofen would be expected to exert less damage to the target tissue in terms of oxidative stress.
The production of IL-1β was not detectable in LPS-activated HAPI microglial cells. The mechanism of release of LPS-induced secretion of IL-1β is unclear as it does not follow the conventional endoplasmic reticulum (ER)-Golgi route (Lopez-Castejon and Brough 2011). Under the stimulation of LPS, IL-1β is first generated as inactive precursor pro-IL-1β which is subsequently cleaved by IL-1 converting enzyme (ICE), a member of caspase-1. Caspase-1 also needs to be activated by the formation of inflammasomes that contain pro-caspase-1 (Lopez-Castejon and Brough 2011). Also, the assembly of inflammasomes is dependent upon the activation of P2X7 receptors on the cell membrane by extracellular adenosine triphosphate (ATP) (Stoffels et al. 2015). Therefore, this might be a reason why IL-1β was not detected through ELISA measurement in this LPS-induced neuroinflammation model. In this model, LPS did not result in acute cell death of HAPI cells, which suggests that there is not a large amount of ATP released following cell membrane damage to activate the P2X7 receptors on the remaining HAPI cells leading to maturation and release of IL-1β (Sanz and Di Virgilio 2000). A different stimulus or a higher concentration of LPS would induce a significant increase in ROS, IL-1 and IL-10. However, this study was designed to mimic the mild inflammatory microenvironment of depression, not the level of inflammation that might be induced in response to a bacterial or viral infection. This is an important differentiation for researchers investigating the relationship between neuroinflammation and depression, rather than more pronounced models of inflammation.
Conclusion
This study has shown the effectiveness of SIRT1 and SIRT2 modulators and how they compare to fluoxetine and ibuprofen in inhibiting LPS-stimulated production of TNF-α and PGE2 in HAPI microglial cells, and protecting SH-SY5Y cells from inflammatory insult. Besides the validation for resveratrol’s potent anti-inflammatory effects in this in vitro model, dual SIRT1/SIRT2 inhibitor sirtinol’s protective effects also need to be considered in future neuroinflammation studies. These findings highlight the importance of further investigating the potential therapeutic effect of SIRT1 and SIRT2 modulators and combination treatments with current antidepressant medication in the animal studies of depression. However, further assessment of the modulators’ efficacy in SIRT1 and SIRT2 activity modulation and broader screening scope of cell lines will provide additional supporting data to guide researchers to choose more effective and selective SIRT1 and SIRT2 modulators. Furthermore, other potential mechanisms besides apoptosis need to be further investigated to warrant SIRT1 and SIRT2 modulators’ protective effects.
References
Abe-Higuchi N, Uchida S, Yamagata H, Higuchi F, Hobara T, Hara K, Kobayashi A, Watanabe Y (2016) Hippocampal sirtuin 1 signaling mediates depression-like behavior. Biol Psychiatry 80:815–826
Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, Colombo L, Manzoni C, Salmona M, Caccia S, Negro A, Forloni G (2009) The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1–42) peptide. J Neurochem 110:1445–1456
Alboni S, Poggini S, Garofalo S, Milior G, El Hajj H, Lecours C, Girard I, Gagnon S, Boisjoly-Villeneuve S, Brunello N, Wolfer DP, Limatola C, Tremblay M, Maggi L, Branchi I (2016) Fluoxetine treatment affects the inflammatory response and microglial function according to the quality of the living environment. Brain Behav Immun 58:261–271
Anoopkumar-Dukie S, Carey JB, Conere T, O’sullivan E, Van Pelt FN, Allshire A (2005) Resazurin assay of radiation response in cultured cells. Br J Radiol 78:945–947
Bi XL, Yang JY, Dong YX, Wang JM, Cui YH, Ikeshima T, Zhao YQ, Wu CF (2005) Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. Int Immunopharmacol 5:185–193
Bockting CL, Ten Doesschate MC, Spijker J, Spinhoven P, Koeter MW, Schene AH (2008) Continuation and maintenance use of antidepressants in recurrent depression. Psychother Psychosom 77:17–26
Cheung YT, Lau WK, Yu MS, Lai CS, Yeung SC, So KF, Chang RC (2009) Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 30:127–135
De Sá Coutinho D, Pacheco MT, Frozza RL, Bernardi A (2018) Anti-inflammatory effects of resveratrol: mechanistic insights. Int J Mol Sci 19:1812
Diaz-Ruiz C, Rodriguez-Perez AI, Beiroa D, Rodriguez-Pallares J, Labandeira-Garcia JL (2015) Reciprocal regulation between sirtuin-1 and angiotensin-II in the substantia nigra: implications for aging and neurodegeneration. Oncotarget 6:26675–26689
Donmez G, Outeiro TF (2013) SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med 5:344–352
Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, John Rush A (2009) What did STAR* D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv 60(11):1439–1445
Guo YJ, Dong SY, Cui XX, Feng Y, Liu T, Yin M, Kuo SH, Tan EK, Zhao WJ, Wu YC (2016) Resveratrol alleviates MPTP-induced motor impairments and pathological changes by autophagic degradation of alpha-synuclein via SIRT1-deacetylated LC3. Mol Nutr Food Res 60:2161–2175
Honda T, Segi-Nishida E, Miyachi Y, Narumiya S (2006) Prostacyclin-IP signaling and prostaglandin E2-EP2/EP4 signaling both mediate joint inflammation in mouse collagen-induced arthritis. J Exp Med 203:325–335
Jiang Y, Liu J, Chen D, Yan L, Zheng W (2017) Sirtuin inhibition: strategies, inhibitors, and therapeutic potential. Trends Pharmacol Sci 38:459–472
Jokela M, Virtanen M, Batty GD, Kivimaki M (2016) Inflammation and specific symptoms of depression. JAMA Psychiat 73:87–88
Köhler O, Krogh J, Mors O, Benros ME (2016) Inflammation in depression and the potential for anti-inflammatory treatment. Curr Neuropharmacol 14:732–742
Kostadinov I, Delev D, Petrova A, Stanimirova I, Draganova K, Kruzliak P, Kostadinova I, Murdjeva M (2015) Study on anti-inflammatory and immunomodulatory effects of fluoxetine in rat models of inflammation. Eur J Inflamm 13:173–182
Krabbe G, Matyash V, Pannasch U, Mamer L, Boddeke HWGM, Kettenmann H (2012) Activation of serotonin receptors promotes microglial injury-induced motility but attenuates phagocytic activity. Brain Behav Immun 26:419–428
Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R (2000) The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci USA 97:5807–5811
Leonard BE (2018) Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr 30:1–16
Liu D, Wang Z, Liu S, Wang F, Zhao S, Hao A (2011) Anti-inflammatory effects of fluoxetine in lipopolysaccharide(LPS)-stimulated microglial cells. Neuropharmacology 61:592–599
Liu W, Yan H, Zhou D, Cai X, Zhang Y, Li S, Li H, Li S, Zhou DS, Li X, Zhang C, Sun Y, Dai JP, Zhong J, Yao YG, Luo XJ, Fang Y, Zhang D, Ma Y, Yue W, Li M, Xiao X (2019) The depression GWAS risk allele predicts smaller cerebellar gray matter volume and reduced SIRT1 mRNA expression in Chinese population. Transl Psychiatry 9:333
Lopez-Castejon G, Brough D (2011) Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev 22:189–195
Lyman M, Lloyd DG, Ji XM, Vizcaychipi MP, Ma DQ (2014) Neuroinflammation: the role and consequences. Neurosci Res 79:1–12
Miller AH, Raison CL (2016) The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16:22–34
Narumiya S (2009) Prostanoids and inflammation: a new concept arising from receptor knockout mice. J Mol Med 87:1015–1022
Paraiso AF, Mendes KL, Santos SH (2013) Brain activation of SIRT1: role in neuropathology. Mol Neurobiol 48:681–689
Penn E, Tracy DK (2012) The drugs don’t work? antidepressants and the current and future pharmacological management of depression. Ther Adv Psychopharmacol 2:179–188
Porcelli S, Salfi R, Politis A, Atti AR, Albani D, Chierchia A, Polito L, Zisaki A, Piperi C, Liappas I, Alberti S, Balestri M, Marsano A, Stamouli E, Mailis A, Biella G, Forloni G, Bernabei V, Ferrari B, Lia L, Papadimitriou GN, De Ronchi D, Serretti A (2013) Association between sirtuin 2 gene rs10410544 polymorphism and depression in Alzheimer’s disease in two independent European samples. J Neural Transm 120:1709–1715
Sanz JM, Di Virgilio F (2000) Kinetics and mechanism of ATP-dependent IL-1 beta release from microglial cells. J Immunol 164:4893–4898
Scuderi C, Stecca C, Bronzuoli MR, Rotili D, Valente S, Mai A, Steardo L (2014) Sirtuin modulators control reactive gliosis in an in vitro model of Alzheimer’s disease. Front Pharmacol 5:89
Stoffels M, Zaal R, Kok N, Van Der Meer JW, Dinarello CA, Simon A (2015) ATP-induced IL-1beta specific secretion: true under stringent conditions. Front Immunol 6:54
Wajant H, Pfizenmaier K, Scheurich P (2003) Tumor necrosis factor signaling. Cell Death Differ 10:45–65
Wang B, Zhang Y, Cao W, Wei X, Chen J, Ying W (2016) SIRT2 plays significant roles in lipopolysaccharides-induced neuroinflammation and brain injury in mice. Neurochem Res 41:2490–2500
World Health Organization (2020) Depression key facts. Retrieved from https://www.who.int/news-room/fact-sheets/detail/depression. Accessed 30 Oct 2020
Zhang F, Zhou H, Wilson BC, Shi J-S, Hong J-S, Gao H-M (2012) Fluoxetine protects neurons against microglial activation-mediated neurotoxicity. Parkinsonism Relat Disord 18:S213–S217
Zhang Y, Anoopkumar-Dukie S, Arora D, Davey AK (2020) Review of the anti-inflammatory effect of SIRT1 and SIRT2 modulators on neurodegenerative diseases. Eur J Pharmacol 867:172847
Zhang Y, Anoopkumar-Dukie S, Davey AK (2021) SIRT1 and SIRT2 modulators: potential anti-inflammatory treatment for depression? Biomolecules 11:353
Funding
We thank the Griffith University Postgraduate Research Scholarship and Griffith University International Postgraduate Research Scholarship for funding this work.
Author information
Authors and Affiliations
Contributions
YZ, SA-D, and AKD designed and conducted this study and prepared the final manuscript. SBM contributed to the establishment of the experiment model.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Anoopkumar-Dukie, S., Mallik, S.B. et al. SIRT1 and SIRT2 modulators reduce LPS-induced inflammation in HAPI microglial cells and protect SH-SY5Y neuronal cells in vitro. J Neural Transm 128, 631–644 (2021). https://doi.org/10.1007/s00702-021-02331-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-021-02331-1