Abstract
Hormonal imbalance may be an important factor in the severity of multiple sclerosis (MS) disease. In this context, hormone therapy has been shown to have immunoregulatory potential in various experimental approaches. There is increasing evidence of potentially beneficial effects of thyroid, melatonin, and sex hormones in MS models. These hormones may ameliorate the neurological impairment through immunoregulatory and neuroprotective effects, as well as by reducing oxidative stress. Expanding our knowledge of hormone therapy may be an effective step toward identifying additional molecular/cellular pathways in MS disease. In this review, we discuss the role of several important hormones in MS pathogenesis in terms of their effects on immunoregulatory aspects and neuroprotection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a multifactorial disease in which environmental, genetic, and epigenetic factors may play causal roles and interact with modifiable risk factors. However, some other fundamental aspects of disease etiopathogenesis remain to be elucidated (Thompson et al. 2018). According to the latest edition of the MS Atlas, the number of MS patients worldwide is expected to increase to 2.8 million by 2020, 30% more than in 2013 (Walton et al. 2020). The above reasons, including the increasing prevalence/incidence of MS, the complex etiology, and the significant economic burden, necessitate a continued quest to understand this disease (Tobore 2020). To counteract the central nervous system (CNS) damage in MS, one of the ideal treatments is to promote remyelination, which could reduce the severity of the disease (Lubetzki et al. 2020). Several studies suggest that hormones from body glands play an important role in the mechanism of immune/CNS cells (Ysrraelit and Correale 2019; Deckx et al. 2013). In this context, there is increasing evidence of the beneficial functions of several important hormones (e.g., thyroid, melatonin, and sex hormones). There are numerous data suggesting that thyroid hormones (TH) might protect the CNS through immunoregulatory effects. Such a reduction in inflammatory responses was shown in a gradual reduction of CD45+ microglial cells, interferon-gamma (IFN-γ), and interleukin-10 (IL-10) after TH therapy in the experimental models (Payghani et al. 2017; Harsan et al. 2008).
Excessive neuroinflammation and oxidative stress have potentially negative effects on biological energy and may lead to axonal degeneration in patients with advanced MS (Pegoretti et al. 2020). Melatonin can inhibit free radicals, intracellular reactive oxygen species (ROS), and thus inflammation by forming antioxidant enzymes and reducing the production of proinflammatory cytokines (e.g., tumor necrosis factor-alpha (TNF-α), IL-1β, and IL-6). Therefore, it acts as a potent antioxidant and anti-inflammatory agent (Tan et al. 2016; Eghbal et al. 2016).
There are prevalence differences between the sexes in autoimmune diseases such as MS, (Ysrraelit and Correale 2019) and the most frequent relapses of MS occur in women (Kamel 2019). Studies show that during pregnancy, placental hormones such as estriol (E3) can reduce the severity of MS by regulating immune cell function. Steroid hormones play a fundamental role in suppressing the immune system and inhibiting immunological responses by controlling leukocyte migration, altering macrophage polarization, and inducing T cell apoptosis (Shimba et al. 2021). Overall, this review aims to provide an overview of the main hormonal effects in MS treatment and the associated challenges of hormonal imbalance and hormone therapy.
MS: a disorder of myelination
MS is primarily an inflammatory disease of the brain and spinal cord in which localized lymphocytic infiltration leads to the destruction of myelin and axons (Azimzadeh et al. 2021; Eslami et al. 2020). In MS patients, myelinated axons may be degraded over time due to a lack of spontaneous remyelination, making delayed differentiation of oligodendrocyte precursor cells (OPCs) (Trapp and Nave 2008; Azimzadeh et al. 2020). Cortical MS lesions were categorized based on their localization. In type I lesions, demyelination occurs at the leukocortical border. Partial perivascular demyelinated areas are evident in type II lesions, and extensive subpial demyelinated plaques in layers III or IV are termed to as type III lesions. Although there is no apparent relationship between lesion location and disease progression, cortical damage is associated with motor/sensory deficits and contributes to cognitive/executive dysfunction which the typical neurological deterioration seen in MS patients (Dutta and Trapp 2011; Rinaldi et al. 2010; Vucic et al. 2012). Remyelination is a complex process that requires not only sufficient numbers of oligodendrocytes but also precise placement (Albornoz et al. 2013). Oligodendrocytes specifically promote the structural and metabolic integrity of CNS neurons. On the other hand, the damaged axons and the reduced potential of the myelin sheath are due to the loss of this support. Neurons have the highest metabolic demand; therefore, their active axons are particularly vulnerable when electrical activity is high. Indeed, axonal injury and poor myelin sheathing can lead to delayed communication at synapses, increasing the brain’s susceptibility to overt neurodegeneration (Saab et al. 2013). Sufficient remyelination at the appropriate time is still considered the most effective strategy to alleviate MS. First, immune regulation alone is not sufficient to treat chronic neurological impairment, although lesion regeneration appears to be successful in MS (Michailidou et al. 2015). Second, MS impairment increases with age, whereas the ability to remyelinate decreases with age. Therefore, early promotion of remyelination is crucial (Brugarolas and Popko 2014). It is hypothesized that promoting remyelination and neuroprotection may be of great benefit in MS (El-Akabawy and Rashed 2015; Brown et al. 2014).
Thyroid hormones
Thyroid hormones and remyelination
Thyroid hormones (TH) act via nuclear receptors on several steps of oligodendrocyte development/myelination and play a positive role in normal myelination (Schoonover et al. 2004; Pagnin et al. 2021). In the presence of a mitogen, TH help OPCs trigger effector mechanism, including disruption of cell division and initiation of differentiation (Tokumoto et al. 2001). TH play an essential role in regulating the maturation of oligodendrocytes, the activation of OPCs, and the protection of the myelin sheath. In addition, TH protect the CNS thoughts immunoregulatory effects, including the gradual reduction of CD45+ microglial cells, IFN-γ reduction, and IL-10 stability following T3 treatment in mice (Harsan et al. 2008; Payghani et al. 2017). Indeed, there are several approaches to TH-induced remyelination, such as increasing neurotrophic factors, simulating oligodendrocyte production, and suppressing apoptosis (Marziali et al. 2015; Picou et al. 2012). Control of neurogenesis through the production of extracellular matrix proteins and growth factors to regulate astrocyte maturation, as well as expression of cytoskeletal proteins during axon growth and involvement in axon/oligodendrocyte interactions are other well-established functions of TH (Trentin 2006; Mohácsik et al. 2011; Talhada et al. 2019; Zendedel et al. 2016). In this regard, TH administration could increase the expression of the transcription factor Olig1 in the optic nerve and induce new OPCs. Similarly, TH restore the ability of mature oligodendrocytes to induce MBP and limit axonal damage in the white/Gy matter of the experimental autoimmune encephalomyelitis (EAE) model (Fernandez et al. 2004) (Fig. 1). In addition, some studies have pointed out the indirect effects of TH on remyelination. For example, it has been suggested that the beneficial effect of TH on MS pathophysiology may be due to its effects on growth hormones and their effectors (e.g. insulin-like growth factor (IGF-1)) by promoting neuron/oligodendrocyte survival and myelination process (Akcali et al. 2017). Harsan and colleagues applied a multidimensional technique to evaluate the effects of TH on endogenous stimulation of neuronal repair mechanisms in mice treated long-term with cuprizone. A significant loss in the subventricular zone (SVZ) and corpus callosum was reversed by TH treatment. It was also found that TH can promote the formation of OPCs and other stages of the oligodendrocyte lineage in chronic demyelination. In other words, TH treatment provides a favorable environment for the development of OPCs and regulates their differentiation (Harsan et al. 2008). These findings have been repeatedly confirmed in other studies (Castelo-Branco et al. 2014; Chaudhary et al. 2021; Zhang et al. 2015); Silvestroff et al. demonstrated that cortical lesions may also benefit from TH treatment by accelerating the cortical remyelination rate in Wistar rats after cuprizone intoxication (Silvestroff et al. 2012).
Is a TH agonist a potential therapeutic candidate for MS?
Current literature suggests that TH employ multiple mechanisms to combat MS progression, including regulation of extracellular glutamate and matrix metalloproteinase 9 (MMP-9) expression as well as regulation of vitamin D levels and oxidative stress (Tobore 2020). TH agonists (selective thyromimetic) have the potential to bind to the TH receptor (TR). Sobetirome, eprotirome, and resmetirom are just a few of the TR-selective T3 analogs that have been developed in the last two decades and has been shown to provide much of the therapeutic benefits of T3 without the negative side effects (Saponaro et al. 2020). Sobetirome is a selective thyromimetic with minimal side effects associated with hyperthyroidism and one of the few thyromimetic that cross the blood-brain barrier (BBB) and reach the CNS (Ferrara et al. 2017; Placzek et al. 2016). Sob-AM2, a CNS-selective prodrug of sobetirome, has been noted to support myelin regeneration. This effect has been associated with significant improvement in neurological clinical symptoms associated with demyelinating CNS diseases.
Melatonin
Antioxidant aspects of melatonin
Melatonin has a small, amphiphilic, and nonpolar molecular structure derived from the amino acid. It is secreted mainly by the pineal gland into the blood or cerebrospinal fluid and is important as an antioxidant in neurological diseases (Alghamdi 2018). Melatonin exerts multifunctional activities in various tissues (membranous or cytoplasmic) through receptor-dependent or receptor-independent mechanisms (Slominski et al. 2012). It deactivates free radical molecules and the hydroxyl anion radical, resulting in a cyclic 3-hydroxy-melatonin derivative that is excreted via the kidneys (Reiter et al. 2018). In in vivo/vitro models, anti-carcinogenic effects of melatonin have been reported, particularly in estrogen-responsive breast tumors (Menéndez-Menéndez and Martínez-Campa 2018).
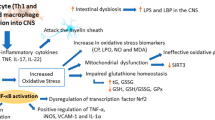
Pathological circumstances characterized by an unfavorable balance of free radicals and elimination mechanisms lead to “oxidative stress,” which increases oxidative damage to biomolecules (Sharifi-Rad et al. 2020). The CNS is extremely vulnerable to oxidative stress due to its increased oxygen consumption, which can increase the production of ROS. Melatonin is known to be a potent antioxidant in diseases associated with oxidative stress (e.g., MS, Alzheimer’s disease, and cancer). In addition, its protective effects including improving remyelination, mitochondrial function, and reducing inflammation in experimental MS models (e.g. IL-1, TNF) have attracted wide attention (Ghareghani et al. 2019). Melatonin directly inhibits mitochondrial dysfunction and ROS production (Kashani et al. 2014; Reiter et al. 2020). Furthermore, specific enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), and glutathione (GSH) are less abundant in the CNS than in other parts of the body (Salim 2017). Indirectly, melatonin as an antioxidant increases the function of these important enzymes (Adamczyk-Sowa et al. 2014) (Fig. 2). Notably, excessive neuroinflammation and oxidative stress have irreversible effects on biological energy. In advanced MS patients, the process that requires energy can be disrupted and lead to axonal degeneration. In particular, inhibition of demyelination and promotion of the enriched environment (ENR) process by adult oligodendrocytes may be effective in the treatment of MS (Kremer et al. 2019). Therefore, the balance between ROS production and the antioxidant activity of enzymes prevents the oxidative stress and increases mitochondrial function (Adamczyk and Adamczyk-Sowa 2016).
Anti-inflammatory effects of melatonin
There is a growing body of data suggesting that the overproduction of pro-inflammatory cytokines, ROS, and nitrogen species (e.g. nitric oxide, hydrogen peroxide, peroxynitrite, and hydroxyl radicals) as a result of T cell, macrophage, and astrocyte activity may be toxic to oligodendrocytes by contributing to demyelination in response to inflammatory stimuli (Wen et al. 2016; Li et al. 2011; Solleiro-Villavicencio and Rivas-Arancibia 2018). In vivo administration of high doses of melatonin stimulated the immune system by increasing regulatory T cell activity (Treg), triggering numerous humoral responses, and preventing age-related thymic involution (Shomali et al. 2021). In this context, a previous study by Pierpaoli and colleagues found that melatonin administration in vitro increased Treg/natural killer (NK) cells activity and IL-2/IFN-γ production in human monocytes (Pierpaoli and Regelson 1994). Melatonin could exert its therapeutic effects by increasing IL-10 levels in Treg cells and suppressing Th17 cell differentiation (Fig. 2). However, there is evidence that NAS (N acetyl serotonin) may be more effective than melatonin in the treatment process due to its better inhibitory effect on Th17 differentiation (Chen et al. 2016). During the relapse phase of MS, CD44 expression increases in circulating T cells. Melatonin has been shown to suppress CD44 expression in T-effector cells while increasing CD44 expression in Treg cells (Ren et al. 2017).
The low dose of melatonin serum levels and clinical signs (e.g. fatigue and sleep disturbance) of MS patients may indicate that melatonin could play an important role as a hormone candidate in MS therapy (Damasceno et al. 2015). In a randomized, placebo-controlled study, 36 individuals with RRMS were treated with interferon beta-1b (IFNβ-1b). The study aimed to find out how well melatonin affects serum pro-inflammatory cytokines and oxidative stress indicators in patients with MS. Melatonin (25 mg/d) was administered orally to the experimental group for 6 months. Thereafter, pro-inflammatory cytokine levels and oxidative stress markers were substantially decreased, suggesting that 6 months of melatonin treatment helps to reduce serum concentrations of pro-inflammatory cytokines and oxidative stress indicators in RRMS patients (Sánchez-López et al. 2018). Unexpectedly, a high dose of melatonin appears to have a pro-inflammatory effect (Escribano et al. 2022). However, these results encourage further research on the safety and efficacy of melatonin administration in MS patients. Moreover, gender might influence melatonin function during the remyelination phase. For example, male mice had a protective effect after melatonin treatment, whereas female mice showed no effect. These results suggest a complicated interplay between melatonin, remyelination, and sex hormones (Taleb and Alghamdi 2020).
Sex hormones
Imbalance of sex hormones in MS
Steroid hormones (divided into corticosteroids and sex steroids) can be evaluated from different points of view, of which “sexual dimorphism” in MS and corticosteroids as first-line therapy for relapses can be mentioned (Adhya et al. 2018; Ozakbas et al. 2017; Frye 2009). MS incidence is more common in young women (20–40 years). In fact, distinct sex differences include earlier disease onset in women and more frequent relapses in men, faster disease progression, and poorer prognosis in women (Coyle 2021). Genetically, sex is not the main factor, suggesting that environmental influences (e.g. unbalanced lifestyle, contraceptive use) contribute to a higher MS risk in women (Ysrraelit and Correale 2019). Puberty, pregnancy, puerperium, and menopause are all hormone-related physiological states in women that have a major impact on MS prevalence and prognosis. Therefore, hormonal and genetic variables are thought to play a key role in disease progression (Ysrraelit and Correale 2019). MS patients are more likely to suffer from physical disability during the premenstrual period than during the ovulatory period (Wingerchuk and Rodriguez 2006). Similarly, there are no sex differences in MS before puberty, although there is evidence that the age of menarche may be associated with the age of onset, further linking sex hormones to MS risk in childhood and adolescence (Jiang et al. 2018; Salpietro et al. 2018; Chitnis 2013).
The discovery of a decrease in relapse rates in women with MS during pregnancy has led to an increase in data supporting the anti-inflammatory effect of estrogens in MS, although the molecular mechanisms are still unknown (Simone et al. 2021). However, Rossi et al. (2018) mentioned that an increase in amino acid (AA) catabolism could be one of the reasons for autoimmunity prevention during pregnancy, which significantly decreases in the postpartum period (McGaha et al. 2012). In the postpartum period, there is a significant increase in relapse rates due to a sudden decrease in estrogen, progesterone, and glucocorticoids levels and a rapid return to pre-pregnancy immunological function (Abu-Raya et al. 2020). Indeed, the postpartum state has been described as an immune reconstitution-like inflammatory phenomenon caused by the comeback of pro-inflammatory cellular activity as a result of fetal/placental birth (Finkelsztejn et al. 2011). At the same time, the increase in hormones involved in breastfeeding processes triggered during this period may potentially contribute to increased disease activity (de Souza Costa et al. 2016). Little is known about the effects of menopause/andropause and hormone replacement therapy (HRT) on MS progression (Miller et al. 2014). Any consequences of hormonal changes during menopause/andropause may be masked by age-related changes in MS disease (Kim et al. 2021; Midaglia et al. 2020). For example, follow-up of patients with late-onset MS (beyond the age of 40) (LOMS) and young onset MS (18–40 years) (YOMS) found that male sex and older age had shortened time to EDSS 6.0 (Alroughani et al. 2016). It appears that andropause along with older age, has a stronger impact on MS progression compared with menopause (Fig. 3). However, MS incidence is higher in female LOMS patients (Naseri et al. 2021).
Immunoregulatory effects of sex hormones on MS
Knowing the immunoregulatory effect of sex hormones on MS, Benedek et al. undertook a concerted effort to elucidate an aspect of estrogen-induced immune regulation in EAE in which regulatory B cells were supported by estrogen-treated M2 macrophages in a feedback loop (Benedek et al. 2017b). In addition, the group investigated the effect of estrogen on gut microbiota composition in EAE mice and associated immune regulation (Benedek et al. 2017a). They found that estrogen prevented EAE-associated inflammation and could improve the immune regulation. Lanello et al. demonstrated that Th17 and Treg cell type-specific regulatory regions (CSRs) are controlled by estrogen receptor alpha (ERα). These CSRs were detected in polarized Th17 cells from healthy donors (HD) and in peripheral blood mononuclear cells, Th17, and Treg cells from MS patients during pregnancy (Iannello et al. 2019). Overall, estrogen is thought to immunomodulate the epigenome of CD4+ T cells in MS; the discovered CSRs could be used as biomarkers to track disease progression or as novel treatment targets.
The pregnancy hormone estriol may improve innate immune cell function by regulating Treg/Th17 balance, inhibiting neutrophil phagocytic activity, increasing indoleamine-2,3-dioxygenase (IDO) activity, decreasing IL-17-producing NK cells, and may play an indispensable role in the development of immune tolerance in the pathogenesis of MS (Nekrasova and Shirshev 2020; Robinson and Klein 2012). Neurosteroids are subtype steroids synthesized by CNS cells (e.g. oligodendrocytes, astrocytes, and microglia) to enhance myelination and anti-inflammatory effects. Decreased expression of the neurosteroids and related enzymes leads to increased expression of pro-inflammatory factors, oxidizing enzymes, and ultimately demyelination (Panzica et al. 2012; Porcu et al. 2016; Leicaj et al. 2018). Further investigations are needed to evaluate the major mechanisms of neurosteroids.
Conclusion
Hormonal dysfunction plays a fundamental role in the pathogenesis of MS disease. The beneficial effects of the most important hormones including thyroid, melatonin, and sex hormones are well-defined in MS experimental models. Besides, these hormones may ameliorate neurological impairment through immunoregulatory and neuroprotective effects, as well as by reducing oxidative stress. Especially, hormone therapy could improve immune system function (e.g., regulation of Treg/Th17 balance, inhibition of phagocytic activity, reduction of NK cells, and development of immune tolerance) in MS pathogenesis. However, the new window/mechanism between hormones and CNS cells in MS therapy needs to be deciphered. In conclusion, expanding the knowledge of hormone therapy may be an effective step towards identifying further molecular and cellular pathways.
Data availability
Not applicable.
Code availability
Not applicable.
References
Abu-Raya B, Michalski C, Sadarangani M, Lavoie PM (2020) Maternal immunological adaptation during normal pregnancy. Front Immunol 11:2627
Adamczyk B, Adamczyk-Sowa M (2016) New insights into the role of oxidative stress mechanisms in the pathophysiology and treatment of multiple sclerosis. Oxidative Med Cell Longev 2016:1973834
Adamczyk-Sowa M, Pierzchala K, Sowa P, Mucha S, Sadowska-Bartosz I, Adamczyk J, Hartel M (2014) Melatonin acts as antioxidant and improves sleep in MS patients. Neurochem Res 39(8):1585–1593
Adhya D, Annuario E, Lancaster MA, Price J, Baron-Cohen S, Srivastava DP (2018) Understanding the role of steroids in typical and atypical brain development: advantages of using a “brain in a dish” approach. J Neuroendocrinol 30(2):e12547
Akcali A, Bal B, Erbagci B (2017) Circulating IGF-1, IGFB-3, GH and TSH levels in multiple sclerosis and their relationship with treatment. Neurol Res 39(7):606–611
Albornoz EA, Carreño LJ, Cortes CM, Gonzalez PA, Cisternas PA, Cautivo KM, Catalán TP, Cecilia Opazo M, Eugenin EA, Berman JW (2013) Gestational hypothyroidism increases the severity of experimental autoimmune encephalomyelitis in adult offspring. Thyroid 23(12):1627–1637
Alghamdi BS (2018) The neuroprotective role of melatonin in neurological disorders. J Neurosci Res 96(7):1136–1149
Alroughani R, Akhtar S, Ahmed S, Behbehani R, Al-Hashel J (2016) Is time to reach EDSS 6.0 faster in patients with late-onset versus young-onset multiple sclerosis? PLoS One 11(11):e0165846
Azimzadeh M, Mahmoodi M, Kazemi M, Hakemi MG, Jafarinia M, Eslami A, Salehi H, Amirpour N (2020) The immunoregulatory and neuroprotective effects of human adipose derived stem cells overexpressing IL-11 and IL-13 in the experimental autoimmune encephalomyelitis mice. Int Immunopharmacol 87:106808
Azimzadeh M, Möhn N, Ezabadi SG, Esfandabadi ZM, Soleimani A, Ranjbar E, Jahromi M, Seyedebrahimi R, Skripuletz T, Kasmaie FM (2021) The immunological therapeutic strategies for controlling multiple sclerosis: considerations during the covid-19 pandemic. Biomolecules 11(9):1372
Benedek G, Zhang J, Nguyen H, Kent G, Seifert HA, Davin S, Stauffer P, Vandenbark AA, Karstens L, Asquith M (2017a) Estrogen protection against EAE modulates the microbiota and mucosal-associated regulatory cells. J Neuroimmunol 310:51–59
Benedek G, Zhang J, Nguyen H, Kent G, Seifert H, Vandenbark AA, Offner H (2017b) Novel feedback loop between M2 macrophages/microglia and regulatory B cells in estrogen-protected EAE mice. J Neuroimmunol 305:59–67
Brown RA, Narayanan S, Arnold DL (2014) Imaging of repeated episodes of demyelination and remyelination in multiple sclerosis. NeuroImage: Clin 6:20–25
Brugarolas P, Popko B (2014) Remyelination therapy goes to trial for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 1(2):e26
Castelo-Branco G, Stridh P, Guerreiro-Cacais AO, Adzemovic MZ, Falcão AM, Marta M, Berglund R, Gillett A, Hamza KH, Lassmann H (2014) Acute treatment with valproic acid and l-thyroxine ameliorates clinical signs of experimental autoimmune encephalomyelitis and prevents brain pathology in DA rats. Neurobiol Dis 71:220–233
Chaudhary P, Marracci GH, Calkins E, Pocius E, Bensen AL, Scanlan TS, Emery B, Bourdette DN (2021) Thyroid hormone and thyromimetics inhibit myelin and axonal degeneration and oligodendrocyte loss in EAE. J Neuroimmunol 352:577468
Chen S-J, Huang S-H, Chen J-W, Wang K-C, Yang Y-R, Liu P-F, Lin G-J, Sytwu H-K (2016) Melatonin enhances interleukin-10 expression and suppresses chemotaxis to inhibit inflammation in situ and reduce the severity of experimental autoimmune encephalomyelitis. Int Immunopharmacol 31:169–177
Chitnis T (2013) Role of puberty in multiple sclerosis risk and course. Clin Immunol 149(2):192–200
Coyle PK (2021) What can we learn from sex differences in MS? J Pers Med 11(10):1006
Damasceno, Alfredo, Adriel Santos Moraes, Alessandro Farias, Benito Pereira Damasceno, Leonilda Maria Barbosa dos Santos, and Fernando Cendes (2015) Disruption of melatonin circadian rhythm production is related to multiple sclerosis severity: a preliminary study. J Neurol Sci 353(1–2):166–168
de Souza Costa TM, de Souza Neto VL, da Cruz Domingos MM, da Silva BCO, Rodrigues IDCV, da Silva RAR (2016) A profile of nursing diagnoses in patients with multiple sclerosis: a cross-sectional study. Online Braz J Nurs 15(3):433–442
Deckx N, Lee W-P, Berneman ZN, Cools N (2013) Neuroendocrine immunoregulation in multiple sclerosis. Clin Dev Immunol 2013:705232
Dutta R, Trapp BD (2011) Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol 93(1):1–12
Eghbal M, Eftekhari A, Ahmadian E, Azarmi Y, Parvizpur A (2016) A review of biological and pharmacological actions of melatonin: oxidant and prooxidant properties. J Pharmacol Rep 1(106):2
El-Akabawy G, Rashed LA (2015) Beneficial effects of bone marrow-derived mesenchymal stem cell transplantation in a non-immune model of demyelination. Annals Anatomy-Anatomischer Anzeiger 198:11–20
Escribano BM, Muñoz-Jurado A, Caballero-Villarraso J, Valdelvira ME, Giraldo AI, Paz-Rojas E, Gascón F, Santamaría A, Agüera E, Túnez I (2022) Protective effects of melatonin on changes occurring in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Mult Scler Relat Disord 58:103520
Eslami A, Dehbashi M, Ashja-Arvan M, Salehi H, Azimzadeh M, Ganjalikhani-Hakemi M (2020) Assessment of ability of human adipose derived stem cells for long term overexpression of IL-11 and IL-13 as therapeutic cytokines. Cytotechnology 72(5):773–784
Fernandez M, Giuliani A, Pirondi S, D'Intino G, Giardino L, Aloe L, Levi-Montalcini R, Calzà L (2004) Thyroid hormone administration enhances remyelination in chronic demyelinating inflammatory disease. Proc Natl Acad Sci 101(46):16363–16368
Ferrara SJ, Matthew Meinig J, Placzek AT, Banerji T, McTigue P, Hartley MD, Sanford-Crane HS, Banerji T, Bourdette D, Scanlan TS (2017) Ester-to-amide rearrangement of ethanolamine-derived prodrugs of sobetirome with increased blood-brain barrier penetration. Bioorg Med Chem 25(10):2743–2753
Finkelsztejn A, Brooks JBB, Paschoal FM Jr, Fragoso YD (2011) What can we really tell women with multiple sclerosis regarding pregnancy? A systematic review and meta-analysis of the literature. BJOG Int J Obstet Gynaecol 118(7):790–797
Frye CA (2009) Steroids, reproductive endocrine function, and affect. A review. Minerva Ginecol 61(6):541–562
Ghareghani M, Scavo L, Jand Y, Farhadi N, Sadeghi H, Ghanbari A, Mondello S, Arnoult D, Gharaghani S, Zibara K (2019) Melatonin therapy modulates cerebral metabolism and enhances remyelination by increasing PDK4 in a mouse model of multiple sclerosis. Front Pharmacol 10:147
Harsan L-A, Steibel J, Zaremba A, Agin A, Sapin R, Poulet P, Guignard B, Parizel N, Grucker D, Boehm N (2008) Recovery from chronic demyelination by thyroid hormone therapy: myelinogenesis induction and assessment by diffusion tensor magnetic resonance imaging. J Neurosci 28(52):14189–14201
Iannello A, Rolla S, Maglione A, Ferrero G, Bardina V, Inaudi I, De Mercanti S, Novelli F, D'Antuono L, Cardaropoli S (2019) Pregnancy epigenetic signature in T helper 17 and T regulatory cells in multiple sclerosis. Front Immunol 9:3075
Jiang X, Olsson T, Alfredsson L (2018) Age at menarche and risk of multiple sclerosis: current progress from epidemiological investigations. Front Immunol 9:2600
Kamel FO (2019) Factors involved in relapse of multiple sclerosis. J Microscopy Ultrastruct 7(3):103
Kashani IR, Rajabi Z, Akbari M, Hassanzadeh G, Mohseni A, Eramsadati MK, Rafiee K, Beyer C, Kipp M, Zendedel A (2014) Protective effects of melatonin against mitochondrial injury in a mouse model of multiple sclerosis. Exp Brain Res 232(9):2835–2846
Kim YJ, Soto M, Branigan GL, Rodgers K, Brinton RD (2021) Association between menopausal hormone therapy and risk of neurodegenerative diseases: Implications for precision hormone therapy. Alzheimer's & Dementia: Translational Research & Clinical Interventions, 7:e12174
Kremer D, Akkermann R, Küry P, Dutta R (2019) Current advancements in promoting remyelination in multiple sclerosis. Mult Scler J 25(1):7–14
Leicaj ML, Pasquini LA, Lima A, Gonzalez MC, Deniselle JM, Pasquini AF, Nicola D, Garay LI (2018) Changes in neurosteroidogenesis during demyelination and remyelination in cuprizone-treated mice. J Neuroendocrinol 30(11):e12649
Li S, Lin W, Tchantchou F, Lai R, Wen J, Zhang Y (2011) Protein kinase C mediates peroxynitrite toxicity to oligodendrocytes. Mol Cell Neurosci 48(1):62–71
Lubetzki C, Zalc B, Williams A, Stadelmann C, Stankoff B (2020) Remyelination in multiple sclerosis: from basic science to clinical translation. Lancet Neurol 19(8):678–688
Marziali LN, Garcia CI, Pasquini JM (2015) Transferrin and thyroid hormone converge in the control of myelinogenesis. Exp Neurol 265:129–141
McGaha TL, Huang L, Lemos H, Metz R, Mautino M, Prendergast GC, Mellor AL (2012) Amino acid catabolism: a pivotal regulator of innate and adaptive immunity. Immunol Rev 249(1):135–157
Menéndez-Menéndez J, Martínez-Campa C (2018) Melatonin: an anti-tumor agent in hormone-dependent cancers. Int J Endocrinol 2018:3271948
Michailidou I, de Vries HE, Hol EM, van Strien ME (2015) Activation of endogenous neural stem cells for multiple sclerosis therapy. Front Neurosci 8:454
Midaglia L, Otero S, Baró F, Montalban X, Tintoré M (2020) Menopause and multiple sclerosis: influence on prognosis and role of disease-modifying drugs and hormonal replacement therapy. Mult Scler J 28(2):173–182
Miller DH, Fazekas F, Montalban X, Reingold SC, Trojano M (2014) Pregnancy, sex and hormonal factors in multiple sclerosis. Mult Scler J 20(5):527–536
Mohácsik P, Zeöld A, Bianco AC, Gereben B (2011) Thyroid hormone and the neuroglia: both source and target. J Thyroid Res 2011: 215718
Naseri A, Nasiri E, Sahraian MA, Daneshvar S, Talebi M (2021) Clinical features of late-onset multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord 50:102816
Nekrasova I, Shirshev S (2020) Estriol in regulation of cell-mediated immune reactions in multiple sclerosis. J Neuroimmunol 349:577421
Ozakbas S, Cinar BP, Kosehasanoğullari G, Kahraman T, Oz D, Kursun BB (2017) Monthly methylprednisolone in combination with interferon beta or glatiramer acetate for relapsing-remitting multiple sclerosis: a multicentre, single-blind, prospective trial. Clin Neurol Neurosurg 160:69–72
Pagnin M, Kondos-Devcic D, Chincarini G, Cumberland A, Richardson SJ, Tolcos M (2021) Role of thyroid hormones in normal and abnormal central nervous system myelination in humans and rodents. Front Neuroendocrinol 61:100901
Panzica GC, Jacques Balthazart CA, Frye LM, Garcia-Segura AEH, Mensah-Nyagan AG, McCarthy MM, Melcangi RC (2012) Milestones on steroids and the nervous system: 10 years of basic and translational research. J Neuroendocrinol 24(1):1–15
Payghani C, Khani F, Zadeh AR, Reisi P, Alaei H, Rashidi B (2017) The effect of levothyroxine on serum levels of interleukin 10 and interferon-gamma in rat model of multiple sclerosis. Adv Biomed Res 6:118
Pegoretti V, Swanson KA, Bethea JR, Probert L, Eisel ULM, Fischer R (2020) Inflammation and oxidative stress in multiple sclerosis: consequences for therapy development. Oxidative Med Cell Longev 2020:7191080
Picou F, Fauquier T, Chatonnet F, Flamant F (2012) A bimodal influence of thyroid hormone on cerebellum oligodendrocyte differentiation. Mol Endocrinol 26(4):608–618
Pierpaoli W, Regelson W (1994) Pineal control of aging: effect of melatonin and pineal grafting on aging mice. Proc Natl Acad Sci 91(2):787–791
Placzek AT, Ferrara SJ, Hartley MD, Sanford-Crane HS, Matthew Meinig J, Scanlan TS (2016) Sobetirome prodrug esters with enhanced blood–brain barrier permeability. Bioorg Med Chem 24(22):5842–5854
Porcu P, Barron AM, Frye CA, Walf AA, Yang S-Y, He X-Y, Morrow AL, Panzica GC, Melcangi RC (2016) Neurosteroidogenesis today: novel targets for neuroactive steroid synthesis and action and their relevance for translational research. J Neuroendocrinol 28(2):12351
Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Zhou XJ, Bing X (2018) Mitochondria: central organelles for melatonin′ s antioxidant and anti-aging actions. Molecules 23(2):509
Reiter RJ, Ma Q, Sharma R (2020) Melatonin in mitochondria: mitigating clear and present dangers. Physiology 35(2):86–95
Ren W, Liu G, Chen S, Yin J, Wang J, Tan B, Guoyao W, Bazer FW, Peng Y, Li T (2017) Melatonin signaling in T cells: functions and applications. J Pineal Res 62(3):e12394
Rinaldi F, Calabrese M, Grossi P, Puthenparampil M, Perini P, Gallo P (2010) Cortical lesions and cognitive impairment in multiple sclerosis. Neurol Sci 31(2):235–237
Robinson DP, Klein SL (2012) Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav 62(3):263–271
Rossi C, Cicalini I, Zucchelli M, Di Ioia M, Onofrj M, Federici L, Del Boccio P, Pieragostino D (2018) Metabolomic signature in sera of multiple sclerosis patients during pregnancy. Int J Mol Sci 19(11):3589
Saab AS, Tzvetanova ID, Nave K-A (2013) The role of myelin and oligodendrocytes in axonal energy metabolism. Curr Opin Neurobiol 23(6):1065–1072
Salim S (2017) Oxidative stress and the central nervous system. J Pharmacol Exp Ther 360(1):201–205
Salpietro V, Polizzi A, Recca G, Ruggieri M (2018) The role of puberty and adolescence in the pathobiology of pediatric multiple sclerosis. Mult Scler Demyelinating Disord 3(1):1–10
Sánchez-López AL, Ortiz GG, Pacheco-Moises FP, Mireles-Ramírez MA, Bitzer-Quintero OK, Delgado-Lara DLC, Ramírez-Jirano LJ, Velázquez-Brizuela IE (2018) Efficacy of melatonin on serum pro-inflammatory cytokines and oxidative stress markers in relapsing remitting multiple sclerosis. Arch Med Res 49(6):391–398
Saponaro F, Sestito S, Runfola M, Rapposelli S, Chiellini G (2020) Selective thyroid hormone receptor-beta (TRβ) agonists: new perspectives for the treatment of metabolic and neurodegenerative disorders. Front Med 7:331
Schoonover CM, Seibel MM, Jolson DM, Stack MJ, Rahman RJ, Jones SA, Mariash CN, Anderson GW (2004) Thyroid hormone regulates oligodendrocyte accumulation in developing rat brain white matter tracts. Endocrinology 145(11):5013–5020
Sharifi-Rad M, Anil NV, Kumar PZ, Varoni EM, Dini L, Panzarini E, Rajkovic J, Fokou PVT, Azzini E, Peluso I (2020) Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front Physiol 11:694
Shimba A, Ejima A, Ikuta K (2021) Pleiotropic effects of glucocorticoids on the immune system in circadian rhythm and stress. Front Immunol 12:4217
Shomali A, Aliniaeifard S, Didaran F, Lotfi M, Mohammadian M, Seif M, Strobel WR, Sierka E, Kalaji HM (2021) Synergistic effects of melatonin and gamma-aminobutyric acid on protection of photosynthesis system in response to multiple abiotic stressors. Cells 10(7):1631
Silvestroff L, Bartucci S, Pasquini J, Franco P (2012) Cuprizone-induced demyelination in the rat cerebral cortex and thyroid hormone effects on cortical remyelination. Exp Neurol 235(1):357–367
Simone IL, Tortorella C, Ghirelli A (2021) Influence of pregnancy in multiple sclerosis and impact of disease-modifying therapies. Front Neurol 12:697974
Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT (2012) Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol 351(2):152–166
Solleiro-Villavicencio H, Rivas-Arancibia S (2018) Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4+ T cells in neurodegenerative diseases. Front Cell Neurosci 12:114
Taleb, Hanin Abdulbaset Abo, and Badrah Saeed Alghamdi (2020) Neuroprotective effects of melatonin during demyelination and remyelination stages in a mouse model of multiple sclerosis. J Mol Neurosci 70(3):386–402
Talhada D, Santos CRA, Gonçalves I, Ruscher K (2019) Thyroid hormones in the brain and their impact in recovery mechanisms after stroke. Front Neurol 10:1103
Tan D-X, Manchester LC, Qin L, Reiter RJ (2016) Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int J Mol Sci 17(12):2124
Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O (2018) Multiple sclerosis. Lancet 391:1622–1636
Tobore TO (2020) Towards a comprehensive etiopathogenetic and pathophysiological theory of multiple sclerosis. Int J Neurosci 130(3):279–300
Tokumoto YM, Tang DG, Raff MC (2001) Two molecularly distinct intracellular pathways to oligodendrocyte differentiation: role of a p53 family protein. EMBO J 20(18):5261–5268
Trapp BD, Nave K-A (2008) Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci 31:247–269
Trentin AG (2006) Thyroid hormone and astrocyte morphogenesis. J Endocrinol 189(2):189–197
Vucic S, Burke T, Lenton K, Ramanathan S, Gomes L, Yannikas C, Kiernan MC (2012) Cortical dysfunction underlies disability in multiple sclerosis. Mult Scler J 18(4):425–432
Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der Mei I (2020) Rising prevalence of multiple sclerosis worldwide: insights from the atlas of MS. Mult Scler J 26(14):1816–1821
Wen J, Ariyannur PS, Ribeiro R, Tanaka M, Moffett JR, Kirmani BF, Namboodiri AMA, Zhang Y (2016) Efficacy of N-acetylserotonin and melatonin in the EAE model of multiple sclerosis. J NeuroImmune Pharmacol 11(4):763–773
Wingerchuk DM, Rodriguez M (2006) Premenstrual multiple sclerosis pseudoexacerbations: role of body temperature and prevention with aspirin. Arch Neurol 63(7):1005–1008
Ysrraelit MC, Correale J (2019) Impact of sex hormones on immune function and multiple sclerosis development. Immunology 156(1):9–22
Zendedel A, Kashani IR, Azimzadeh M, Pasbakhsh P, Omidi N, Golestani A, Beyer C, Clarner T (2016) Regulatory effect of triiodothyronine on brain myelination and astrogliosis after cuprizone-induced demyelination in mice. Metab Brain Dis 31(2):425–433
Zhang M, Zhan XL, Ma ZY, Chen XS, Cai QY, Yao ZX (2015) Thyroid hormone alleviates demyelination induced by cuprizone through its role in remyelination during the remission period. Exp Biol Med 240(9):1183–1196
Author information
Authors and Affiliations
Contributions
A.S, S.G: Wrote the manuscript. Z.M: Contributed to the literature search and editing of the manuscript. N.M, Z.K, T.S: Revised the manuscript. M.A: Supervised the manuscript preparation. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article contains no original data from studies with human participants or animals performed by any of the authors, apart from the data already published and quoted herein.
Conflicts of interest/Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soleimani, A., Ezabadi, S.G., Möhn, N. et al. Influence of hormones in multiple sclerosis: focus on the most important hormones. Metab Brain Dis 38, 739–747 (2023). https://doi.org/10.1007/s11011-022-01138-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01138-7