Abstract
Adipose-derived stem cells (ADSCs) are a type of mesenchymal stem cells with the therapeutic effects that make them one of the best sources for cell therapy. In this study, we aimed to assess the ability of human ADSCs for constant expression of IL-11 and IL-13, simultaneously. In this study, the characterized hADSCs were transduced with a lentiviral vector (PCDH-513B) containing IL-11 and IL-13 genes, and the ability of long-term expression of the transgenes was evaluated by ELISA technique on days 15, 45 and 75 after transduction. Our results indicated a high rate of transduction (more than 90%) in the isolated hADSCs. Our data showed the highest rate of expression on days 75 after transduction which was 242.67 pg/ml for IL-11 and 303.6 pg/ml for IL-13 compared with 35.2 pg/ml and 35.6 pg/ml in untreated cells, respectively (p = 0.001). Besides, MTT assay showed transduction of hADSCs with lentiviral viruses containing IL-11 and IL-13 had no adverse effect on hADSCs proliferation (p-value = 0.89). Finally, we successfully constructed a hADSC population stably overexpressing IL-11 as the neurotrophic cytokine and IL-13 as the anti-inflammatory cytokine and this transduced cells can be used for further studies in EAE mice model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mesenchymal stem cells (MSCs) as multipotent stem cells can be derived from various sources including bone marrow, amniotic fluid, dental caries, adipose tissue, umbilical cord, synovial membranes, and peripheral blood (Dargahi et al. 2017). Adipose mesenchymal stem cells or adipose-derived stem cells (ADSCs) are a type of MSCs that are an appropriate candidate for cell therapy in some disorders such as Multiple sclerosis (MS) (Kimiskidis and Fassas 2013; Constantin et al. 2009). ADSCs can migrate to the inflammatory site across the blood–brain barrier (BBB) to release anti-inflammatory cytokines that are involved in remyelination. They also stimulate the proliferation and survival of nerve cells (Ghasemi 2015). One of the limitations of stem cells transplantation is graft-versus-host disease (GVHD) via stimulation of immune cells but ADSCs express a relatively low level of MHC-I and MHC-II and they don’t express co-stimulatory molecules such as CD40, CD40L, CD80, and CD86 and induce anergy in T lymphocytes (Winkelmann et al. 2014; Machado et al. 2013). As mentioned the advantages of ADSCs make them an appropriate candidate for the treatment of various diseases such as autoimmune disease (Maria et al. 2017). Multiple sclerosis (MS) is an autoimmune disease that affects the central nervous system. Activation of T lymphocyte against auto-antigens such as myelin sheath antigens leads to malignant neuronal damage and myelin deficiency. Also, the infiltration of lymphocytes and macrophages, as well as the death of oligodendrocytes are the main causes of myelin destruction in MS (McLaughlin and Wucherpfennig 2008; Patel and Balabanov 2012). Common therapies for MS such as interferon beta, glutylamer acetate, fingolimod, triplonomide, dimethyl fumarate, mitoxantrone, and natalizumab usually act as anti-inflammatory and immunosuppressive drugs and do not promote remyelination. Thus, these drugs are not capable of ameliorating the neuronal damages in MS patients (Tavazzi et al. 2014). Besides, a long period of administration and complications during the treatment are some of the problems in the application of these drugs.
Current therapy for MS often targets inflammation and cannot directly stop the degeneration of nerve tissue. So using stem cells with the ability of regulation neural immunity and neuroprotection can be a promising treatment for MS (Regmi et al. 2019). Including stem cells with these properties are ADSCs that can be genetically engineered to enhance their anti-inflammatory and neurotrophic properties (Vizoso et al. 2017). One of the anti-inflammatory cytokines is interleukin (IL)-13, which produced by TH2, microglia, and neurons. IL-13 reduces the production of TH1 inflammatory cytokines and stimulates the activation of microglia and protective macrophages (M2a) (Sochocka et al. 2017). In addition, IL-13 inhibits severe loss of oligodendrocytes and demyelination process in MS mice models (Way et al. 2015). In a study was done by Dooley et al. mesenchymal stem cell (MSCs) carrying IL-13 transferred to the injured spinal cord mice and converted macrophages phenotype to M2a followed by reduced the number of axon-invading macrophages (Dooley et al. 2016).
IL-11 is a member of the CNTF family (ciliary neurotrophic factor) with a neuro-regenerative effect which is derived from astrocytes (Liu et al. 2007). In vitro and in vivo studies have shown that IL-11 improves the survival and maturation of oligodendrocytes. It has been reported that the IL-11 receptor expressed on the oligodendrocytes and administration of recombinant IL-11 reduces the clinical neuropathy of experimental autoimmune encephalomyelitis (EAE) (Gurfein et al. 2009; Maheshwari et al. 2013).
Previous studies have shown that IL-11 significantly reduces the production of activated T-cell cytokines and decreases the apoptosis of Oligodendrocyte Precursor Cells (OPCs) leading to increased mitosis in OPCs (Peferoen et al. 2014). IL-11 also inhibits CD11c+ dendritic cells and reduces CD4+ (TH1/TH17) lymphocytes, which play an important role in the pathogenesis of MS (Zhang et al. 2011). Therefore, we suggested that using the stem cells derived from adipose tissue engineered by IL-13 as an anti-inflammatory, and IL-11 as a neurotrophic cytokine can be a promising candidate for the treatment of MS. In the current study, we overexpressed IL-13 and IL-11 genes in hADSCs via a lentiviral vector and evaluated the ability of transduced cells for the simultaneous expression of these cytokines for a long period.
Materials and methods
Ethics approval and consent
Adipose tissue was obtained from adult women who were subjected for liposuction surgery with informed consents. This study was performed following the Declaration of Helsinki for ethics in medical researches and ethics committee of Isfahan University of Medical Sciences (Association GA 2014) (No.IR.MUI.MED.REC.1397.026).
Bioinformatics designing of the cytokines expressing construct
To make a construct expressing human IL-11 and IL-13 cytokines, firstly, their protein sequences were retrieved from http://www.uniprot.org (P01574 and P15018, respectively) and converted to the DNA sequence by EMBOSS Backtranseq (http://www.ebi.ac.uk/Tools/st/emboss_backtranseq/). DNA sequences were confirmed using NCBI refseq (http://www.ncbi.nlm.nih.gov) database.
Then, E2A sequence was incorporated between the IL-11 and IL-13 sequences as the ribosome jumping element. Kozak sequence (GCCACC) was inserted at the 5′ end of the construct to enhance expression of transgenes. In addition, in order to end of translation, TAG sequence was added at the 3′ end of the construct. After checking the restriction maps using NEBcutter2 (http://nc2.neb.com/NEBcutter2/), XbaI (5′ end) and NotI (3′ end) were selected as restriction enzymes, and their relevant cutting sites were designed at the two ends of the construct.
The construct sequence was ordered to be synthesized by General Biosystem Company (United States) based on pCDH-513B (System Bioscience, Mountain View, CA, United States) as the transfer vector.
Separation and expansion of human adipose-derived mesenchymal stem cells
Adipose tissues were washed with phosphate buffer (PBS) containing 1% penicillin/streptomycin and spliced to very small pieces by mechanical method. Then, chemical digestion was done using 0.075% collagenase type I (Sigma-Aldrich, USA) in PBS buffer at 37 °C for 30 min. Next, the suspension was centrifuged for 10 min at 1800 rpm and after pouring out the supernatant, hADSCs were isolated from cells precipitate. Isolated hADSCs were cultured in DMEM (low glucose Sigma-Aldrich, USA) medium containing 1% penicillin/streptomycin and 10% fetal bovine serum (FBS) (Wang et al. 2017). After 24 h, the medium was refreshed and dead cells were removed. After 3 days when the cells reached 85% cellular confluency, sub-culture was performed for adherent cells. During 2 weeks, the cells were reached the third passage and used for subsequent experiments.
Identification hADSCs using flow cytometry
Briefly, 1 × 105 of isolated hADSCs in the third passage and transduced hADSCs in the fifth passage were studied for expressing the specific surface markers of the mesenchymal stem cells. Detached cells were suspended in 500 μl PBS and staining was done using 2 μl of monoclonal antibodies against CD45-PerCP and CD14-PerCP as negative markers and CD73-FITC and CD105- PE as positive markers. Then, incubation was done in 4 °C for 30 min. Finally, stained cells were evaluated by The FACS Calibur flow cytometer (BD biosciences, USA), and data were analyzed by Flowjo software (Trees tar Inc., USA).
Differentiation of ADSCs
We evaluated the ability of hADSC to differentiate into osteocytes and adipocytes before and after transduction. In order to differentiate into adipocytes, 2 × 104 cells were seeded in 24-well-plates in presence of 5 Mm insulin (Sigma-Aldrich, St. Louis, MO) and 10 M dexamethasone. Then, Oil red O staining was performed after 21 days cell culture.
For differentiation of hADSC into osteocytes, 2 × 104 cells were seeded in 24-well-plates in presence of 10 mM sodium β-glycerophosphate, 50 µg/ml ascorbic acid and 10 M dexamethasone. Then, Alizarin Red staining was performed after 21 days of cell culture (Ciuffreda et al. 2016).
Lentivirus production
In this study, pCDH-513B-1, a HIV-1-based VSV-G pseudotyped lentiviral vector (System Biosciences, USA) containing cPPT-CMV-GFP-eF1-pur was used as the transfer vector. The ps-PAX2.2 (Addgene, USA) and pMD2.G (Addgene, USA) were applied as packaging and envelope vector, respectively. Lentivirus production was performed based on Prof. Trono lab protocols with modifications (Trono lab) (Klages et al. 2000). Three plasmids were co-transfected into the Lenti-X 293T as packaging cell line by the calcium phosphate precipitation procedure. Briefly, 2 × 106 Lenti-X 293T cells were seeded into the T75 flask in high glucose DMEM medium containing 10% FBS and 1% penicillin/streptomycin to achieve the confluency of 70–80%. After 24 h, the medium was replaced with 5 ml of fresh medium. Then, three vectors including 21 µg transfer vector, 21 µg of psPAX, and 10.5 µg pMD2.G were added to 11 μLTE (1×), 35 μL CaCl2 (2.5 M) and 350 μL HBSS 2× (HEPES-Buffered Saline Solution). Afterward, buffered water added to reach the total volume of 251.5 µL and finally added to the medium. The medium was replaced with 8 ml of fresh medium containing 10% FBS 14–16 h post-transfection. The supernatant was collected every 8 to 12 h up to 3 days.
Concentration and titration of lentiviral vectors
Collected supernatants were centrifuged for 5 min at 1500 rpm and filtration was done using 0.45 μm filter to remove cell debris. Pooled supernatant containing virus particles was concentrated by PEG-8000 based on Tronolab protocols with modifications (Klages et al. 2000). Following the concentration of viruses by PEG8000, 6 × 104 Lenti-X 293T cells were seeded in 24-well culture plate in 250 µl high glucose DMEM medium containing 10% FBS and 1% penicillin/streptomycin. After 24 h, 1 µl, 4 µl and 16 µl of concentrated viruses were added into each well. The determination of viral titration based on the expression level of green fluorescent protein (GFP) was performed using flow cytometry method 72 h After transduction. All steps were previously described in details Tronolab protocols (Klages et al. 2000; Barde et al. 2010).
Transduction and selection of hADSCs
Characterized hADSC were seeded at a density of 6 × 104 cells in 6-well culture plate in 2 ml low glucose DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin and 6 µg/ml polybrene. After 24 h, virus particles were added to cultured cells with a multiplicity of infection (MOI) of 100. Then, the plate was centrifuged at 2000 rpm for 1 h at 25 °C and incubated in a CO2 incubator overnight. Finally, transduced cells expressing GFP were elevated by using fluorescence microscopy and flow cytometry. In order to select transduced stem cells, 2 µg/ml puromycin was added to transduced cells which contained the puromycin resistance gene. Finally, selected cells were elevated for GFP expressing (Zuk 2011). In this study, we confirmed our results by comparing them with two control groups, including “untreated cells” which refers to hADSCs without lentiviral vector, and “mock” which refers to hADSCs transduced with lentiviral vector without the transgene construct.
MTT assay
Transduced hADSCs were cultured in 96 well culture plate at a density of 8 × 103 cells per well containing 200µL low glucose DMEM medium supplemented by 10% FBS and 1% penicillin/streptomycin and incubated for 24 h in a CO2 condition. Then, cultured cells were incubated in 37 °C for 4 h in the presence of 5 mg/ml MTT dye (Sigma Aldrich, USA). The reaction was stopped by adding 200µL DMSO (Sigma Aldrich, USA) and absorbance was read in 570 nm by a microplate reader (Bio-Rad, USA).
Polymerase chain reaction (PCR)
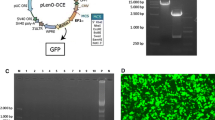
To assure of the presence of the transgene in hADSCs, the PCR technique was performed by using of the specific primer including: 5′-GCCATCCACGCTGTTTTGAC3′ as the forward primer which designed based on the vector backbone sequence and 5′-TCAGCAGGGCGTAGTTGGT3′ as the reverse primer which designed for a part of the transgene. Altogether DNA was extracted by GENET BIO kit (South Korea) and PCR was performed in 30 cycles at 95 °C for 20 s, 60 °C for 40 s and 72 °C for 1 min with an initial holding at 95 °C for 2 min. The PCR product was run on 1% agarose gel for further assessment.
SDS-PAGE
To investigate and confirm the overexpression of IL-11 and IL-13 by transduced hADSC cells, SDS-PAGE was performed on a 12% polyacrylamide gel with collected supernatants at 15, 45, and 75 days post transduction. Then, protein bands were visible with silver nitrate staining.
Enzyme-linked immunosorbent assay (ELISA)
Transduced ADSCs supernatants were collected at 15, 45, and 75 days post transduction to assure the secretion of IL-11 and IL-13 cytokines, and ELISA technique was performed with Human IL-11 Human IL-13 ELISA Kits (R&D systems, USA) according to the manufacturer’s instructions.
Statistical analysis
In the present study, all experiments were performed independently as three identical repeats and the data are presented as their mean ± SD. Statistical analyses were performed using SPSS 20 software (SPSS Company, Chicago, IL, USA) and charts were designed with GraphPad Prism 7 (GraphPad, San Diego, CA). The probability of normal distribution of data was accessed with Kolmogorov–Smirnov and Shapiro tests and groups comparisons were done with the one-way ANOVA test and p-value < 0.05 was considered as statistical significance.
Results
Confirmation of hADSCs identity
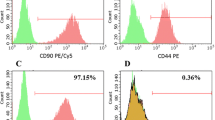
The hADSCs were evaluated for the expression of CD45, CD14, CD73 and CD105 markers before and after transduction with the flow cytometry method. Based on the obtained results from the freshly isolated stem cells, 90.1 and 62.2% of the cells were positive for CD73 and CD105, respectively, while only 5.98% of the cells were positive for CD45 and CD14. For transducted hADSCs the expression levels of CD73 and CD105 were 81.5 and 57.6, respectively, while expression level of CD45 and CD14 was only 1.493% (Fig. 1, Part I).
I Flow cytometric analysis. (A) Flow cytometry analysis for untreated hADSCs expressed high level of CD73 and CD105 as positive markers of and low level of CD45 and CD14 as negative markers of mesenchymal stem cells. (B) Flow cytometry analysis for transducted hADSCs showed that the transduced cell still was positive for CD73 and CD105 and negative for CD45 and CD14. II assessment of differentiation potential of human Adipose-derived stem cells (hADSCs) before and after transduction. A) The osteogenic differentiation potential of untreated and transduced hADSCs (A1 and A2, respectively) after 21 days culture in specific medium was confirmed with calcium deposition which stained with Alizarin red. A3) control group (100X magnification). B) The adipogenic The differentiation potential of untreated and transduced hADSCs (B1 and B2, respectively) after 21 days culture in specific medium was confirmed with lipid droplets which stained with Oil Red O (100X magnification)
Differentiation potential of hADSCs
Transduced and un-transduced hADSCs were cultured in a specific media in order to differentiate to osteocytes and adipocytes for 21 days. Differentiation into osteocytes and adipocytes lineages was successfully performed as confirmed by specific staining of calcium deposits with Alizarin Red and lipid vacuoles with Oil Red O. (Fig. 1, Part II).
Transduction of hADSCs with lentiviral vector expressing IL-11 and IL-13
Following co-transfection of the three vectors, the transfection efficiency was studied with a fluorescence microscope (brand, country), and a high level of green irradiation was observed (Fig. 2, Part I). After the concentration of viruses by PEG8000, the viral titration was measured by flow cytometry method. Based on the flow cytometry analysis, 13.1% of the Lenti-X 293T cells were successfully transduced by the virus (Figure x) and the titer of virus was calculated as a 625 × 105 TU/ml (Fig. 2, Part II).
I The transfection efficiency of Lenti-X 293T cells with Co-transfection of the three vectors by Calcium phosphate precipitation method (×40). (A1) fluorescence microscopy of Lenti-X 293T cells 24 h after transfection showed a high level of green irradiation. (A2) Light microscopy of Lenti-X 293T cells 24 h after transfection. II Flow cytometry analysis, show that 13.1% of the Lenti-X 293T cells were successfully transduced by the virus. III Transduction of hADSCs. (A1) fluorescence microscopy of transducted hADSCs72h after transduction show a high level of GFP expression (×100). (A2) Light microscopy of transducted hADSCs72 h after transduction (×100). (B) Flow cytometry analysis of transduced hADSCs indicate that more than 90% of cells were expressing GFP
Here we used the 3rd passage of hADSCs for transduction with a lentiviral vector expressing IL-11 and IL-13. Fluorescence microscopy of transduced cells after selection with puromycin showed that more than 90% of cells were expressing GFP. To approve this observation, quantification of the number of transduced cells was done by flow cytometry and the data revealed that 93.5% of the transduced hADSC were expressing GFP (Fig. 2, Part III).
Assessment of viral cytotoxicity on hADSC cells was evaluated with MTT assay and the result showed there was no significant difference between the viability of transduced hADSCs and untreated hADSCs as the negative control (p-value = 0.89) (Fig. 3, Part I).
I Evaluation of the effects of viral cytotoxicity on hADSC cells with MTT assay. Untreated hADSC and Mock cells use as the controls. MTT assay indicate no significant difference between transduced hADSCs and untreated hADSCs (p-value = 0.89). Also, the Mock cells has shown a high proliferation even more than untreated cells that indicated transduction with lentiviral vectors have no harmful effect on hADSCs (p-value = 0.59). II Confirmation of the Presence of the transgenes in transduced hADSCs as a 676 bp band
Presence of IL-11/IL-13 as the transgene
The PCR technique was performed on extracted genomic DNA from transduced hADSCs with special primers. After electrophoresis on the 1% agarose gel, the presence of IL-11/IL-13 transgene has been confirmed as a 676 bp visible band versus a GeneRuler 1 kb DNA size marker (Fig. 3, Part II).
The hADSCs Expressed IL-11 and IL-13 cytokines
Overexpression of IL-11 and IL-13 proteins bands were detected and confirmed using SDS-PAGE (Fig. 4, Part I).
I SDS page of transduced cells supernatant. Silver nitrate staining of a Poly acrylamide gel. A distinct band is found in the range of 15 and 20 kDa compared with untreated hADSCs which indicates the overexpression of IL-13 and IL-11 with molecular weights of 15.8 and 19.1 kDa, respectively. II ELISA of transduced cells supernatant. (A) Quantification of human IL-11 and IL-13 secreted by untreated hADSCs, MOCK cells and transduced hADSCs. Cell culture supernatants were collected after 15.45 and75 h of culture and IL-11 and IL-13 secretion determined by ELISA. Data represent mean ± SD of three independent samples assayed in triplicate. *p-value < 0.01,**p-value < 0.001, ***p-value < 0.0001. (A1) Overexpretion of IL-11 compared with the untreated and mock groups. Data show a significant increase of IL-11 on day 15 (0.0001) that is still significant on day 45 and 75 (0.001). (A2) Overexpretion of IL-13 secretion compared with the untreated and mock groups. Data indicate a significant high level of IL-13 secretion on day 15 and 45 (0.0001) and 75 (0.001)
To confirm the secretion of IL-11 and IL-13 cytokines, the cell supernatants were collected in 15, 45, and 75 days after transduction, and IL-11 and IL-13 concentrations were quantified by the ELISA method. The results showed that the transduced hADSCs expressed significantly higher levels of IL-11 and IL-13 compared with Mock and untreated cells (p = 0.001) (Fig. 4, Part II). Our results also showed that there was no significant difference between Mock and untreated cells in terms of IL-11 and IL-13 secretion (p = 0.998 and p = 0.844, respectively).
Discussion
Adipose derived mesenchymal stem cells (ADSCs) are a type of mesenchymal stem cells (MSCs) with the ability to secrete anti-inflammatory cytokines and stimulation of the proliferation of nerve cells (Mazini et al. 2019). The advantages of ADSCs make them an interesting tool for cell therapy in some disorders such as Multiple sclerosis (MS) (Dulamea 2015). In a study done by Payne et al. the effect of three types of MSCs isolated from bone marrow (BM), umbilical cord and adipose tissue on EAE mice models was elevated and their results showed that ADSCs were more effective in reducing EAE severity than the types of stem cells (Dulamea 2015). One of the ways to use MSCs is their genetic engineering with specific genes. The gene modified MSCs with lentiviral vector has several advantages compare with other delivery methods. For example, transduction with lentiviral vector provides a stable amount of the drug while injectable proteins have a low half-life that leads to reduce their clinical effects (Meyerrose et al. 2008; Payne et al. 2013). Due to the advantages of ADSCs and the lentiviral vectors’ delivery system, we decided to overexpress IL-11 and IL-13 in ADSCs via a lentiviral vector. Briefly, hADSCs were isolated from adipose tissues and flow cytometry and differentiation assays showed that transduction of hADSCs with PCDH-513B1 lentiviral vector did not have significant effects on cell surface markers expression, cell growth, and differentiation (Rostami et al. 2018; Guan et al. 2015; Van Vollenstee et al. 2016). In this study, we used a precipitation method based on PEG8000 for viral concentration. Previous studies have suggested that PEG reduces or eliminates the effects of viral compounds. Thus, it can be said that using this procedure can make the application of the lentiviral vectors easier by reducing their side effects (Colombet et al. 2007).
Here, we successfully transduced ADSCs, and the evaluation of the transduced hADSCs showed almost all of the transduced cells were expressing GFP in line with prior reports (Alizadeh et al. 2015). Previous researches have reported that lentiviruses provide a stable expression of the transgene in the long term via integration into the host cells genome (Liu et al. 2012). Cytokines have a short half-life in vivo. Therefore, in order to achieve a stable amount of IL-11 and IL-13, continuous production of these two cytokines by transduced cells was important. In this study, sub-culturing was performed when the cells reached 85% cellular confluency. After the transduction of hADSCs with lentiviruses containing IL-11 and IL-13, it was observed that although there was no significant difference between the viability of transduced hADSCs and controls, the cell proliferation rate was decreased. So that the transduced cells needed to be sub-cultured for the first time after 15 days and in subsequent cases, every 30 days. Based on mentioned, secretion of IL-11 and IL-13 was assessed 15,45,75 days after transduction by ELISA technique to ensure long-term overexpression and high expression levels of these two cytokines at least for 75 days was a confirmation of previous studies (Payne et al. 2012, 2013).
As mentioned, the use of ADSCs in the treatment of some inflammatory diseases such as multiple sclerosis (MS) has been studied, so far. Multiple sclerosis is a central nervous system (CNS) inflammatory autoimmune disease. The therapeutic strategy for MS can be divided into two main ways. First, immuno-therapeutic treatments that designed to reduce or prevent new CNS lesions and other treatments designed to repair previous neurological damages (Münzel and Williams 2013; Cuascut and Hutton 2019). The combination of both approaches may introduce effective therapies for CNS inflammatory demyelination. IL-11 is a cytokine with neuro-regenerative effects derived from astrocytes (Nair et al. 2008; Zhang et al. 2006). Pervious data have indicated that IL-11 signaling may provide a treatment for CNS diseases such as MS (Gurfein et al. 2009). Some researchers have shown that IL-11 injections directly reduce myelin phagocytosis and overexpression of IL-11 by lentiviruses in demyelinating brain areas significantly improves spontaneous remyelination in cuprizone-induced demyelination mice (Maheshwari et al. 2013). IL-13 is another interesting cytokine that its high levels in cerebrospinal fluid may have a neuroprotective effect. It has also been observed that glutarimer acetate which is currently used to treatment of MS, significantly increases IL-13 level in MS patients (Mori et al. 2016). In addition, the ability of IL-13 to switch the inflammatory to anti-inflammatory environment can make it a suitable drug for MS and other inflammatory diseases (Ochoa-Repáraz et al. 2008; Guglielmetti et al. 2016; Kolosowska et al. 2019).
All in all, the results of this study, indicated that human adipose derived stem cells are a suitable vehicle for transduction and expression of specific genes. Due to the properties of hADSCs and the synergistic effects of overexpression of IL-11 and IL-13, transduced hADSCs consisting these two cytokines can provide a potential new treatment for MS patients.
Conclusion
In the current study, we successfully produced hADSCs overexpressing IL-13 and IL-11, simultaneously. Our study showed that hADSCs cells can be considered as a tool for long-term expression of IL-11 and IL-13 as a transgene. The effect of the simultaneous expression of IL-11 and IL-13 in MS requires further studies by the application of these transduced cells in the EAE mice.
Availability of data and materials
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Alizadeh A et al (2015) Lentiviral mediated overexpression of NGF in adipose-derived stem cells. Clon Trans 4:3
Association GA (2014) World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. J Am College Dent 81:14
Barde I, Salmon P, Trono D (2010) Production and titration of lentiviral vectors. Curr Protocols Neurosci 53:1–23
Ciuffreda MC et al (2016) Protocols for in vitro differentiation of human mesenchymal stem cells into osteogenic, chondrogenic and adipogenic lineages mesenchymal stem cells. Springer, Berlin, pp 149–158
Colombet J et al (2007) Virioplankton ‘pegylation’: use of PEG (polyethylene glycol) to concentrate and purify viruses in pelagic ecosystems. J Microbiol Methods 71:212–219
Constantin G et al (2009) Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem cells 27:2624–2635
Cuascut FX, Hutton GJ (2019) Stem cell-based therapies for multiple sclerosis: current perspectives. Biomedicines 7:26
Dargahi N et al (2017) Multiple sclerosis: immunopathology and treatment update. Brain Sci 7:78
Dooley D et al (2016) Cell-based delivery of interleukin-13 directs alternative activation of macrophages resulting in improved functional outcome after spinal cord injury. Stem Cell Reports 7:1099–1115
Dulamea A (2015) Mesenchymal stem cells in multiple sclerosis-translation to clinical trials. J Med Life 8:24
Ghasemi N (2015) Therapeutic effects of adipose derived mesenchymal stem cells on remyelination process in inflammatory demyelinating diseases. J Histol Histopathol 2:8
Guan J et al (2015) Bone morphogenetic protein 2 gene transduction enhances the osteogenic potential of human urine-derived stem cells. Stem Cell Res Ther 6:5
Guglielmetti C et al (2016) Interleukin-13 immune gene therapy prevents CNS inflammation and demyelination via alternative activation of microglia and macrophages. Glia 64:2181–2200
Gurfein BT et al (2009) IL-11 regulates autoimmune demyelination. J Immunol 183:4229–4240
Kimiskidis V, Fassas A (2013) Stem cell-based therapies in multiple sclerosis. J Genet Syndr Gene Ther S 3:2
Klages N, Zufferey R, Trono D (2000) A stable system for the high-titer production of multiply attenuated lentiviral vectors. Mol Ther 2:170–176
Kolosowska N et al (2019) Peripheral administration of IL-13 induces anti-inflammatory microglial/macrophage responses and provides neuroprotection in ischemic stroke. Neurotherapeutics 1:16
Liu X, Clark AF, Wordinger RJ (2007) Expression of ciliary neurotrophic factor (CNTF) and its tripartite receptor complex by cells of the human optic nerve head. Mol Vision 13:758
Liu Y et al (2012) Lentiviral-mediated gene transfer into human adipose-derived stem cells: role of NELL1 versus BMP2 in osteogenesis and adipogenesis in vitro. Acta Biochim Biophys Sin 44:856–865
Machado CV, Telles PD, Nascimento IL (2013) Immunological characteristics of mesenchymal stem cells. Revista brasileira de hematologia e hemoterapia 35:62–67
Maheshwari A et al (2013) Local overexpression of interleukin-11 in the central nervous system limits demyelination and enhances remyelination. Mediat Inflamm 2013:685317
Maria AT et al (2017) Adipose-derived mesenchymal stem cells in autoimmune disorders: state of the art and perspectives for systemic sclerosis. Clin Rev Allergy Immunol 52:234–259
Mazini L et al (2019) Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int J Mol Sci 20:2523
McLaughlin KA, Wucherpfennig KW (2008) B cells and autoantibodies in the pathogenesis of multiple sclerosis and related inflammatory demyelinating diseases. Adv Immunol 98:121–149
Meyerrose TE et al (2008) Lentiviral-transduced human mesenchymal stem cells persistently express therapeutic levels of enzyme in a xenotransplantation model of human disease. Stem Cells 26:1713–1722
Mori S, Maher P, Conti B (2016) Neuroimmunology of the interleukins 13 and 4. Brain Sci 6:18
Münzel EJ, Williams A (2013) Promoting remyelination in multiple sclerosis—recent advances. Drugs 73:2017–2029
Nair A, Frederick TJ, Miller SD (2008) Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci 65:2702
Ochoa-Repáraz J et al (2008) IL-13 production by regulatory T cells protects against experimental autoimmune encephalomyelitis independently of autoantigen. J Immunol 181:954–968
Patel J, Balabanov R (2012) Molecular mechanisms of oligodendrocyte injury in multiple sclerosis and experimental autoimmune encephalomyelitis. Int J Mol Sci 13:10647–10659
Payne NL et al (2012) Early intervention with gene-modified mesenchymal stem cells overexpressing interleukin-4 enhances anti-inflammatory responses and functional recovery in experimental autoimmune demyelination. Cell Adhes Migr 6:179–189
Payne NL et al (2013) Human adipose-derived mesenchymal stem cells engineered to secrete IL-10 inhibit APC function and limit CNS autoimmunity. Brain Behav Immun 30:103–114
Peferoen L et al (2014) Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 141:302–313
Regmi S et al (2019) Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur J Cell Biol 98:5–8
Rostami M, Haidari K, Shahbazi M (2018) The Human IL-23 Decoy Receptor Inhibits T-Cells Producing IL-17 by Genetically Engineered Mesenchymal Stem Cells. Int J Cell Biol. https://doi.org/10.1089/cell.2018.0006
Sochocka M, Diniz BS, Leszek J (2017) Inflammatory response in the CNS: friend or foe? Mol Neurobiol 54:8071–8089
Tavazzi E, Rovaris M, La Mantia L (2014) Drug therapy for multiple sclerosis. CMAJ 186:833–840
Van Vollenstee FA et al (2016) Human adipose derived mesenchymal stromal cells transduced with GFP lentiviral vectors: assessment of immunophenotype and differentiation capacity in vitro. Cytotechnology 68:2049–2060
Vizoso FJ et al (2017) Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci 18:1852
Wang JM et al (2017) Isolation, culture and identification of human adipose-derived stem cells. Exp Ther Med 13:1039–1043
Way SW et al (2015) Pharmaceutical integrated stress response enhancement protects oligodendrocytes and provides a potential multiple sclerosis therapeutic. Nat Commun 6:6532
Winkelmann A et al (2014) Multiple sclerosis treatment and infectious issues: update 2013. Clin Exp Immunol 175:425–438
Zhang Y et al (2006) Interleukin-11 potentiates oligodendrocyte survival and maturation, and myelin formation. J Neurosci 26:12174–12185
Zhang J et al (2011) Promoting myelin repair and return of function in multiple sclerosis. FEBS Lett 585:3813–3820
Zuk PA (2011) Viral transduction of adipose-derived stem cells adipose-derived stem cells. Springer, Berlin, pp 345–357
Funding
This study was supported by Isfahan University of Medical Sciences, Isfahan, Iran (Grant No: 397053).
Author information
Authors and Affiliations
Contributions
AE: Experimental procedures, and preparation of the manuscript; MAA: Experimental procedures, and preparation of the manuscript; MD: Experimental procedures; MA: Experimental procedures; HS: Supervision of the cell culture procedures and study design; MGH: Supervision of the study, data analysis and finalizing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study is approved by the ethics committee of the Isfahan University of Medical Sciences, Isfahan, Iran (IR.MUI.MED.REC.1397.026).
Consent to participate
All authors agree to participate in this research study.
Consent for publication
All authors agree to this publication.
Competing interest
There are no conflicts of interest among the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eslami, A., Dehbashi, M., Ashja-Arvan, M. et al. Assessment of ability of human adipose derived stem cells for long term overexpression of IL-11 and IL-13 as therapeutic cytokines. Cytotechnology 72, 773–784 (2020). https://doi.org/10.1007/s10616-020-00421-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-020-00421-8