Abstract
Regarding the low quality of life due to the cognitive complications in the patients with hepatic cirrhosis (HC), the goal of this study was to examine the possible neuroprotective effect of pioglitazone (PIO) on the electrophysiological alterations of hippocampus, a major area of cognition, in the experimental model of bile duct ligation (BDL). We used adult male Wistar rats in the present study to perform BDL or sham surgery. Pioglitazone was administered in BDL rats two weeks after the surgery for the next continuous four weeks. The effects of pioglitazone on BDL-induced electrophysiological alterations of the CA1 pyramidal neurons in the hippocampus were evaluated by whole-cell patch clamp recordings. Our findings demonstrated that chronic administration of PIO could not reverse the electrophysiological changes in the CA1 pyramidal neurons of the hippocampus in BDL rats but could improve the hepatic dysfunction.
Together, the results of this study suggest that PIO administration cannot counteract altered intrinsic properties of the hippocampal neurons which has been shown recently as an involved mechanism of the cognitive impairments in hepatic encephalopathy (HE).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatic encephalopathy (HE) is a common serious disorder, in a range of 20–80% in patients with hepatic cirrhosis (HC) (Lizardi-Cervera et al. 2003; Rose et al. 2020) with various grades of neurological complications particularly neuropsychiatric, cognitive and motor dysfunction (Schuppan and Afdhal 2008; Felipo 2013). These cerebral dysfunctions are mainly caused by hyperammonemia and neuroinflammation, which are of relevance to the known pathomechanisms of HE (Rodrigo et al. 2010). In addition, the alterations of neurotransmitters following HC have been illustrated in several studies (Çelik et al. 2005; Dhanda and Sandhir 2015; Cauli et al. 2006, 2009; Llansola et al. 2013). In our previous study, prominent changes in electrophysiological characteristics of the CA1 area of the hippocampus in the animal model of bile duct ligation (BDL) was observed (Tahamtan et al. 2017).
BDL (Leke et al. 2013; Magen et al. 2009; Aghaei et al. 2015; Tahamtan et al. 2017), portacaval shunt (Erceg et al. 2005; Méndez et al. 2008; Cauli et al. 2007; Monfort et al. 2007), and thioacetamide intoxication (Méndez et al. 2008) are among animal models for HC in the experimental studies (Butterworth et al. 2009), which lead to behavioral dysfunctions including learning, memory and motor impairments very similar to clinical manifestations. Regarding the cognitive abnormalities in HC individuals, experimental evidences discriminate impairments in hippocampal long-term potentiation (LTP) following hyperammonemia and HE (Monfort et al. 2007; Muñoz et al. 2000). LTP which plays a significant role in memory storage is a major feature of the hippocampus (Lynch et al. 1990; Bliss and Collingridge 1993). The glutamatergic neurotransmission as a prominent pathway in LTP induction is impaired due to hyperammonemia (Hermenegildo et al. 1998; Aguilar et al. 2000; Monfort et al. 2001, 2004).
To date, there is no effective strategies against brain deficits following HE, therefore, further studies are necessary to find an effective treatment to promote functional improvement after HE. In the light of the several studies, pioglitazone (PIO), which is commonly an antidiabetic drug, has neuroprotective and neuroregenerative effects (Zhao et al. 2005; Bordet et al. 2006; Swanson et al. 2011; Blackburn et al. 2020, 2022; Ulusoy et al. 2011; Zakaria et al. 2019; Bonato et al. 2018; Aghaei et al. 2019) in different cerebral dysfunctions including stroke, ischemia and autism (Capano et al. 2018; Serghides et al. 2014). One important action mechanism of PIO is through anti-inflammatory pathways including prevention of immune cells or expression of proinflammatory cytokines that led to gaining increased attention in the study of this peroxisome proliferator activated receptors (PPARs) agent (Ribeiro et al. 2019; Kielian and Drew 2003). PPAR activation can reduce neuronal death and also protect vascular function through inhibition of oxidative or inflammatory mechanisms and endothelial dysfunction which are identified following brain injuries (Bordet et al. 2006). PPARs can induce vascular protection by inhibition of adhesion proteins (Bordet et al. 2006). PIO also can present neuroprotective effects by regulation of reactive oxygen species production through increase in paraoxonase-2 expression (Blackburn et al. 2020). Our previous study showed the neuroprotective effects of PIO against motor and cognitive problems following BDL (Aghaei et al. 2014).
Based on these reports, in line with our previous behavioral and electrophysiological research, we designed the present study with this main goal to assess the possible neuroprotective effect of PIO against electrophysiological changes in the CA1 area of the hippocampus in BDL rats.
Materials and methods
Animals and experimental design
Male Wistar rats (n=40) weighing 180–220 g were housed in a controlled room (21 ± 3 °C, 12 h light/dark cycle) with free access to standard food and water. All procedures and protocols were approved by the Ethics Committee of the Kerman Medical University (IR.KMU.REC.1395.792). Four groups were involved to assess biochemical parameters (n = 10 rats in each group).
-
1.
The sham group rats underwent all surgical procedures without bile duct ligation.
-
2.
The sham + PIO group (20 mg/kg, i.p., based on the pilot study and our previous studies with dosages 10, 20 and 50 mg/kg; we reached 20 mg/kg (Aghaei et al. 2014, 2019).
-
3.
BDL group rats that were subjected to bile duct ligation.
-
4.
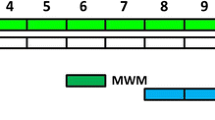
BDL + PIO group (20 mg/kg, i.p.). Pioglitazone hydrochloride (Sigma–Aldrich, USA) was dissolved in saline and administered intraperitoneally to the rats two weeks after BDL induction and continued for the next four weeks, once a day. Based on our biochemical and behavioral data, the rats were allocated to these three groups to assess electrophysiological properties: sham, BDL and BDL + PIO groups (Fig. 1).
Bile duct ligation surgery
The bile duct ligation procedure was carried out as described previously (Aghaei et al. 2014). The rats were generally anesthetized by using ketamine (70 mg/kg) and xylazine (10 mg/ kg) injected intraperitoneally and underwent abdominal incision and the segregation of the hepatic ligament. The common bile duct was ligated with two 4–0 nonabsorbent surgical sutures. The first suture was placed below the junction of the biliary hepatic ducts and the second suture was placed above the entrance of the pancreatic ducts. The common bile duct was resected between the two ligatures. The abdominal incisions were closed with 4 − 0 silk sutures under sterile condition. For the sham control group, all the procedures were performed in similar manner except the bile duct ligation. The animals were then allowed to recover with free access to food and water for 6 weeks following surgery.
Assessment of hepatic function
Following plasma biochemistry parameters were measured by a blinded lab technician to assess the hepatic function in four experimental groups (sham, sham + PIO, BDL and BDL + PIO groups): total bilirubin, direct bilirubin, alkaline phosphatase and hepatic enzymes (ALT, AST). Plasma samples were analyzed by using a commercially available kit (Zistshimi, Tehran, Iran).
Electrophysiological assessment
Slice preparation
Under ether anesthesia, the sham, BDL and BDL + PIO rats were decapitated 6 weeks after surgery. Brains were dissected rapidly and hippocampal transverse slices were cut in an ice-cold artificial cerebrospinal fluid (aCSF) containing in mM: 206 sucrose, 26 NaHCO3, 10 D-glucose, 2.8 KCl, 2MgSO4, 1.25 NaH2PO4, 1 CaCl2, and 1MgCl2; 290–300 mOsm (pH justified to 7.4 with 95%O2 and 5% CO2) by using a vibrating microtome (752 M, Campden Instruments Ltd., UK). Slices were incubated in aCSF containing: 124 NaCl, 26 NaHCO3, 10 D-glucose, 2.8 KCl, 2 CaCl2, 2 MgSO4, 1.25 NaH2PO4; 295 mOsm, pH 7.4, for at least 60 min at 36 °C and thereafter maintained at room temperature until used.
Whole cell patch clamp recording
We evaluated the electrophysiological properties of the CA1 pyramidal neurons by using whole-cell patch clamp recordings to examine whether chronic administration of PIO can protect against the alterations of neuronal function induced by bile duct ligation. Intracellular recordings in current clamp mode from pyramidal neurons were made, as described previously by Razavinasab et al. (Razavinasab et al. 2016), under direct visual control using differential interference contrast optics (Olympus; BX 51WI). CA1 pyramidal neurons were visualized with a 40× water immersion objective using Nomarski-type differential interference contrast imaging with infrared illumination. Images were captured with a CCD camera (Hmamatsu, ORSA,Japan). The hippocampal slices were continuously perfused with normal aCSF (2 ml/min) at room temperature (22–25 °C) in a recording chamber. Whole cell current clamp recordings were obtained from CA1 pyramidal neurons using Multiclamp 700B amplifiers (Axon Instruments, Foster City, CA) equipped with Digidata 1440 A/D converter (Axon Instruments, Foster City, CA). Micropipettes were pulled with an electrode puller (PC10; Narishige, Tokyo, Japan) and had a resistance of 4–9 MΩ when filled with an internal solution. The composition of the internal solution was as follows (in mM): 0.33GTPtris, 125 K-gluconate, 5 KCl, 5 BAPTA, 0.5 CaCl2, 5 MgATP, and 10 HEPES. The final pH of the internal solution was adjusted to 7.2 by adding 1M KOH; the final osmolarity was adjusted to 280–285 mOsm. Cells with a seal < 1 GΩ before rupture of the membrane were discarded and the test seal function was constantly monitored throughout the recording to ensure that the seal was stable. Spontaneous firing properties of CA1 cells including resting membrane potential (RMP), spontaneous firing frequency and membrane input resistance were measured. To investigate the excitability of neurons, action potentials were induced in pyramidal cells from V holding = − 70 mV in 100 ms duration current steps ranging from − 500 to + 500 pA in 100 pA increments. Before positive current steps, a negative prepulse protocol current with 300 PA was also identified. We sought to reveal the effect of PIO on excitability changes induced by BDL, by examining these parameters: the number of action potentials and action potentials rebound generated by these current injections. The first spike latency was defined as the time between the offset of the negative current steps and the peak of the first spike.
Statistical analysis
Results are presented as the mean ± SEM. To investigate the characterization of spontaneous and evoked electrophysiological activities of CA1 pyramidal neurons in the experimental groups, statistical data were first analyzed for normality using a Kolmogorov-Smirnov (K-S) test. Results that found to be normally distributed (p > 0.05 in K-S test) were evaluated using one-way ANOVA. Results that were not normally distributed (p < 0.05 in K-S test) were assessed using a Kruskal Wallis test. P < 0.05 was considered statistically significant.
Results
The effect of chronic administration of PIO on the biochemical parameters following induction of BDL in rats
As demonstrated in Table 1, BDL group rats had higher plasma concentration of ALT than sham, sham + PIO and BDL + PIO groups (F (3, 36) = 2.1, p < 0.05). Also Alk.ph level increased in BDL group compared to the sham (F (3, 36) = 7.3, p < 0.001) and PIO could reverse this effect of BDL significantly in BDL + PIO group (p < 0.01). The mean level of direct and total bilirubin increased in BDL compared to sham and sham + PIO groups and were not reversed in BDL + PIO group (p < 0.001). The albumin level had lower level in BDL and BDL + PIO rats than sham and sham + PIO groups (F (3, 36) = 5.8, p < 0.001). No significant difference was observed in the level of AST amongst four groups of study (Table 1).
The effect of chronic administration of PIO on the electrophysiological activities of hippocampal cells following induction of BDL in rats
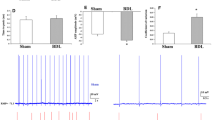
As mentioned in the methods section, we designed our electrophysiological assessments in the following three groups: Sham, BDL and BDL + PIO groups, because we found no significant differences between sham and sham + PIO groups in the biochemical and also our previous published behavioral data (Aghaei et al. 2014). Therefore, we removed sham + PIO group from the electrophysiological evaluation. Compared with sham animals (-60.01 ± 1.22), the mean resting membrane potential parameter is significantly more negative in the BDL (-71.4 ± 2.01) and BDL + PIO (-75 ± 2.27) group rats (Fig. 2, p < 0.05). Only 5 from 14 recorded hippocampal cells of the sham group showed spontaneous firing frequency, while in BDL and BDL + PIO groups, no spontaneous firing frequency from 12 recorded cells was reported all during the experiment. No difference were seen in the membrane input resistance among three experimental groups (F (2, 27) = 0.83, p > 0.05).
The effect of chronic administration of PIO on the evoked electrophysiological properties of hippocampal cells following induction of BDL in rats
Five trains of depolarizing and hyperpolarizing current pulses (0.1–0.5 and − 0.1– −0.5 nA, 520 ms) were used to evaluate the possible neuroprotective effects of PIO on the evoked firing responses of hippocampal neurons of rats subjected to BDL (Figs. 3 and 4). The relationship between the number of spikes evoked per pulse and first spike latency was examined in the sham, BDL and BDL + PIO animals. In negative current pulses injection (Fig. 3 A-D), we found a significant increase in delay in the first spike latency in the first three pulses and steady state (in the last three pulses) in the BDL and BDL + PIO group rats compared to the sham operated animals (Fig. 3 A, C, p < 0.01 and p < 0.05 respectively). Compared with sham operated animals sag voltage amplitude had a significant reduction in the BDL and BDL + PIO group rats in the last three pulses (Fig. 3D, p < 0.05). Following 520 ms negative and positive current protocol, the number of rebound action potential (AP) in the BDL and BDL + PIO groups decreased compared to the sham group animals (Figs. 3B and 4 A, p < 0.001 and p < 0.01 respectively).
Effect of PIO administration on alterations in first spike latency (A), rebound (B), steady state (C) and sag voltage (D) following negative current pulses injection in BDL animals. The traces indicate the response of the hippocampal cells from − 0.1 to − 0.5 nA negative current pulses injection. (Sham) Blue color traces, (BDL) green color traces and (BDL + PIO) red color traces. #p < 0.05, ##p < 0.01 and ###p < 0.001 as compared represent significant differences between the sham group vs. the BDL group. $p < 0.05, $$p < 0.01 and $$$p < 0.001 represent significant differences between the sham group vs. the BDL + PIO group. Data represent the mean ± S.E.M
Effect of PIO administration on alterations in the evoked firing responses of the hippocampal cells following positive current pulses injection (Fig. 3 A) and 5 repeated depolarizing pulses (Fig. 3B) in BDL animals. The relationship between firing rate and different positive current injection with fixed step amplitude A; the relationship between firing rate and repetitive current injection with fixed step amplitude B. (Sham) Blue color traces, (BDL) green color traces and (BDL + PIO) red color traces. #p < 0.05 and ##p < 0.01 represent significant differences between the sham group vs. the BDL group. $p < 0.05 and $$p < 0.01 represent significant differences between the sham group vs. the BDL + PIO group. Data represent the mean ± S.E.M
Firing properties were changed in BDL and PIO treated rats as shown in Fig. 4B for all repetitive currents with a fixed duration (0.2 s) and amplitude (200 pA). The number of action potentials in the BDL group evoked by this protocol decreased compared to that in the sham group and were not reversed by PIO administration in rats subjected to BDL (Fig. 4B, p < 0.05). In negative pre-pulse injection following 0.3 nA test pulse, significant increased delay in the first spike latency in the BDL and BDL + PIO groups was indicated compared to the sham group (Fig. 5 A, p < 0.001, p < 0.01, p < 0.05 following − 0.1 nA to -0.3 nA pre pulses). Also firing properties were altered in BDL and BDL + PIO rats as shown in Fig. 4B for all five current pre pulses. The number of AP in the BDL and BDL + PIO groups evoked by this protocol decreased significantly compared to the sham group (Fig. 5B, p < 0.05 following − 0.1 nA and p < 0.01 for all four current pre pulses).
Effect of PIO administration on alterations in delay in the first spike latency (A) and rate of action potential (B), by negative current prepulse injection (− 0.1 to − 0.5 nA) following 0.3 nA test pulse injection. (Sham) Blue color traces, (BDL) green color traces and (BDL + PIO) red color traces. #p < 0.05, ##p < 0.01 and ###p < 0.001 as compared represent significant differences between the sham group vs. the BDL group. $p < 0.05, $$p < 0.01 and $$$p < 0.001 represent significant differences between the sham group vs. the BDL + PIO group. Data represent the mean ± S.E.M
Discussion
The main goal of the current study was to examine the potential neuroprotective effect of pioglitazone against electrophysiological alterations induced by BDL in the CA1 area of the hippocampus by using whole cell patch clamp technique. Our findings indicated that PIO could not reverse these intrinsic electrophysiological alterations. Also, we measured biochemical parameters to evaluate whether PIO can protect hepatic function in BDL rats. PIO could reverse diminished hepatic function due to BDL. These biomedical findings is in consistent with our previous study (Aghaei et al. 2014).
Patients with HC suffer from various neurological and neuropsychiatric problems. Motor, learning and memory impairments seem to currently occur following HC (Aghaei et al. 2014, 2015; Weissenborn et al. 2005; Pflugrad et al. 2015; Arias et al. 2014).
Many studies have used BDL as an animal model for induction of HC (Butterworth et al. 2009). BDL exhibits cognitive deficiencies very similar to neurological symptoms in human. Our previous published study reported impaired balance, learning and spatial memory in rotarod, passive avoidance learning task and Morris water maze in BDL animals, respectively (Aghaei et al. 2014). A significant cognitive dysfunction was observed in Morris water maze and novel object recognition tests in BDL rats probably by reducing the expression of brain derived neurotrophic factor (BDNF) (Dhanda et al. 2018). Another study reported cognitive problem in the eight arm maze and the T-maze tests and also locomotor dysfunction in the open field test following BDL (Magen et al. 2009).
Decreasing the brain deficiencies can increase the quality of life in HC patients, therefore discovering the involved exact mechanisms is valuable. Previous studies showed several pathways which lead to behavioral dysfunctions following HE. Underlying pathophysiological mechanisms of HE are not well understood, but over production of ammonia (Cauli et al. 2006), deficit in neurotransmitter systems particularly GABAergic (Cauli et al. 2009), glutamatergic (Llansola et al. 2013) and dopaminergic pathways (Dhanda and Sandhir 2015; El Hiba et al. 2013), oxidative stress, neural apoptosis, and excitotoxicity (Felipo 2013; Javadi-Paydar et al. 2013) contribute in pathomechanism of HE. Since the cerebellum (Rodrigo et al. 2010) and hippocampus (Ahmadi et al. 2015) are two main vulnerable regions compared to other brain regions to hyperammonemia, so these two regions are of great interest of many studies. The electrophysiological alterations were observed in the cerebellum (Aghaei et al. 2016) and hippocampus (Tahamtan et al. 2017) in animals subjected to BDL procedure. In the cerebellar Purkinje cells, BDL caused hyperexcitability, larger AHP amplitude, and shortened action potentials compared to sham animals (Aghaei et al. 2016). In CA1 neurons of the hippocampus, lower excitability in BDL animals is indicated when compared to the sham group by alteration in the frequency of AP, amplitude of AHP, AP duration at half-amplitude and first spike latency. The AHP amplitude was significantly larger in the hippocampal neurons of the BDL group than that of sham rats. Increased levels in KV2 and KV3 channel activity (McKay and Turner 2004), direct activation of voltage gated K+ channels, including fast transient (A-type) and large conductance calcium activated K+ channels by NH4+ (Allert et al. 1998) are suggested that participate in these mentioned electrophysiological changes.
Impaired LTP and reduced effectiveness of excitatory synaptic transmission in hippocampal slices of rats, due to dysfunction in AMPA or NMDA receptors and soluble guanylate cyclase (Szerb and Butterworth 1992; Monfort et al. 2005, 2007; Muñoz et al. 2000; Rodrigo et al. 2005; ElMlili et al. 2010; El-Mlili et al. 2008), the block of action potential conduction in the presynaptic terminals in the cat spinal cord (Raabe 1990) are suggested as consequences of hyperammonemia.
Significance of understanding the involved mechanisms of HE certainly is finding more effective treatment strategies against cognitive and motor impairments following BDL. There are a vast body of evidences showing neuroprotective effect of PIO through anti-inflammatory by lowering neutrophilia, TNFα, C-reactive protein and IL-6, anti-apoptotic and also interaction with oxidative stress pathways by the activation of PPAR receptors, maintaining mitochondrial respiration, induction of constitutive nitric oxide synthase, against different cerebral diseases including Parkinson’s, traumatic brain injury, ischemia, spinal cord injury and Huntington’s disease (Napolitano et al. 2011; Zhao et al. 2005; Park et al. 2007; Swanson et al. 2011; Barbiero et al. 2011; Kumar et al. 2009; Xing et al. 2007; Sauerbeck et al. 2011; Schuppan and Afdhal 2008; Kapadia et al. 2008; Bordet et al. 2006; Patel et al. 2017; Shafaroodi et al. 2012; Agarwal 2006; Sharma et al. 2009). Our previous findings demonstrate the neuroprotective effects of PIO against cognitive and motor dysfunction following BDL compared to sham rats (Aghaei et al. 2014). Pioglitazone could improve deficits in spatial learning and also passive avoidance learning which caused by BDL (Aghaei et al. 2014).
In addition, PIO could reverse the electrophysiological alterations induced by BDL in cerebellar Purkinje cells (Aghaei et al. 2016). In the current study, we found that PIO could not reverse the electrophysiological changes induced by BDL in the CA1 neurons of the hippocampus. We suggest that further studies should be done to examine more aspects of the study. For example, different dosages and duration of pioglitazone treatment may be effective and contract the BDL induced electrophysiological alterations in the CA1 region of the hippocampus.
Conclusions
Although, PIO could protect the rats from biochemical and behavioral (Aghaei et al. 2014) dysfunctions and also reversed the electrophysiological alterations induced by BDL in purkinje cells (Aghaei et al. 2016) but could not exert as a protective agent in CA1 neurons of the hippocampus of BDL rats against electrophysiological alterations.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
All software applications used are included in this article.
References
Agarwal R (2006) Anti-inflammatory effects of short-term pioglitazone therapy in men with advanced diabetic nephropathy. Am J Physiology-Renal Physiol 290:F600–F605
Aghaei I, Shabani M, Doustar N, Nazeri M, Dehpour A (2014) Peroxisome proliferator-activated receptor-γ activation attenuates motor and cognition impairments induced by bile duct ligation in a rat model of hepatic cirrhosis. Pharmacol Biochem Behav 120:133–139
Aghaei I, Nazeri M, Shabani M, Mossavinasab M, Mirhosseini FK, Nayebpour M, Dalili A (2015) Erythropoietin ameliorates the motor and cognitive function impairments in a rat model of hepatic cirrhosis. Metab Brain Dis 30:197–204
Aghaei I, Hajali V, Dehpour A, Haghani M, Sheibani V, Shabani M (2016) Alterations in the intrinsic electrophysiological properties of Purkinje neurons in a rat model of hepatic encephalopathy: Relative preventing effect of PPARγ agonist. Brain Res Bull 121:16–25
Aghaei I, Hajali V, Haghani M, Vaziri Z, Moosazadeh M, Shabani M (2019) Peroxisome proliferator-activated receptor-γ activation attenuates harmaline-induced cognitive impairments in rats. J Clin Neurosci 59:276–283
Aguilar M, Miñarro J, Felipo V (2000) Chronic moderate hyperammonemia impairs active and passive avoidance behavior and conditional discrimination learning in rats. Exp Neurol 161:704–713
Ahmadi S, Poureidi M, Rostamzadeh J (2015) Hepatic encephalopathy induces site-specific changes in gene expression of GluN1 subunit of NMDA receptor in rat brain. Metab Brain Dis 30:1035–1041
Allert N, Köller H, Siebler M (1998) Ammonia-induced depolarization of cultured rat cortical astrocytes. Brain Res 782:261–270
Arias N, Fidalgo C, Vallejo G, Arias JL (2014) Brain network function during shifts in learning strategies in portal hypertension animals. Brain Res Bull 104:52–59
Barbiero JK, Santiago RM, Lima MM, Ariza D, Morais LH, Andreatini R, Vital MA (2011) Acute but not chronic administration of pioglitazone promoted behavioral and neurochemical protective effects in the MPTP model of Parkinson’s disease. Behav Brain Res 216:186–192
Blackburn JK, Curry DW, Thomsen AN, Roth RH, Elsworth JD (2020) Pioglitazone activates paraoxonase-2 in the brain: a novel neuroprotective mechanism. Exp Neurol 327:113234
Blackburn JK, Jamwal S, Wang W, Elsworth JD (2022) Pioglitazone transiently stimulates paraoxonase-2 expression in male nonhuman primate brain: Implications for sex-specific therapeutics in neurodegenerative disorders. Neurochem Int 152:105222
Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39
Bonato JM, Bassani TB, Milani H, Vital MABF (2018) & R. M. W. de Oliveira Pioglitazone reduces mortality, prevents depressive-like behavior, and impacts hippocampal neurogenesis in the 6-OHDA model of Parkinson’s disease in rats. Experimental neurology, 300, 188–200
Bordet R, Ouk T, Petrault O, Gele P, Gautier S, Laprais M, Deplanque D, Duriez P, Staels B, Fruchart J (2006) PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem Soc Trans 34:1341–1346
Butterworth RF, Norenberg MD, Felipo V, Ferenci P, Albrecht J, Blei AT (2009) Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int 29:783–788 o. E. M. o. HE]
Capano L, Dupuis A, Brian J, Mankad D, Genore L, Hastie Adams R, Smile S, Lui T, Odrobina D, Foster JA (2018) A pilot dose finding study of pioglitazone in autistic children. Mol autism 9:1–14
Cauli O, Llansola M, Erceg S, Felipo V (2006) Hypolocomotion in rats with chronic liver failure is due to increased glutamate and activation of metabotropic glutamate receptors in substantia nigra. J Hepatol 45:654–661
Cauli O, Rodrigo R, Piedrafita B, Boix J, Felipo V (2007) Inflammation and hepatic encephalopathy: ibuprofen restores learning ability in rats with portacaval shunts. Hepatology 46:514–519
Cauli O, Mansouri MT, Agusti A, Felipo V (2009) Hyperammonemia increases GABAergic tone in the cerebellum but decreases it in the rat cortex. Gastroenterology 136:1359–1367e2
Çelik T, Gören MZ, Çınar K, Gürdal H, Önder FO, Tan A, Terzioğlu B, Bozdayı AM, Bozkaya H, Uzunalimoğlu Ö (2005) Fatigue of cholestasis and the serotoninergic neurotransmitter system in the rat. Hepatology 41:731–737
Dhanda S, Sandhir R (2015) Role of dopaminergic and serotonergic neurotransmitters in behavioral alterations observed in rodent model of hepatic encephalopathy. Behav Brain Res 286:222–235
Dhanda S, Gupta S, Halder A, Sunkaria A, Sandhir R (2018) Systemic inflammation without gliosis mediates cognitive deficits through impaired BDNF expression in bile duct ligation model of hepatic encephalopathy. Brain Behav Immun 70:214–232
El Hiba O, Gamrani H, Chatoui H, Ahboucha S (2013) Loss of tyrosine hydroxylase expression within the nigro-striato-cortical pathways in the cirrhotic rat: the possible restorative effect of the neurosteroid dehydroepiandrosterone sulfate. Acta Histochem 115:637–645
El-Mlili N, Rodrigo R, Naghizadeh B, Cauli O, Felipo V (2008) Chronic hyperammonemia reduces the activity of neuronal nitric oxide synthase in cerebellum by altering its localization and increasing its phosphorylation by calcium‐calmodulin kinase II. J Neurochem 106:1440–1449
ElMlili N, Boix J, Ahabrach H, Rodrigo R, Errami M, Felipo V (2010) Chronic hyperammonemia induces tonic activation of NMDA receptors in cerebellum. J Neurochem 112:1005–1014
Erceg S, Monfort P, Hernández-Viadel M, Rodrigo R, Montoliu C, Felipo V (2005) Oral administration of sildenafil restores learning ability in rats with hyperammonemia and with portacaval shunts. Hepatology 41:299–306
Felipo V (2013) Hepatic encephalopathy: effects of liver failure on brain function. Nat Rev Neurosci 14,:851–858
Hermenegildo C, Montoliu C, Llansola M, Muñoz MD, Gaztelu JM, Miñana MD, Felipo V (1998) Chronic hyperammonemia impairs the glutamate–nitric oxide–cyclic GMP pathway in cerebellar neurons in culture and in the rat in vivo. Eur J Neurosci 10:3201–3209
Javadi-Paydar M, Ghiassy B, Ebadian S, Rahimi N, Norouzi A, Dehpour AR (2013) Nitric oxide mediates the beneficial effect of chronic naltrexone on cholestasis-induced memory impairment in male rats. Behav Pharmacol 24:195–206
Kapadia R, Yi J-H, Vemuganti R (2008) Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front bioscience: J virtual Libr 13:1813
Kielian T, Drew PD (2003) Effects of peroxisome proliferator-activated receptor‐γ agonists on central nervous system inflammation. J Neurosci Res 71:315–325
Kumar P, Kaundal RK, More S, Sharma SS (2009) Beneficial effects of pioglitazone on cognitive impairment in MPTP model of Parkinson’s disease. Behav Brain Res 197:398–403
Leke R, Oliveira DL, Forgiarini LF, Escobar TD, Hammes TO, Meyer FS, Keiding S, Silveira TR, Schousboe A (2013) Impairment of short term memory in rats with hepatic encephalopathy due to bile duct ligation. Metab Brain Dis 28:187–192
Lizardi-Cervera J, Almeda P, Guevara L, Uribe M (2003) Hepatic encephalopathy: a review. Ann Hepatol 2:122–130
Llansola M, Montoliu C, Cauli O, Hernández-Rabaza V, Agustí A, Cabrera-Pastor A, Giménez-Garzó C, González-Usano A, Felipo V (2013) Chronic hyperammonemia, glutamatergic neurotransmission and neurological alterations. Metab Brain Dis 28:151–154
Lynch G, Kessler M, Arai A, Larson J (1990) Chapter The nature and causes of hippocampal long-term potentiation. Prog Brain Res 83:233–250
Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R, Berry EM (2009) Cannabidiol ameliorates cognitive and motor impairments in mice with bile duct ligation. J Hepatol 51:528–534
McKay B, Turner R (2004) Kv3 K + channels enable burst output in rat cerebellar Purkinje cells. Eur J Neurosci 20:729–739
Méndez M, Méndez-López M, López L, Aller M, Árias J, Cimadevilla JM, Árias JL (2008) Spatial memory alterations in three models of hepatic encephalopathy. Behav Brain Res 188:32–40
Monfort P, Corbalán R, Martinez L, López-Talavera J-C, Córdoba J, Felipo V (2001) Altered content and modulation of soluble guanylate cyclase in the cerebellum of rats with portacaval anastomosis. Neuroscience 104:1119–1125
Monfort P, Muñoz Ma-D, Felipo V (2004) Hyperammonemia impairs long-term potentiation in hippocampus by altering the modulation of cGMP-degrading phosphodiesterase by protein kinase G. Neurobiol Dis 15:1–10
Monfort P, Muñoz MD, Felipo V (2005) Chronic hyperammonemia in vivo impairs long-term potentiation in hippocampus by altering activation of cyclic GMP‐dependent‐protein kinase and of phosphodiesterase 5. J Neurochem 94:934–942
Monfort P, Erceg S, Piedrafita B, Llansola M, Felipo V (2007) Chronic liver failure in rats impairs glutamatergic synaptic transmission and long-term potentiation in hippocampus and learning ability. Eur J Neurosci 25:2103–2111
Muñoz M-D, Monfort P, Gaztelu J-M, Felipo V (2000) Hyperammonemia impairs NMDA receptor-dependent long-term potentiation in the CA1 of rat hippocampus in vitro. Neurochem Res 25:437–441
Napolitano M, Costa L, Palermo R, Giovenco A, Vacca A, Gulino A (2011) Protective effect of pioglitazone, a PPARγ ligand, in a 3 nitropropionic acid model of Huntington’s disease. Brain Res Bull 85:231–237
Park S-W, Yi J-H, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R (2007) Thiazolidinedione class of peroxisome proliferator-activated receptor γ agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther 320:1002–1012
Patel SP, Cox DH, Gollihue JL, Bailey WM, Geldenhuys WJ, Gensel JC, Sullivan PG, Rabchevsky AG (2017) Pioglitazone treatment following spinal cord injury maintains acute mitochondrial integrity and increases chronic tissue sparing and functional recovery. Exp Neurol 293:74–82
Pflugrad H, Bronzlik P, Raab P, Tryc AB, Goldbecker A, Barg-Hock H, Strassburg CP, Ding XQ, Lanfermann H, Weissenborn K (2015) Cerebral white matter lesions in patients with cirrhosis–causative for hepatic encephalopathy or bystanders? Liver Int 35:1816–1823
Raabe W (1990) Effects of NH4 + on reflexes in cat spinal cord. J Neurophysiol 64:565–574
Razavinasab M, Moazzami K, Shabani M (2016) Maternal mobile phone exposure alters intrinsic electrophysiological properties of CA1 pyramidal neurons in rat offspring. Toxicol Ind Health 32:968–979
Ribeiro NQ, Santos APN, Emídio ECP, Costa MC, Freitas GJC, Carmo PHF, Silva MF, de Brito CB, de Souza DG (2019) Pioglitazone as an adjuvant of amphotericin B for the treatment of cryptococcosis. Int J Antimicrob Agents 54:301–308 & T. A. Paixão
Rodrigo R, Erceg S, Felipo V (2005) Neurons exposed to ammonia reproduce the differential alteration in nitric oxide modulation of guanylate cyclase in the cerebellum and cortex of patients with liver cirrhosis. Neurobiol Dis 19:150–161
Rodrigo R, Cauli O, Gomez–Pinedo U, Agusti A, Hernandez–Rabaza V, Garcia–Verdugo JM, Felipo V (2010) Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology 139:675–684
Rose CF, Amodio P, Bajaj JS, Dhiman RK, Montagnese S, Taylor-Robinson SD, Vilstrup H, Jalan R (2020) Hepatic encephalopathy: Novel insights into classification, pathophysiology and therapy. J Hepatol 73:1526–1547
Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, Sullivan PG (2011) Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp Neurol 227:128–135
Schuppan D, Afdhal NH (2008) Liver cirrhosis. The Lancet 371:838–851
Serghides L, McDonald CR, Lu Z, Friedel M, Cui C, Ho KT, Mount HT, Sled JG, Kain KC (2014) PPARγ agonists improve survival and neurocognitive outcomes in experimental cerebral malaria and induce neuroprotective pathways in human malaria. PLoS Pathog 10:e1003980
Shafaroodi H, Moezi L, Ghorbani H, Zaeri M, Hassanpour S, Hassanipour M, Dehpour AR (2012) Sub-chronic treatment with pioglitazone exerts anti-convulsant effects in pentylenetetrazole-induced seizures of mice: The role of nitric oxide. Brain Res Bull 87:544–550
Sharma R, Kaundal RK, Sharma SS (2009) Amelioration of pulmonary dysfunction and neutrophilic inflammation by PPARγ agonist in LPS-exposed guinea pigs. Pulm Pharmacol Ther 22:183–189
Swanson CR, Joers V, Bondarenko V, Brunner K, Simmons HA, Ziegler TE, Kemnitz JW, Johnson JA, Emborg ME (2011) The PPAR-γ agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J Neuroinflamm 8:1–14
Szerb JC, Butterworth RF (1992) Effect of ammonium ions on synaptic transmission in the mammalian central nervous system. Prog Neurobiol 39:135–153
Tahamtan M, Aghaei I, Pooladvand V, Sheibani V, Khaksari M, Shabani M (2017) Characterization of the CA1 pyramidal neurons in rat model of hepatic cirrhosis: insights into their electrophysiological properties. Metab Brain Dis 32:881–889
Ulusoy GK, Celik T, Kayir H, Gürsoy M, Isik AT, Uzbay TI (2011) Effects of pioglitazone and retinoic acid in a rotenone model of Parkinson’s disease. Brain Res Bull 85:380–384
Weissenborn K, Bokemeyer M, Krause J, Ennen J, Ahl B (2005) Neurological and neuropsychiatric syndromes associated with liver disease. Aids 19:S93–S98
Xing B, Liu M, Bing G (2007) Neuroprotection with pioglitazone against LPS insult on dopaminergic neurons may be associated with its inhibition of NF-κB and JNK activation and suppression of COX-2 activity. J Neuroimmunol 192:89–98
Zakaria A, Rady M, Mahran L, Abou-Aisha K (2019) Pioglitazone attenuates lipopolysaccharide-induced oxidative stress, dopaminergic neuronal loss and neurobehavioral impairment by activating Nrf2/ARE/HO-1. Neurochem Res 44:2856–2868
Zhao Y, Patzer A, Gohlke P, Herdegen T, Culman J (2005) The intracerebral application of the PPARγ-ligand pioglitazone confers neuroprotection against focal ischaemia in the rat brain. Eur J Neurosci 22:278–282
Acknowledgements
Funding for this study was provided by Kerman University of Medical Sciences and Jiroft University of Medical Sciences as a grant for the PhD thesis.
Author information
Authors and Affiliations
Contributions
MT, IA and MSH have conceived and designed the concept and road map of the study, searched the literature, designed the concept map and figures, and drafted the manuscript. VP and AN have critically reviewed the manuscript for its content, originality, usage of English language, and accuracy of interpreted data. MR designed the study, helped in manuscript preparation, and critically reviewed the manuscript. MR is the archival author and attests to the integrity of the original data and the analysis reported in this manuscript. All authors have made substantive contribution and attest to approving the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest and declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval
All procedures performed in studies were in accordance with the ethical standards of the ethical committee of Kerman University of Medical Sciences (Ethical approval number: IR.KMU.REC.1395.792, Reg. No. 95000573).
Consent for publication
Not applicable.
Conflict of Interest statement
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tahamtan, M., Aghaei, I., Shabani, M. et al. Peroxisome proliferator-activated receptor-γ doesn’t modify altered electrophysiological properties of the CA1 pyramidal neurons in a rat model of hepatic cirrhosis. Metab Brain Dis 37, 2687–2697 (2022). https://doi.org/10.1007/s11011-022-01057-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01057-7