Abstract
We investigate changes in gene expression of GluN1 subunit of N-Methyl-D-Aspartate (NMDA) receptor in the prefrontal cortex (PFC), hippocampus and striatum in a rat model of hepatic encephalopathy (HE). We used male Wistar rats in which HE was induced after a common bile duct ligation (BDL). The animals were divided into three sets, and each set included three groups of control, sham operated and BDL. In the first set of animals, blood samples collected for biochemical analysis on day 21 of BDL. In the second set, changes in nociception threshold was assessed on day 21 of BDL using a hotplate test. In the third set, whole brain extracted, and the PFC, the hippocampus and the striatum in each rat were immediately dissected. We used a semi-quantitative RT-PCR method for evaluating the GluN1 gene expression. The biochemical analyses showed that plasma levels of ammonia and bilirubin in BDL rats were significantly increased compared to the sham control group on day 21 of BDL (P < 0.01). Nociception threshold was also increased in rats with BDL compared to sham group (P < 0.001). The results revealed that the GluN1 gene expression at mRNA levels in BDL group was decreased by 19 % in the PFC (P < 0.05) but increased by 82 % in the hippocampus (P < 0.01) compared to the sham control group; however, no significant change was observed in the striatum. It can be concluded that HE affects the GluN1 gene expression in rat brain with a site-specific pattern, and the PFC and hippocampus are more sensitive areas than striatum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatic encephalopathy (HE) is a term that is used to describe the alterations in cerebral function that result from liver failures (Butterworth 2008). There are different reports that HE is primarily induced by hyperammonemia followed by liver failure and subsequently causes morphological and physiological changes in astrocytes (Butterworth 2008; Rodrigo et al. 2010). In addition, it has been reported that HE affects neurons and neurotransmitter systems including gamma-aminobutyric acid (GABA) and glutamate, which may play main roles in the pathogenesis of the neurological alterations in HE (Cauli et al. 2009b; Felipo 2013; Llansola et al. 2013). Glutamate is the main excitatory neurotransmitter in the central nervous system that involved in many cerebral and neurological functions (Paoletti and Neyton 2007; Rousseaux 2008). It has been shown that blocking of N-methyl-D-aspartate (NMDA) subtype of glutamate receptors improves alterations induced by HE in rats and delays their death (Cauli et al. 2014; Felipo 2013; Vogels et al. 1997).
NMDA receptors play key roles in excitatory synaptic transmission and are also thought to be potential targets for the treatment of pathological changes of HE (Felipo 2013). According to molecular data, functional NMDA receptors are tetramers composed of different subunits namely GluN1, GluN2A-D, GluN3A-B, which were formerly known as NR1, NR2A-D and NR3 A-B in the old nomenclatures (Glasgow et al. 2015; Paoletti 2011). Different assemblies of NMDA receptor subunits have been reported in different sites of brain but GluN1 is an obligatory subunit of all endogenous NMDA receptors (Sanz-Clemente et al. 2013). It has been shown that changes in expression of the GluN1 subunit modulate NMDA receptor function, which subsequently may set the tone of the nervous system’s response to noxious stimuli and tissue injury (Da Silva et al. 2010).
Rats with common bile duct ligation (BDL) has been accepted and used as a model of chronic liver failure and HE (Butterworth et al. 2009; Rodrigo et al. 2010). In case of chronic liver failure and the subsequent HE, concentration of ammonia in circulation increases and it has been proposed that the reserve mechanisms of detoxification of ammonia in astrocytes may turn on (Felipo 2013; Rodrigo et al. 2010). Astrocytes turn ammonia into glutamine, which in turn it is transported to neurons where glutaminase deaminate glutamine into glutamate (Olde Damink et al. 2009). Therefore, it is possible that an increase in glutamate and excessive activation of NMDA receptors subsequently could also contribute to changes by which HE affects neural functions (Cauli et al. 2009b).
NMDA receptors are highly expressed in the forebrain structures including the PFC, hippocampus and striatum (Paoletti 2011). Considering the importance of GluN1 as an obligatory subunit of NMDA receptors, the aim of this study was to investigate changes in the GluN1 gene expression in the PFC, hippocampus and striatum in a rat model of HE to correlate changes in gene expression of NMDA receptors within these areas with HE. We also aimed to propose a shared link between possible changes in expression of NMDA receptors in the above areas of rat brain with nociception processing.
Materials and methods
Subjects
In this study, we used 60 male Wistar rats weighing 300–350 g. The animals were kept in an animal house at a constant temperature (22 ± 2 °C) under a 12 h light/dark cycle (light beginning at 7:00 a.m.). They had free access to food and water except for during the experiments. Experimental groups consisted of either eight rats in biochemical analysis of the serum and hotplate test or four rats in the gene expression studies, respectively. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (2011), prepared by the National Academy of Sciences’ Institute for Laboratory Animal Research.
Surgical laparotomy
Three groups of control, sham and rats with BDL were used in each part of the study. Sham and BDL groups were undergone laparotomy under anesthesia induced by intraperitoneal (i.p.) injection of a mixture of ketamine/xylazine (50 and 5 mg/kg, respectively). Sham operation consisted of laparotomy, bile duct identification and manipulation but without ligation and resection. In BDL rats, common bile duct was exposed, ligated at two points with approximately 5 mm apart, and then transected at the midpoint between the ligatures. Each rat received injection of 1 ml normal saline (i.p.) after closure of the abdominal wound and moved to a clean box until complete recovery. Mortality due to operation was about six percent during and/or after laparotomies.
Experimental design
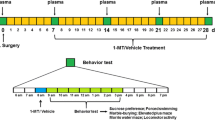
Three sets of animals were used to complete this study. In the first set, a total number of 24 rats in three groups of control, sham and BDL (n = 8, in each group) were used to examine plasma levels of ammonia and bilirubin. In the second set, three groups of control, sham and BDL (n = 8, in each group) were used to study nociception threshold using a hotplate test. In the third set, three groups of control, sham and BDL (n = 4, in each group) were used to examine the GluN1 gene expression in the PFC, hippocampus and striatum. These animals were independent of those that we used in our previous study (Ahmadi et al. 2015).
Biochemical analysis
Twenty one days of bile duct ligation (BDL) rats of the first set of animals were anaesthetized and blood samples collected from heart for biochemical analysis. Plasma levels of ammonia was determined using a Biorex Ammonia Assay Kit (Biorex Diagnostics, Muckamore, Antrim, UK). In this assay, ammonia combines with α-ketoglutarate and NADPH in the presence of glutamate dehydrogenase to produce glutamate and NADP. This reaction results in a decrease in NADPH absorbance measured at 340 nm which is proportional to the concentration of ammonia in plasma. Total and direct bilirubin levels in plasma were also measured using standard kits (Darmankav, Esfahan, Iran). These assays are based on the reaction of bilirubin with diazotized sulphanilic acid in acidic medium to form the pink colored azobilirubin. The intensity of absorbance of the colored produced at 578 nm for total bilirubin and at 546 nm for direct bilirubin are directly proportional to their concentration present in the sample. Indirect bilirubin was indirectly calculated from difference of total and direct bilirubin.
Measurement of nociception
We used the second set of animals including control, sham and BDL groups to assess pain behavior using a hotplate test according to the method that we have earlier reported (Ahmadi et al. 2015). In brief, time elapse between placement of each animal on the hotplate (52 ± 0.1 °C) and licking one of the hind paws or first jumping was measured as an index of pain reaction latency. First, baseline latency was measured 1 day before surgical laparotomies. Second, different experimental groups (control, sham and BDL) on day 21 of BDL were tested to measure a test latency on the hotplate apparatus. A cutoff time of 80 s was set to prevent tissue damage. Finally, the two measured latencies were converted to percentage maximum possible effect (%MPE) using the following formula: % MPE = [(test latency – baseline latency)/(cut − off time – baseline latency)] × 100 (Keil and Delander 1995; Ossipov et al. 1990). An increase in %MPE means an elevation in nociception threshold.
Dissection of the PFC, hippocampus and striatum from rat brains to examine the GluN1 gene expression on day 21 of BDL
Three groups of control, sham and BDL rats (n = 4, in each group) were used to examine the GluN1 gene expression in the PFC, hippocampus and striatum. Twenty-one days of BDL, each rat was sacrificed, whole brain was quickly removed from the skull and the PFC, the hippocampus and the striatum were immediately dissected on an ice-chilled sterile surface according to previous reported method (Chiu et al. 2007). Then, each tissue was immediately moved into a tube in which it was submerged in RNAlater RNA Stabilization Reagent (QIAGEN, USA) and incubated overnight at 4 °C. Then, the RNAlater solution was drained after 24 h and the tubes containing the tissues were stored at −80 °C until further analysis.
Total RNA extraction and cDNA synthesis
Total RNA was extracted from 70 mg of each tissue using a TRIZOL method according to our previous study (Ahmadi et al. 2015). In brief, each tissue sample homogenized for 60 s in a tube containing 1 ml lysis buffer (RNX+ reagent, Cinagen, Tehran, Iran), with a high speed homogenizer (Silent Crusher S., Heidolph, Germany), then was subjected to a total RNA extraction according to manufacturer’s manual (Cinagen, Tehran, Iran). Quality of the extracted total RNA was assessed by electrophoresis on 1 % agarose gel to visualize 28 s and 18 s ribosomal RNA. The quantities of total RNAs were also measured spectrophotometerically (Specord210, Analytic Jena, Germany). Synthesis of cDNA from the total RNAs was performed using a Viva 2-step RT-PCR Kit according to manufacturer’s protocol (Vivantis Technologies, Selangor Darul Ehsan, Malaysia).
Polymerase chain reaction (PCR)
We used a semi-quantitative RT-PCR method to evaluate the GluN1 gene expression in the brain areas (Marone et al. 2001). Related cDNAs of the β-actin (as control) and the GluN1 genes were amplified using thermal cycling (C1000 Thermal Cycler, BIO-RAD, USA). Primers for both genes were designed at exon-exon junctions to prevent amplifications of genomic DNA fragments of the genes. The accession numbers were NM-031144 for β-actin gene and NM-017010 for the GluN1 subunit of NMDA receptor gene. For primer designing, we have selected a sequence of the GluN1 gene that was shared between its different isoforms. Primers had the following sequences: the β-actin forward primer, 5′-CTGGGTATGGAATCCTGTGGC-3′; the β-actin reverse primer, 5′-AGGAGGAGCAATGATCTTGATC-3′; the GluN1 forward primer, 5′-TGGCATCATCGGACTTCAG-3′; the GluN1 reverse primer, 5′- TCTGGTGGACATCTGGTATC-3′.
PCR was carried out in a reaction volume of 20 μl consisting of 10 μl of PCR Master Mix (Thermo Scientific), 2 μl of cDNA, 3 μl of upstream and downstream mix of the GluN1 primers (10 μM), 2 μl of upstream and downstream mix of the β-actin primers (10 μM), and nuclease free water up to 20 μl. According to a pilot PCR optimization process, thermal cycling was initiated with a first denaturation step of 95 °C for 3 min, followed by 27 cycles of thermal cycling of 94 °C for 30 s, 61 °C for 30 s, 72 °C for 30 s, and finally followed by 10 min of a final extension step at 72 °C and was terminated at 4 °C for 5 min. Finally, the PCR products were subsequently analyzed on 2 % agarose gel electrophoresis and the bands were quantified with densitometry using Image J software.
Statistical analysis
Plasma levels of ammonia and bilirubin data failed to pass normality and/or equal variance tests, so these data were analyzed by Kruskal-Wallis one-way non-parametric ANOVA. Then, pairwise comparisons were done with Mann-Whitney’s U-test and Holmes-Bonferroni’s correction test was used after multiple comparisons. The obtained data for hotplate test and the quantified values for the GluN1 gene expression passed normality and equal variance tests and they were analyzed with one-way ANOVA. Then, after a significant F value, Holm-Sidak’s test was used for pairwise comparisons. The quantified value for the GluN1 gene expression in each sample were normalized as percentage of the GluN1 to β-actin ratio and then was set at 100 % as the relative the GluN1 gene expression in the control group. P < 0.05 was considered statistically significant level.
Results
Plasma levels of ammonia and all types of bilirubin were increased in BDL rats
Kruskal–Wallis one-way ANOVA revealed that plasma levels of ammonia was significantly altered between experimental groups on day 21 of BDL [H (2) = 15.68, P < 0.001]. In addition, all types of bilirubin were significantly altered between experimental groups on day 21 of BDL [direct bilirubin, H (2) = 16.23, P < 0.001; indirect bilirubin, H (2) = 15.4, P < 0.001; and total bilirubin H (2) = 15.39, P < 0.001]. Pairwise comparisons with Mann-Whitney’s U-test showed a significant increase (P < 0.01) in plasma levels of ammonia and all types of bilirubin in the group with BDL compared to the sham control group (Table 1).
Nociception threshold was increased in rats with BDL
One-way ANOVA revealed that nociception threshold in experimental groups was significantly altered on day 21 of BDL [F (2, 21) = 59.18, P < 0.001]. The post hoc test revealed that nociception threshold in rats with BDL compared to the sham group was significantly increased (Fig. 1).
The GluN1 gene expressions at mRNA levels were significantly decreased in the PFC, increased in the hippocampus and remained without significant change in the striatum
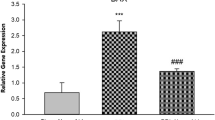
One-way ANOVA showed that the GluN1 gene expression in the PFC was altered between the experimental groups [F (2, 9) = 7.87, P < 0.05], and in the hippocampus [F (2, 9) = 11.36, P < 0.01]. However, no significant change in the GluN1 gene expression was observed between experimental groups in the striatum [F (2, 9) = 0.72, P > 0.05]. Post hoc test with Holm-Sidak’s method revealed a significant decrease of the GluN1 gene expression by 19 % in the PFC (Fig. 2a) but a significant increase by 82 % in the hippocampus (Fig. 2b) compared to the sham control group after 21 days of BDL. However, post hoc test revealed no significant change for the GluN1 gene expression in the striatum (Fig. 2c).
The GluN1 gene expression on day 21 of BDL in the prefrontal cortex (a), in the hippocampus (b) and in the striatum (c) of control, sham and BDL groups (n = 4). Each bar represents mean ± S.E.M of the GluN1 gene expression normalized to β-actin as control gene. *P < 0.05 and **P < 0.01 compared to the GluN1 gene expression in the respective sham control group
Discussion
The results of biochemical analyses in this study showed that plasma levels of ammonia and all types of bilirubin including direct, indirect and total bilirubin were significantly increased in rats with BDL. An increase in plasma bilirubin levels is a common result of BDL that has been widely reported across literatures (Adler et al. 1977; Maillette de Buy Wenniger and Beuers 2010; Rodriguez-Garay 2003). The increased levels of bilirubin in plasma confirmed obstruction of common bile duct during the surgical laparotomy, which in turn might cause chronic liver failure (Butterworth et al. 2009; Magen et al. 2009; Wright et al. 2010). In addition, chronic liver failure may induce hyperammonemia and HE, which has been previously reported by other investigators (Cauli et al. 2009a; Rodrigo et al. 2010).
According to the previous researches, accumulations of ammonia and bilirubin and other toxic substances in the liver and plasma could affect functions of not only the liver but also the brain (Benedetti et al. 1997; Garcia-Ayllon et al. 2008; Huang et al. 2004). It has been also reported that liver failure alters brain functions via affecting neurotransmitter systems (Garcia-Ayllon et al. 2008; Lozeva et al. 2004). During hyperammonemia in HE, astrocytes detoxify ammonia into glutamine. Then, glutamine is transported to neurons and is converted into glutamate by glutaminase (Olde Damink et al. 2009). Therefore, it is possible that an increase in glutamate levels in neurons during HE may lead to imbalances in neural circuits that may finally affect neural functions.
The result of the hotplate test in the present study revealed that nociception threshold was increased in rats with HE on day 21 of BDL. This may reflect slowing of sensory processing in HE. It has been shown that plasma total opioid levels in cholestatic liver disease is increased, which may underlie changes including pruritus and analgesia after BDL (Alemi et al. 2013; Bergasa et al. 1995; Nicoll et al. 2005). In support of the involvement of a opioid system in the antinociception induced by BDL, we have recently reported that it was almost completely prevented by injection of a mu-opioid receptor antagonist, naloxone, on day 21 of BDL (Ahmadi et al. 2015). In addition, NMDA receptor pathway has been shown to be involved in modulation of cholestasis-induced antinociception in rats (Hasanein et al. 2007). According to previous researches, NMDA receptors play a key role not only in pain signal transmission in the spinal cord but also in pain perception at higher brain centers (Piovesan et al. 2008; Rodriguez-Munoz et al. 2012; Zhou et al. 2011). Therefore, we propose possible changes in NMDA receptor system may account for, at least partly, alteration in nociception threshold in the BDL model of HE.
To test changes in NMDA receptor expression in HE, we examined changes in gene expression of GluN1 as an obligatory subunit of NMDA receptors in some forebrain areas including the PFC, hippocampus and striatum. The results revealed that the GluN1 gene expression was significantly decreased in the PFC but was increased in the hippocampus; however, it was remained without significant changes in the striatum. It has been reported that alterations in certain neurotransmitters including GABA and glutamate play a main role in the pathogenesis of the neurological alterations in HE (Cauli et al. 2009b). According to previous reports, the process of neurotransmission can be altered in HE at different steps (Wen et al. 2013). We propose that hyperammonemia in HE may alter expression of NMDA receptors at mRNA levels. It has been reported that for many genes, transcript and protein levels do not correlate well (Tian et al. 2004). However, there are some reports that transcript levels of the GluN1 subunit are correlate with its protein levels (Jayanthi et al. 2014; Priya et al. 2013). In addition, it has been recently reported that complete deletion of the obligatory GluN1 subunit of the NMDA receptors in hippocampal slice cultures completely eliminates NMDA receptors (Incontro et al. 2014). Therefore, it can be proposed that changes in the GluN1 gene expression in the present study may importantly affect the amount of NMDA receptors in neurons of the examined brain areas, which subsequently may affect neural functions in HE.
Furthermore, according to the present results, it is possible that changes in the GluN1 gene expression in a specific brain area in rats with HE cannot be extrapolated to other areas or to the whole brain. These results may be result from special pattern of afferents and interneurons in each area of the brain. In support of the different changes of the GluN1 expression in different brain areas, Cauli et al. (2009a) have also reported that GABAergic tone is increased in cerebellum of rats with chronic hyperammonemia but reduced in cerebral cortex of the same animal (Cauli et al. 2009a). One may propose that hyperammonemia increases glutamate in the PFC, which subsequently induces a decrease in the GluN1 gene expression. On the other hand, an increase in the GluN1 gene expression in the hippocampus may result from imbalances in neurotransmitter systems that in turn may cause cognitive dysfunctions in HE (Rodrigo et al. 2010). However, the results of the GluN1 gene expression in the striatum further indicate that cellular adaptation in different brain areas in HE may be a site-specific process.
The results of a recent research from our laboratory revealed that the mu-opioid receptor 1 (MOR1) gene expression was also influenced by BDL in the PFC, hippocampus and hypothalamus but not in the striatum (Ahmadi et al. 2015). We proposed that the observed changes in the MOR1 gene expression might directly or indirectly result from an increase in endogenous opioids. Alternatively, one may propose that changes in opioid systems due to cholestasis may also influence neural circuits affecting NMDA receptor systems. Therefore, alteration in the GluN1 gene expression could also be a consequence of changes in opioid systems and their subsequent resulting changes in rats with HE. However, more complementary experiments needs to be done to reveal the exact mechanism of these changes.
Conclusion
In summary, our results showed that hyperammonemia in a rat model of HE decreased the GluN1 gene expression in the PFC but increased it in the hippocampus, and remained without significant changes in the striatum. These results may propose that changes in the GluN1 gene expression in rat brain with HE have a site-specific pattern and consequence of these changes may underlie slowness of brain functions including sensory perception (for example nociception) and cognitive dysfunctions in HE.
References
Adler RD, Wannagat FJ, Ockner RK (1977) Bile secretion in selective biliary obstruction. Adaptation of taurocholate transport maximum to increased secretory load in the rat. Gastroenterology 73:129–136
Ahmadi S, Karami Z, Mohammadian A, Khosrobakhsh F, Rostamzadeh J (2015) Cholestasis induced antinociception and decreased gene expression of MOR1 in rat brain. Neuroscience 284:78–86
Alemi F et al (2013) The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest 123:1513–1530
Benedetti A et al (1997) Cytotoxicity of bile salts against biliary epithelium: a study in isolated bile ductule fragments and isolated perfused rat liver. Hepatology 26:9–21
Bergasa NV, Sabol SL, Young WS 3rd, Kleiner DE, Jones EA (1995) Cholestasis is associated with preproenkephalin mRNA expression in the adult rat liver. Am J Physiol 268:G346–G354
Butterworth RF (2008) Pathophysiology of hepatic encephalopathy: the concept of synergism. Hepatol Res 38(Suppl 1):S116–S121
Butterworth RF, Norenberg MD, Felipo V, Ferenci P, Albrecht J, Blei AT (2009) Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int 29:783–788
Cauli O, Mansouri MT, Agusti A, Felipo V (2009a) Hyperammonemia increases GABAergic tone in the cerebellum but decreases it in the rat cortex. Gastroenterology 136:1359–1367, e1351–1352
Cauli O et al (2009b) Glutamatergic and gabaergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab Brain Dis 24:69–80
Cauli O et al (2014) Blocking NMDA receptors delays death in rats with acute liver failure by dual protective mechanisms in kidney and brain. Neuromol Med 16:360–375
Chiu K, Lau WM, Lau HT, So KF, Chang RC (2007) Micro-dissection of rat brain for RNA or protein extraction from specific brain region. J Vis Exp 7:269
Da Silva LF, Walder RY, Davidson BL, Wilson SP, Sluka KA (2010) Changes in expression of NMDA-NR1 receptor subunits in the rostral ventromedial medulla modulate pain behaviors. Pain 151:155–161
Felipo V (2013) Hepatic encephalopathy: effects of liver failure on brain function. Nat Rev Neurosci 14:851–858
Garcia-Ayllon MS et al (2008) Brain cholinergic impairment in liver failure. Brain 131:2946–2956
Glasgow NG, Siegler Retchless B, Johnson JW (2015) Molecular bases of NMDA receptor subtype-dependent properties. J Physiol 593:83–95
Hasanein P, Parviz M, Keshavarz M, Javanmardi K, Allahtavakoli M, Ghaseminejad M (2007) Modulation of cholestasis-induced antinociception in rats by two NMDA receptor antagonists: MK-801 and magnesium sulfate. Eur J Pharmacol 554:123–127
Huang LT, Hsieh CS, Chou MH, Chuang JH, Liou CW, Tiao MM, Lai MC (2004) Obstructive jaundice in rats: cause of spatial memory deficits with recovery after biliary decompression. World J Surg 28:283–287
Incontro S, Asensio CS, Edwards RH, Nicoll RA (2014) Efficient, complete deletion of synaptic proteins using CRISPR. Neuron 83:1051–1057
Jayanthi S et al (2014) Methamphetamine downregulates striatal glutamate receptors via diverse epigenetic mechanisms. Biol Psychiatry 76:47–56
Keil GJ 2nd, Delander GE (1995) Time-dependent antinociceptive interactions between opioids and nucleoside transport inhibitors. J Pharmacol Exp Ther 274:1387–1392
Llansola M et al (2013) Chronic hyperammonemia, glutamatergic neurotransmission and neurological alterations. Metab Brain Dis 28:151–154
Lozeva V, Montgomery JA, Tuomisto L, Rocheleau B, Pannunzio M, Huet PM, Butterworth RF (2004) Increased brain serotonin turnover correlates with the degree of shunting and hyperammonemia in rats following variable portal vein stenosis. J Hepatol 40:742–748
Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R, Berry EM (2009) Cannabidiol ameliorates cognitive and motor impairments in mice with bile duct ligation. J Hepatol 51:528–534
Maillette de Buy Wenniger L, Beuers U (2010) Bile salts and cholestasis. Dig Liver Dis 42:409–418
Marone M, Mozzetti S, De Ritis D, Pierelli L, Scambia G (2001) Semiquantitative RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biol Proced Online 3:19–25
National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) Guide for the care and use of laboratory animals. 8th edition. National Academies Press (US), Washington (DC)
Nicoll J, Axiotis CA, Bergasa NV (2005) The delta opioid receptor 1 is expressed by proliferating bile ductules in rats with cholestasis: implications for the study of liver regeneration and malignant transformation of biliary epithelium. Med Hypotheses 65:1099–1105
Olde Damink SW, Jalan R, Dejong CH (2009) Interorgan ammonia trafficking in liver disease. Metab Brain Dis 24:169–181
Ossipov MH, Harris S, Lloyd P, Messineo E, Lin BS, Bagley J (1990) Antinociceptive interaction between opioids and medetomidine: systemic additivity and spinal synergy. Anesthesiology 73:1227–1235
Paoletti P (2011) Molecular basis of NMDA receptor functional diversity. Eur J Neurosci 33:1351–1365
Paoletti P, Neyton J (2007) NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol 7:39–47
Piovesan EJ et al (2008) Influence of NMDA and non-NMDA antagonists on acute and inflammatory pain in the trigeminal territory: a placebo control study. Arq Neuropsiquiatr 66:837–843
Priya A, Johar K, Wong-Riley MT (2013) Specificity protein 4 functionally regulates the transcription of NMDA receptor subunits GluN1, GluN2A, and GluN2B. Biochim Biophys Acta 1833:2745–2756
Rodrigo R, Cauli O, Gomez-Pinedo U, Agusti A, Hernandez-Rabaza V, Garcia-Verdugo JM, Felipo V (2010) Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology 139:675–684
Rodriguez-Garay EA (2003) Cholestasis: human disease and experimental animal models. Ann Hepatol 2:150–158
Rodriguez-Munoz M, Sanchez-Blazquez P, Vicente-Sanchez A, Berrocoso E, Garzon J (2012) The mu-opioid receptor and the NMDA receptor associate in PAG neurons: implications in pain control. Neuropsychopharmacology 37:338–349
Rousseaux C (2008) A review of glutamate receptors I: current understanding of their biology. J Toxicol Pathol 21:25–21
Sanz-Clemente A, Nicoll RA, Roche KW (2013) Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist Rev J Neurobiol Neurol Psychiatry 19:62–75
Tian Q et al (2004) Integrated genomic and proteomic analyses of gene expression in Mammalian cells. Mol Cell Proteomics MCP 3:960–969
Vogels BA, Maas MA, Daalhuisen J, Quack G, Chamuleau RA (1997) Memantine, a noncompetitive NMDA receptor antagonist improves hyperammonemia-induced encephalopathy and acute hepatic encephalopathy in rats. Hepatology 25:820–827
Wen S, Schroeter A, Klocker N (2013) Synaptic plasticity in hepatic encephalopathy—a molecular perspective. Arch Biochem Biophys 536:183–188
Wright G et al. (2010) Role of aquaporin-4 in the development of brain oedema in liver failure. J Hepatol 53:91–97
Zhou HY, Chen SR, Pan HL (2011) Targeting N-methyl-D-aspartate receptors for treatment of neuropathic pain. Expert Rev Clin Pharmacol 4:379–388
Funding
This study was not funded by any grant and/or financial support.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
“All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmadi, S., Poureidi, M. & Rostamzadeh, J. Hepatic encephalopathy induces site-specific changes in gene expression of GluN1 subunit of NMDA receptor in rat brain. Metab Brain Dis 30, 1035–1041 (2015). https://doi.org/10.1007/s11011-015-9669-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-015-9669-x