Abstract
The purpose of the present study is to determine the effects of gallic acid (GA) on sodium arsenite (iAS)-induced behavior deficits and memory alteration in male rats. Thirty six animals were divided in to 6 groups (six animals in each) (i) saline+saline; (ii) saline+GA (50 mg/kg); (iii) saline+ GA (100 mg/kg) (iv) iAS + saline; (v) iAS + GA(50 mg/kg); (vi) iAS + GA (100 mg/kg). Animals were treated with iAS (2.5 mg/kg/ml); GA (50 and 100 mg/kg/ml) and saline (0.9%; 1 ml/kg) for 4 weeks. Repeated administration of iAS increases immobility time in forced swim test and decreases time spent in open arm (elevated plus maze) and light box (light dark activity box test) suggests depression like and anxiety-like symptoms respectively. On the other hand, animals treated with iAS + GA decreases immobility time and increases time spent in open arm and light box than saline+iAS treated animals suggests anxiolytic and antidepressant-like behavior of GA. Repeated administration of iAS also involves in memory impairment as observed in the Morris water maze test that is reversed by co-administration of GA, indicates that GA also involves in the enhancement of memory. Brain malondialdehyde (MDA) levels, antioxidant enzymes and acetylcholinesterase (AChE) activities also observed in the present study. Results show that iAS produces oxidative stress by increasing lipid peroxidation and decreasing antioxidant enzyme activity. Conversely co-administration of GA produces antioxidant effects by normalization of oxidative stress induced by iAS. Alteration in iAS induced AChE activity is also reversed by GA. It is suggested that GA via its antioxidant potential, has protective effects on iAS induced behavioral deficits and memory alteration. The findings have a strong implication on iAS induced neurological diseases, such as depression, anxiety, Alzheimer’s disease and dementia etc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arsenic (iAS) is an element that naturally occurring in food, soil, and water. Exposure of iAS has become a global human health apprehension of utmost significance. Acute and chronic exposure of iAS has been associated with various toxic indices. Drinking water (0.01–3.7 mg/l) is the major source of iAS exposure in living organisms. iAS can contribute to a spectrum of diseases like cancers (skin, lung, bladder, liver) and other chronic effects like diabetes, keratosis, hyperkeratosis, hepatopathy, neuropathy and gastrointestinal disorders either by acute or chronic exposure. Excessive generation of reactive oxygen species (ROS) is the main cause of various diseases that caused genetic inequity and malfunctioning of body’s antioxidant defence mechanism (Hei et al.1998). Studies on experimental animals have shown that iAS cause neurotoxicity and deteriorate myelin sheath, vanishing of axons, vacuolar disintegration, and loss formation of the synapse (Piao et al. 2011) and leads various neurological diseases including memory impairment, anxiety and depression (Nutt and Stein 2006; Boyer 2000).
ROS, which causes oxidative stress (OS) causing an imbalance between the antioxidant defence system levels and the production of free radicals, are produced by environmental toxicants to prompt various types of neurological diseases (Coyle and Puttfarcken 1993; Jomova et al. 2011). Neuronal injuries have long been established to play a vital role in toxicant-induced OS leading to neuronal cells death and impairment of brain functions, because the brain relies on aerobic respiration, utilizes a huge amount of oxygen, and has high poly-unsaturated lipid contents, making it vulnerable to OS deterioration (Loh et al. 2006). iAS directly inhibits mitochondrial complex I resulting in increased ROS generation and thiol oxidation (Nutt et al. 2005). The blockade of complex II has been shown to result in reduced ATP production and increased OS and membrane depolarization, triggering cell death pathways (Brouillet et al. 1999). Mitochondria have been shown to play a crucial role in the regulation of cell death pathways. Moreover, many recent studies have provided evidence that OS is significantly related to the development of toxic metals-induced pathophysiological progress of neurodegenerative diseases, including iAS (Ahmed et al. 2011). A significant relationship between neuronal cell death and impaired brain function related to iAS-induced ROS overproduction and production of inflammatory cytokines (Yu et al. 2017) which involved in the progression of neurological diseases such as anxiety, depression and memory (Więdłocha et al. 2018; Bharathi Ravid and Rao 2006). Moreover, iAS-induced OS is also linked with the activation of MAPKs/PI3KAkt/NF kappa β/mTOR/endoplasmic reticulum stress-regulated signaling pathways that lead to diverse cellular responses such as cell growth, differentiation, apoptosis and stress responses to environmental stimuli (Estan et al. 2012; Yen et al. 2012).
There have been fabulous struggles to thrive valuable components from medicinal plants with the purpose to accomplish an ideal level of neuroprotection. Consideration has been waged to an extensive variation of natural antioxidants that can hunt free radicals and shield cells from oxidative deterioration, such as resveratrol, quercetin, curcumin and catechins (Han et al. 2004; Lee et al. 2004). Gallic acid (GA) or 3,4,5-trihydroxybenzoic acid and its derivatives are polyphenol natural products mainly found in processed beverages such as red wine and green tea (Graham 1992). GA induces antioxidant, anti-inflammatory, anti-microbial, and anti-cancer activities (Bachrach and Wang 2002; Borges et al. 2013; Kubo et al. 2001) that are associated with different signaling pathways including MAPKs/NF kappa β/TGF-β (Huang et al. 2016, 2017) GA-containing plant extracts have been reported to contain anti-diabetic, anti-angiogenic and anti-melanogenic effects in addition to reducing the incidence of myocardial infarction, and oxidative liver and kidney damages (Constat 1997; Jadon et al. 2007; Kim 2007). GA also protects neural cells against in vitro β-amyloid peptide (Aβ)-induced death (Bastianetto et al. 2006). It can be used further as an antioxidant in foods, cosmetics, and in pharmaceuticals (Zhao et al. 2011). GA is nontoxic to mammals at pharmacological doses. The lethal dose at which 50% of the animals died (LD50) for GA is 5 g/kg body weight in rats (Shahrzad et al. 2001).
Based on the antioxidant effects of GA, many in vitro studies on cell lines have been reported (Serrano et al. 2010). We hypothesized that, being a polyphenol, GA may produce protective effects against iAS induced alteration in behavioral activity and memory function. In view the role of GA against intoxication of iAS, the present study aims to explore the protective effects of GA on iAS induced oxidative stress (OS), behavioral deficits and memory impairment in rats.
Material and method
Animals
Due to hormonal cycle variation in female and male; extensive studies reported on male rats. In the present study, Sprague Dawley male rats (weighing 170–190 g, 4–5 months by age) were confined alone 3 days prior to the start of the experiment. The entire study is approved by the Institutional Ethics and Animal Care Committee.
Chemicals and reagents
Sodium arsenite (iAS), GA, Thiobarbituric acid (TBA), H2O2 stock (35%) solution, Nitroblue tetrazolium (NBT), Trichloroacetic acid (TCA) and Dithio-bisnitrobenzoic acid (DTNB) and all other analytical grade reagents were bought from Sigma chemicals Co. (St. Louis, USA).
Experimental
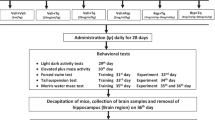
Thirty-six rats were at random alienated into 6 groups (6 animals in every set). (i) saline+saline (ii) saline+GA (50 mg/kg/ml) (iii) saline+GA (100 mg/kg/ml) (iv) iAS + saline (v) iAS + GA (50 mg/kg/ml) (vi)iAS + GA (100 mg/kg/ml). iAS (2.5 mg/kg/ml; Ola-Davies and Ajani 2016), GA (50 and 100 mg/kg/ml; Sarkaki et al. 2014) and saline (0.9%; 1 ml/kg) administered intraperitoneally (i.p), one by one as per group division with no interval as reported in other studies (Haleem et al. 2007; Samad et al. 2007; Samad and Haleem 2014). The drugs were administered daily for 4 weeks. Assessment of behavioral activities (Light dark activity box, Elevated plus maze, Forced swim test and Morris water maze) were conducted on next day. Animals were killed after the behavioral analysis following 4 weeks administration of iAS and GA to the gather the whole brain as done formerly by Samad and Saleem (2018). The samples were preserved at a set of temperature of −20 °C for the biochemical estimations. Malondialdehyde [MDA; lipid peroxidation (LP)] acetylcholinesterase (AChE) and antioxidant enzyme activity i.e. superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) in the brain.
Behavioral tests
Elevated plus maze (EPM) test
The plus maze tools used in the current analysis was comprised of four arms in which two were closed and two were opened as described before (Naqvi et al. 2012). The arms were of the same length (50 cm) and width (10 cm). Arms were connected by middle area of 5 cm2. The maze was high from the floor at an elevation of 60 cm. To find out the commotion rat was positioned in the middle of the plus maze and time used up (spent) in the open arms was recorded for 300 s.
Light-dark activity box (LDA) test
The experiment was performed in a home-made box (Samad et al. 2005). The partition of equal size (26x26x26cm), with an entrance (12x12cm) between the compartments, differed in their sensory properties. Walls of on compartment were transparent and other Black. Rat positioned in this box probable to get ahead of more time in the dark box. To find out the commotion rat was launched via the black box. Time spent in the transparent box was recorded for a mean time of 300 s.
Forced swim test (FST)
The FST apparatus contained a glass chamber with 56 cm height and 30 cm width, which limited water at the height of 22 cm and temperature of 25 °C. In this glass chamber animals were independently forced to swim for 300 s. The height of water was designated so that animal was barred from moving the bottom of the glass chamber and to avertits escape from the glass chamber. The FST is usually used as standard pharmacological model for appraising depression like symptoms in rats/mice (Porsolt 1981). When the mice/rat is placed in an inevitable chamber which is filled with water then the progress of the state of immobility imitates the termination of determined escape directed behavior. In this test animal’s swimming behavior was monitored which can be defined as movement throughout the swim chamber (glass tank). The immobility time was observed. The animal is considered immobile when it makes no further tries to escape andonly tries to keep its head above the water.
Morris water maze (MWM) test
Morris Water Maze (MWM) test was done to observe the effects on spatial memory as designated by Haider et al. (2011). We have evaluated learning acquisition, long-term memory (LTM) and short-term memory (STM) in terms of expectancy to locate the escape platform. The test is based on two segments: the training segment and the test segment. Memory functions of the ratwere verified by observing down the holding latency. The cut off time was 120 s for each session. Primarily, the training session (once; Samad and Saleem 2018) was attained throughout which each rat has positioned into the water in such a way that their face was to the wall of the tank. Each animal was given 120 s to find and stand onto the concealed platform by using distal extra maze indications. Indications must be observable and useful to mice. They must be far enough to require the rat to use spatial analysis, rather than connotation, to resolve the task. If the rat positioned the platform it was permitted to stay on it for 10 s. Time on the platform must be enough for them to feel the location and to see the meticulous position. If it unsuccessful to locate the platform during the owed time, then it was guided moderately onto the platform. The test involved three trials: training, STM and LTM. After training of animals, STM was evaluated 60 min after a training session, and LTM was assessed after 24 h of training.
Biochemical analysis
Determination of LP
LP was evaluated by determining levels of MDA in the whole brain of mice. MDA was evaluated by calculating thiobarbituric reactive species using the method of Chow and Tappel (1972) in which the thiobarbituric acid reactive substances react with thiobarbituric acid to produce a red colored complex having peak absorbance at 532 nm.
Determination of activity of SOD
The SOD was assessed by the method of Naskar et al. (2011). An aliquot of brain homogenate (10%) was treated with 0.75 ml of ethanol and 0.15 ml of ice chilled chloroform then centrifuged. Then 0.5 ml of EDTA (0.6 mM) and 1.0 ml of carbonate-bicarbonate (0.1 M; pH 10.2) buffer was added in 0.5 ml of supernatant. The reaction was started by adding 0.5 ml of epinephrine (1.8 mM) and the absorbance was measured for 3 min at 480 nm. Blank contained all reagents except supernatant. Finally, the percent inhibition of SOD was calculated.
Determination of activity of CAT
CAT was estimated using a previously reported method (Pari and Latha 2004). Brain homogenate (10%) in 0.01 M phosphate buffer (pH 7.0) was prepared and filtered. Then 0.1 ml of filtrate was mixed with 1.4 ml of a reaction mixture that contained 0.4 ml of 2 M hydrogen peroxide and 1 ml of same phosphate buffer. The reaction was terminated after 1 min by adding 2.0 ml of dichromate-acetic acid reagent. Blank was contained distilled water in place of filtrate. The absorbance of both test and blank were measured at 620 nm to calculate percent inhibition of CAT.
Determination of activity of GPx
GPx activity was measured by the procedure of Flohe and Gunzler (1984). One ml of the reaction mixture was prepared which contained 0.3 ml of phosphate buffer (0.1 M, pH 7.4), 0.2 ml of reduced glutathione (2 mM), 0.1 ml of sodium azide (10 mM), 0.1 ml of H2O2 (1 mM), and 0.3 ml of brain supernatant. After incubation at 37 °C for 15 min, the reaction was terminated by addition of 0.5 ml 5% TCA. Tubes were centrifuged at 1500×g for 5 min, and the supernatant was collected. Phosphate buffer 0.2 ml (0.1 M, pH 7.4) and DTNB 0.7 ml (0.4 mg/ml) were added to 0.1 ml of reaction supernatant. After mixing, absorbance was recorded at 420 nm. Activity of GPx was expressed as μmol/min/g of the brain.
Determination of activity of AChE
The activity of AChE in homogenate was determined according to the method of Ellman et al. (1961) using ATC as a substrate. The reaction mixture contained 0.4 ml brain homogenate (0.02 g/ml), 2.6 ml phosphate buffer (0.1 M, pH 8.0), 100 μl DTNB. The reaction mixture was mixed by bubbling air and placed in the spectrophotometer. Once the reaction content was stable, the absorbance was noted at 412 nm for the basal reading followed by addition of 5.2 μl of ATC to this cuvette. Any change in absorbance was recorded from zero time followed by 10 min at 25 °C. The activity of AChE was expressed as μmol/min/g of brain tissue.
Statistical analysis
All the data statistically analyzed by Tukey’s test followed by two-way ANOVA. P < 0.05 was taken as significant.
Results
Effect of GA on iAS induced anxiety observed in elevated plus maze activity
Figure 1 shows the effects of GA on anxiety profile in iAS treated rats observed in EPM. Data for time spent in open arm analyzed by two-way ANOVA revealed that the significant effect of iAS [F1,30 = 11.11, p < 0.01], GA [F2,30 = 274.15, p < 0.01], and interaction between iAS× GA [F2,30 = 124.80, p < 0.01]. Tukey’s test showed that sub-chronic administration of iAS substantially decreased time spent in the open arm than control animals. Time spent in open arm substantially increased in GA (50 and 100 mg/kg) + saline and GA(50 and 100 mg/kg/ml) + iAS treated than their counterparts. Time spent in open arm substantially greater in GA + iAS than GA + saline treated animals. The result suggested that GA (50 and 100 mg/kg) have the potential to attenuate iAS induced anxiety in rats.
Effect of administration of iAS on time spent in open arm in Elevated plus maze test for the saline and GA treated rats. Values are mean ± SD (n = 6). Data was analyzed by Tukey’s test following two-way ANOVA. Statistical difference is represented as**p < 0.01 and *p < 0.05 versus respective control and ++p < 0.01 versus saline + saline and saline+GA (50 and 100 mg/kg) treated animals
Effect of GA on iAS induced anxiety observed in light dark box activity
Figure 2 shows the effects of GA on anxiety profile in iAS treated rats observed in LDA. Data for time spent in open arm analyzed by two-way ANOVA revealed that the effect of iAS (F1,30 = 0.64 p > 0.05) was not significant. Whereas effect of GA [F2,30 = 180.09, p < 0.01], and interaction between iAS× GA [F2,30 = 24.03, p < 0.01] were significant. Tukey’s test showed that sub-chronic administration of iAS substantially decreased the time spent in light box than control animals. Co administration of GA increased time spent in light box of saline and iAS treated animals than saline+saline and iAS + saline treated rats respectively. It is indicated that co-administration of GA prevented iAS induced anxiety-like behaviors.
Effect of administration of iAS on time spent in light box in light dark box test in animals treated with saline and GA. Values are mean ± SD (n = 6). Data was analyzed by Tukey’s test following two-way ANOVA. Statistical difference is represented as**p < 0.01 versus respective control and ++p < 0.01 versus saline+saline treated rats
Effect of GA on iAs induced depression-like symptoms observed in the forced swim test
Figure 3 shows the effects of GA administration on immobility time observed in FST in iAS treated rats. Data for time spent in open arm analyzed by two-way ANOVA revealed that the significant effect of iAS [F1,30 = 45.94, p < 0.01], GA [F2,30 = 341.99, p < 0.01], and interaction between iAS× GA [F2,30 = 82.81, p < 0.01]. Tukey’s test showed that sub-chronic administration of iAS substantially increased immobility time than saline treated animals. GA (50 and 100 mg/kg) + saline and GA(50 and 100 mg/kg) + iAS treated rats exhibit decreased immobility time than their counterparts. The result suggested that co administration of GA has antidepressant effects.
Effect of administration of iAS on immobility time in Forced swim test in animals treated with saline and GA. Values are mean ± SD (n = 6). Data was analyzed by Tukey’s test following two-way ANOVA. Statistical difference is represented as**p < 0.01 versus respective control and ++p < 0.01 versus saline+saline treated rats
Effects of co-administration of GA on iAS induced memory impairment
MWM was conducted to asses learning and memory function in animals treated with GA and iAS. Latency escape in MWM activity is conducted immediately after training (acquisition, Fig. 4a), 1 h (short term memory, Fig. 4b) and 24 h (long term memory, Fig. 4c). Results on acquisition analyzedby two-way ANOVA revealed that significant effect of iAS [F1,30 = 68.03, p < 0.01], GA [F2,30 = 81.37, p < 0.01], and iAS× GA [F2,30 = 31.00, p < 0.01]. Two-way ANOVA for short term memory showed that significant effect of iAS [F1,30 = 85.08, p < 0.01], GA [F2,30 = 115.63, p < 0.01], and iAS× GA [F2,30 = 29.03, p < 0.01]. Two-way ANOVA for long term memory showed that significant effect of iAS [F1,30 = 40.72, p < 0.01], GA [F2,30 = 61.43, p < 0.01], and iAS× GA [F2,30 = 16.21, p < 0.01]. Tukey’s test demonstrated that administration of iAS substantially increased latency escape, while GA (50 and 100 mg/kg) + iAS and while GA (100 mg/kg) + saline treated showed decreased latency escapethan their respective control during acquisition. Analysis of short term memory revealed that time to reach hidden platform was significantly greater in iAS + saline than saline+saline treated animals. This time substantially reduced byGA (50 and 100 mg/kg) + iAS and GA (50 and 100 mg/kg) + saline when compared to saline+iAS and saline+saline respectively. GA (50 mg/kg) + iAS showed greater latency escape than GA (50 mg/kg) + saline injected rats. Evaluation of long term memory revealed that latency escape was significantly greater in iAS + saline than saline+saline treated animals. The latency escapes substantially reduced by GA (50 and 100 mg/kg) + iAS than saline+iAS. GA (100 mg/kg) + saline showed smaller latency escape than saline+saline treated rats. Results showed that the co-administration of GA has protective effects on iAS induced disturbed short term and long term memory.
Effect of administration of iAS on acquisition a short term memory b and long term memory c in terms of escape latency (s) assessed by Morris water maze in animals treated with saline and GA. Values are mean ± SD (n = 6). Data was analyzed by Tukey’s test following two-way ANOVA. Statistical difference is represented as**p < 0.01and *p < 0.05 versus respective control and ++p < 0.01 versus saline+saline and saline+GA (50 mg/kg) treated groups
Effect of GA on iAS induced increased brain LP
Figure 5 shows the effects of GA on brain levels of MDA (LP) in iAS treated rats. Data analyzed by two-way ANOVA revealed that the significant effect of iAS [F1,30 = 68.47, p < 0.01], GA [F2,30 = 138.78, p < 0.01], and interaction between iAS× GA [F2,30 = 27.74, p < 0.01]. Tukey’s test showed that sub-chronic administration of iAS substantially increased LP. GA (50 and 100 mg/kg) + saline and GA (50 and 100 mg/kg) + iAS treated rats exhibit decreased MDA levels than their counterparts. It is indicated that GA reduced brain LP or oxidative stress.
Effect of administration of iAS on brain MDA level in animals treated with saline and GA. Values are mean ± SD (n = 6). Data was analyzed by Tukey’s test following two-way ANOVA. Statistical difference is represented as**p < 0.01 versus respective control and ++p < 0.01versus saline+saline treated rats
Effect of co-administration of GA on iAS induced alteration in brain antioxidant enzymes activity
Figure 6 shows the effects of GA on brain antioxidant enzyme activity in iAS treated rats. Data on SOD activity (6a) analyzed by two-way ANOVA revealed that the significant effect of iAS [F1,30 = 30.11, p < 0.01], GA [F2,30 = 127.66, p < 0.01], and interaction between iAS × GA [F2,30 = 15.84, p < 0.01]. Tukey’s test showed that sub-chronic administration of iAS substantially decreased % inhibition of SOD. GA (50 and 100 mg/kg) + iAS treated rats exhibit increased % inhibition of SOD than their counterparts.
Effect of administration of iAS on brain SOD (a), CAT (b) and GPx (c) activity in animals treated with saline and GA. Values are mean ± SD (n = 6). Data was analyzed by Tukey’s test following two-way ANOVA. Statistical difference is represented as**p < 0.01 versus respective control and ++p < 0.01and + p < 0.05 versus saline+saline treated animals
Data on CAT activity (6b) analyzed by two-way ANOVA revealed that the significant effect of iAS [F1,30 = 12.21, p < 0.01], GA [F2,30 = 85.95, p < 0.01], while interaction between iAS× GA [F2,30 = 2.62, p > 0.05] was not significant. Tukey’s test showed that GA (50 and 100 mg/kg) + saline and GA (50 and 100 mg/kg) + iAS treated animals exhibit increased CAT levels than saline+saline and saline+iAS treated rats.
Data on GPx activity (6c) analyzed by two-way ANOVA revealed that the significant effect of iAS [F1,30 = 19.83, p < 0.01], GA [F2,30 = 38.21, p < 0.01], while interaction between iAS× GA [F2,30 = 0.68, p > 0.05] was not significant. Tukey’s test showed that sub-chronic administration of iAS substantially decreased activity of GPx. GA (50 and 100 mg/kg) + saline and GA (50 and 100 mg/kg) + iAS treated rats exhibit increased GPx levels than their counterparts. Results showed that GA via is potential antioxidant activity enhanced the activity of antioxidant enzyme in the brain.
Effect of GA on brain acetylcholinesterase activity
Figure 7 shows the effects of GA on brain AChE activity in iAS treated rats. Data on AChE activity analyzed by two-way ANOVA revealed that the significant effect of iAS [F1,30 = 15.03, p < 0.01], GA [F2,30 = 68.94, p < 0.01], and interaction between iAS × GA [F2,30 = 14.05, p < 0.01]. Tukey’s test showed that sub-chronic administration of iAS substantially increased the activity of AChE. GA (50 and 100 mg/kg) + saline and GA (50 and 100 mg/kg) + iAS treated animals exhibit the decreased activity of AChE than their respective controls. It is suggested that GA has a role in memory function by decreasing AChE activity.
Effect of administration of iAS on brain AChE activity in animals treated with saline and GA. Values are mean ± SD (n = 6). Data was analyzed by Tukey’s test following two-way ANOVA. Statistical difference is represented as**p < 0.01 versus respective control and ++P < 0.01 versus saline+saline treated animals
Discussion
AS is an element found in the earth’s crust and biosphere and has been known as a human poison for centuries (Banu et al. 2009). A large quantity of AS results in the prospect of daily exposures to humans, which may be via ingestion through drinking water (major route) or through inhalation and skin absorption (minor route) (Shi et al. 2004). The present study, for the first time, evaluated the behavioral and biochemical effects of GA in iAS-induced OS. Clinical studies demonstrated that arsenicosis patients show psychiatric illnesses such as depression, mixed anxiety, and depressive disorder (Sen and Biswas 2012). In animal studies, perinatal exposure to relatively low levels of AS (0.05 mg/L) also significantly increase learned helplessness and immobility in a forced swim task that predisposes affected offspring to depressive-like behavior in the affected adult C57BL/6 J mouse model (Martinez et al. 2008). In the present work, we confirmed that sub-chronic administration of iAS induces anxiety-like and depression-like behaviors in control rats. After 4 weeks of iAS administration, the animal showed obvious anxiety-like behaviors in both behavioral tests for anxiety (EPM, LDA) (Figs. 1 and 2). Similarly, sub-chronic treatment of iAS also showed obvious depression like behavior in the FST (Fig. 3). On the contrary, the reversal of both anxiety- and depression-like behaviors occurred by co-treatment with GA (50 and 100 mg/kg).
An imbalance between OS biomarkers and antioxidants has also been known in a stress situation. Extensive studies reported that stress (anxiety and depression) diminished levels of antioxidant enzymes in human (Liu et al. 2015; Daglia et al. 2017) and animal (Samad et al. 2018) as well. Furthermore, it has also been reported that antidepressant (Liu et al. 2015), anxiolytic (de Almeida et al. 2014), various plant extracts (Samad et al. 2018) and their active components (Haider et al. 2015; Samad et al. 2018) can regulate the mechanism of antioxidant enzymes such as SOD, CAT and GPx, suppress inflammatory markers and inhibit OS. Previously it has reported that GA produces its antidepressant (Can et al. 2017), anti-anxiety (Mansouri et al. 2014) properties via potential antioxidant mechanism (Popovic-Milenkovic et al. 2014). The present study also shows that iAS induced depression and anxiety-like behaviors (Figs. 1, 2 and 3) overturned by co-administration of GA (50 and 100 mg/kg) through its antioxidant potential.
OS has a significant importance in iAS toxicity. Cellular antioxidant mechanism become impairs due to the large production of ROS and RNS that involves in the generation of inflammatory markers including TNF-α, IL-1β and IL-6 (Ingawale et al. 2014) and deteriorate the cellular components (Manna et al. 2008). OS is indicative by LP. MDA is the final product of LP, and its quantity can quantitatively imitate the level of LP in vivo (Całyniuk et al. 2016). The sub-chronic treatment of iAS elevates the levels of MDA (Fig. 5) which is an indicative of OS. AS-induced ROS production may be the thru consequence of metabolites of AS that may play a role as free radicals and involved in the depletion of antioxidants (Flora 2011). Antioxidant enzymes are also badly distorted by AS-induced toxicity, which diminished their action by obligatory to Thiol group (Isuzugawa et al. 2001). Antioxidant enzymes are weighed to be the first line of cellular defense against oxidative harm. An antioxidant metalloenzyme SOD lessens superoxide radicals to Water and Oxygen (McCord et al. 1976). A hemoprotein CAT that lessens hydrogen peroxide to oxygen and water (Gutteridge 1995). An increase in the production of superoxide radicals was observed following treatment of AS, due to the lower levels of SOD in the brain (Yamanaka et al. 1991). The activity of CAT was also reduced in the brain of the animal following intoxication of iAS. GPx is also important for the lessening of organic hydroperoxides (Flora 1999). So, in bulk generation of ROS can enhance the level of inflammatory markers and cease the activity of these antioxidant enzymes. In our study the % inhibion of SOD and activity of GPx was decreased following administration of iAS, while the activity of CAT is comparable to control (Fig. 6), confirms that iAS involves in the induction of OS by increasing LP and decreasing antioxidant enzymes mechanism.
Extensive studies have evidenced that, GA showed its antioxidant activity (Bachrach and Wang 2002; Moghadas et al. 2016) by hunting the ROS, decreasing the inflammatory activity (Saygin et al. 2016), inhibiting the activation of MAPKs/NFkappa β/TGF-β (Ahad et al. 2015) or correcting the antioxidant enzyme activity (Ker et al. 2013). `It is reported earlier that SOD is the first line of defence against OS (Samad et al. 2018). The free radical hunting activity of SOD is active only when it is tracked by the actions of CAT and GPx, as the dismutase activity of SOD produces hydrogen peroxide from the superoxide ion, which is more lethal than oxygen derived free radicals and requires to be hunted further by CAT and GPx (Blake et al. 1987). We observed that GA recovers the iAS-induced behavioral commotion (Figs. 1, 2 and 3) by regularizing the activity of SOD and withdrawing the negative properties of iAS toxicity on CAT, resulting in regular activity of GPx (Fig. 6). The results showed that co-treatment by GA (50 and 100 mg/kg) diminished the OS (Fig. 5) with enhancement in the activity of antioxidant enzymes (Fig. 6). These properties may be ascribed to the minimized OS subsequent in lessening of adverse effects of iAS induced toxicity on behaviors. Literature cited has shown that reduction of OS (Ker et al. 2013) may also suppress inflammatory responses (Saygin et al. 2016) with inhibition of activated signaling pathways (Ahad et al. 2015) by GA. On the basis of earlier reported protective effects of GA, results of the present study are also indicated that, the antidepressant and anxiolytic activity of GA is due to its antioxidant potential.
Co-administration of GA reduced escape latency during memory assessment (STM and LTM) in saline and iAS treated rats, indicating memory improving effects of GA (Fig. 4). Escape latency was increased in STM as well as LTM examine in iAS + saline treated rats, that is representing iAS as a toxic element that impairs the memory (Fig. 4). Acetylcholine is one of the neurotransmitters involves in memory function. Acetylcholine is not only present in central nervous system it also involves in other functions at the periphery level. AChE (a biomarker) which is used to determine the cholinergic functions (Lian et al. 2017; Emad et al. 2017; Papandreou et al. 2011). Previously it has been reported that sub-chronic (Wang et al. 2018) and chronic (Sun et al. 2015) administration of iAS disturbs the cognitive function (Wang et al. 2018; Sun et al. 2015) and increases AChE activity (Chandravanshi et al. 2014). Our presented data is also consistent with these results of increased AChE activity (Fig. 7) and impaired learning and memory (Fig. 4) following sub-chronic administration of iAS, most likely by falling of acetylcholine at the synapse (Kaufer et al. 1998). GA on the other hand increased the STM (Fig. 4b) and LTM (Fig. 4c) as well as acquisition (Fig. 4a), which may be ascribed to the decreased AChE activity (Fig. 7) in iAS treated animals. Various reports have shown that taurine (Guan et al. 2017), curcumin (Yadav et al. 2011) enhanced memory in AS-treated rats with decreased activity of AChE and inflammatory surge. In view of previous report, we assume that GA improves the neuronal plasticity (Hajipour et al. 2016) by reduction in inflammatory responses (Yadav et al. 2018) and inhibition of MAPK (Ma et al. 2018), NF-kβ (Khare et al. 2017) signaling pathways, which in turn affects the brain neurotransmitter levels. Hence an increase in the brain acetylcholine levels, the substrate for the enzyme, could be the cause of decreased AChE activity that was observed in the present study (Fig. 7), however, a novel finding is that GA may increase cognition beside iAS induced toxicity via its antioxidant potential.
In conclusion, the results of this present study support the previous studies of the antioxidant ability of GA. The present study,consequently,emphasizes the use of dietary sources rich in GA contents and/or supplementation of GA as an effective remedy for intoxication of iAS and associated disorders.
References
Ahad A, Ahsan H, Mujeeb M, Siddiqui WA (2015) Gallic acid ameliorates renal functions by inhibiting the activation of p38 MAPK in experimentally induced type 2 diabetic rats and cultured rat proximal tubular epithelial cells. Chem Biol Interact 240:292–303
Ahmed S, Mahabbat-e Khoda S, Rekha RS, Gardner RM, Ameer SS, Moore S, Ekstrom EC, Vahter M, Raqib R (2011) Arsenic-associated oxidative stress,inflammation, and immune disruption in human placenta and cord blood. Environ Health Perspect 119:258–264
Bachrach U, Wang YC (2002) Cancer therapy and prevention by green tea: role of ornithine decarboxylase. Amino Acids 22(1):1–13
Banu GS, Kumar G, Murugesan AG (2009) Effects of leaves extract of Ocimum sanctum L. on arsenic induced toxicity in wistar albino rats. Food Chem Toxicol 47(2):490–495
Bastianetto S, Yao ZX, Papadopoulos V, Quirion R (2006) Neuroprotective effects of green and black teas and their catechin gallate esters against beta-amyloid- induced toxicity. Eur J Neurosci 23(1):55–64
Bharathi Ravid R, Rao KS (2006) Role of metals in neuronal apoptosis: challenges associated with neurodegeneration. Curr Alzheimer Res 3:311–326
Blake DR, Allen RE, Lunee J (1987) Free radicals in biological systems: a review oriented to the inflammatory process. Br Med Bull 43(2):371–385
Borges A, Ferreira C, Saavedra MJ, Simoes M (2013) Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb Drug Resist 19(4):256–265
Boyer P (2000) Do anxiety and depression have a common pathophysiological mechanism? Acta Psychiatr Scand Suppl 102(406):24–29
Brouillet E, Conde F, Beal MF, Hantraye P (1999) Replicating Huntington’s disease phenotype in experimental animals. Prog Neurobiol 59:427–468
Całyniuk B, Grochowska-Niedworok E, Walkiewicz K, Kawecka S, Popiołek E, Fatyga E (2016) Malondialdehyde (MDA) -product of lipid peroxidation as marker of homeostasis disorders and aging. Ann Acad Med Silesiensis 70(4):224–228
Can ÖD, Turan N, DemirÖzkay Ü, Öztürk Y (2017) Antidepressant-like effect of gallic acid in mice: dual involvement of serotonergic and catecholaminergic systems. Life Sci 190(4):110–117
Chandravanshi LP, Yadav RS, Shukla RK, Singh A, Sultana S, Pant AB, Parmar D, Khanna VK (2014) Reversibility of changes in brain cholinergic receptors and acetylcholinesterase activity in rats following early life arsenic exposure. Int J Dev Neurosci 34:60–75
Chow CK, Tappel AL (1972) An enzymatic protective mechanism against lipidperoxidation damage to lungs of ozone-exposed rats. Lipids 7:518–524
Constat J (1997) Alcohol, ischemic heart disease, and the French paradox. Coron Artery Dis 8(10):645–649
Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262:689–695
Daglia M, Di Lorenzo A, Nabavi SF, Sureda A, Khanjani S, Moghaddam AH, Braidy N, Nabavi SM (2017) Improvement of antioxidant defences and mood status by Oral GABA tea administration in a mouse model of post-stroke depression. Nutrients 9(5):446. https://doi.org/10.3390/nu9050446
de Almeida AA, de Carvalho RB, Silva OA, de Sousa DP, de Freitas RM (2014) Potential antioxidant and anxiolytic effects of (+)-limonene epoxide in mice after marble-burying test. Pharmacol Biochem Behav 118:69–78
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Emad S, Qadeer S, Sadaf S, Batool Z, Haider S, Perveen T (2017) Attenuation of stress induced memory deficits by nonsteroidal anti-inflammatory drugs (NSAIDs) in rats: Role of antioxidant enzymes. Pharmacol Rep 69(2):300–305
Estan MC, Calvino E, de Blas E, Boyano-AdanezMdel C, Mena ML, Gomez-Gomez M, Rial E, Aller P (2012) 2-Deoxy-d-glucose cooperates with arsenictrioxide to induce apoptosis in leukemia cells: involvement of IGF-1R-regulatedAkt/mTOR, MEK/ERK and LKB-1/AMPK signaling pathways. Biochem Pharmacol 84:1604–1616
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Flora SJS (1999) Arsenic-induced oxidative stress and its reversibility following combined administration of N-acetylcysteine and meso 2,3-dimercaptosuccinic acid in rats. Clin Exp Pharmacol Physiol 26(11):865–869
Flora SJS (2011) Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med 51(2):257–281
Graham HN (1992) Green tea composition, consumption, and polyphenol chemistry. Prev Med 21(3):334–350
Guan H, Qiu Z, Zhou X, Li S, Liu X, Zhang C, Piao F (2017) Protection of Taurine against impairment in learning and memory in mice exposed to arsenic. Adv Exp Med Biol 975 (Pt 1:255–269
Gutteridge JMC (1995) Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem 41(12 Pt 2):1819–1828
Haider S, Tabassum S, Perveen T, Ali S, Saleem S, Khan AK, Haleem DJ (2011) Age-related decrease in striatal DA produces cognitive deficits in male rats. J Pharm Nutr Sci 1:20–27
Haider S, Naqvi F, Batool Z, Tabassum S, Sadir S, Liaquat L, Naqvi F, Zuberi NA, Shakeel H, Perveen T (2015) Pretreatment with curcumin attenuates anxiety while strengthens memory performance after one short stress experience in male rats. Brain Res Bull 115:1–8
Hajipour S, Sarkaki A, Farbood Y, Eidi A, Mortazavi P, Valizadeh Z (2016) Effect of Gallic acid on dementia type of Alzheimer disease in rats: electrophysiological and histological studies. Basic Clin Neurosci 7(2):97–106
Haleem DJ, Samad N, Haleem MA (2007) Reversal of haloperidol-induced tardive vacuous chewing movements and supersensitive somatodendritic serotonergic response by buspirone in rats. Pharmacol Biochem Behav 87(1):115–121
Han YS, Zheng WH, Bastianetto S, Chabot JG, Quirion R (2004) Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. Br J Pharmacol 141(6):997–1005
Huang L, Hou L, Xue H, Wang C (2016) Gallic acid inhibits inflammatory response of RAW264.7 macrophages by blocking the activation of TLR4/NF-κB induced by LPS. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 32(12):1610–1614
Huang Y, Chen J, Jiang T, Zhou Z, Lv B, Yin G, Fan J (2017) Gallic acid inhibits the release of ADAMTS4 in nucleus pulposus cells by inhibiting p65 phosphorylation and acetylation of the NF-κB signaling pathway. Oncotarget 8(29):47665–47674
Ingawale DK, Mandlik SK, Naik SR (2014) Models of hepatotoxicity and the underlying cellular, biochemical and immunological mechanism(s): a critical discussion. Environ Toxicol Pharmacol 37:118–133
Kaufer D, Friedman A, Seidman S, Soreq H (1998) Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature 393(6683):373–377
Isuzugawa K, Inoue M, Ogihara Y (2001) Catalase contents in cells determine sensitivity to the apoptosis inducer gallic acid. Biol Pharm Bull 24(9):1022–1026
Jadon A, Bhadauria M, Shukla S (2007) Protective effect of Terminalia belerica Roxb and gallic acid against carbon tetrachloride induced damage in albino rats. J Ethnopharmacol 109(2):214–218
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodesd CJ, Valkoc M (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31:95–107
Ker YB, Peng CC, Lin CH, Chen KC, Hsieh CL, Peng RY (2013) In vitro polyphenolics erythrocyte model and in vivo chicken embryo model revealed gallic acid to be a potential hemorrhage inducer: physicochemical action mechanisms. Chem Res Toxicol 26(3):325–335
Khare P, Datusalia AK, Sharma SS (2017) Parthenolide, an NF-κB inhibitor ameliorates diabetes-induced behavioral deficit, neurotransmitter imbalance and neuroinflammation in type 2 diabetes rat model. NeuroMolecular Med 19(1):101–112
Kim YJ (2007) Antimelanogenic and antioxidant properties of gallic acid. Biol Pharm Bull 30(6):1052–1055
Kubo I, Xiao P, Fujita K (2001) Antifungal activity of octyl gallate: structural criteria and mode of action. Bioorg Med Chem Lett 11(3):347–350
Lee H, Bae JH, Lee SR (2004) Protective effect of green tea polyphenol EGCG against neuronal damage and brain edema after unilateral cerebral ischemia in gerbils. J Neurosci Res 77(6):892–900
Lian W, Fang J, Xu L, Zhou W, Kang D, Xiong W, Jia H, Liu AL, Du GH (2017) DL0410 Ameliorates memory and cognitive impairments induced by scopolamine via increasing cholinergic neurotransmission in mice. Molecules 22(3):410
Liu T, Zhong S, Liao X, Chen J, He T, Lai S, Jia Y (2015) A meta analysis of oxidative stress markers in depression. PLoS One 10(10):e0138904
Loh KP, Huang SH, De Silva R, Tan BK, Zhu YZ (2006) Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res 3:327–337
Ma KG, Lv J, Yang WN, Chang KW, Hu XD, Shi LL, Zhai WY, Zong HF, Qian YH (2018) The p38 mitogen activated protein kinase regulates β amyloid protein internalization through the α7 nicotinic acetylcholine receptor in mouse brain. Brain Res Bull 137:41–52
Manna P, Sinha M, Sil PC (2008) Arsenic-induced oxidative myocardial injury: protective role of arjunolic acid. Arch Toxicol 82(3):137–149
Mansouri MT, Soltani M, Naghizadeh B, Farbood Y, Mashak A, Sarkaki A (2014) A possible mechanism for the anxiolytic-like effect of gallic acid in the rat elevated plus maze. Pharmacol Biochem Behav 117:40–46
Martinez EJ, Kolb BL, Bell A, Savage DD, Allan AM (2008) Moderate perinatal arsenic exposure alters neuroendocrine markers associated with depression and increases depressive like behaviors in adult mouse offspring. NeuroToxicology 29(4):647–655
McCord JM, Keele BB, Fridovich I (1976) An enzyme-based theory of obligate anaerobis: the physiological functions of superoxide dismutase. Proc Natl Acad Sci U S A 68(5):1024–1031
Moghadas M, Edalatmanesh MA, Robati R (2016) Histopathological analysis from Gallic acid administration on hippocampal cell density, depression, and anxiety related behaviors in a Trimethyltin intoxication model. Cell J 17(4):659–667
Naqvi F, Haider S, Batool Z, Perveen T, Haleem DJ (2012) Subchronic exposure to noise affects locomotor activity and produces anxiogenic and depressive like behavior in rats. Pharmacol Rep 64(1):64–69
Naskar S, Islam A, Mazumder UK, Saha P, Haldar PK, Gupta M (2010) In vitro and in vivo antioxidant potential of hydromethanolic extract of Phoenix dactylifera fruits. J Sci Res 2:144–157
Nutt DJ, Stein DJ (2006) Understanding the neurobiology of comorbidity in anxiety disorders. CNS Spectr 11(12):13–20
Nutt LK, Gogvadze V, Uthaisang W, Mirnikjoo B, McConkey DJ, Orrenius S (2005) Indirect effects of Bax and Bak initiate the mitochondrial alterations that lead to cytochrome c release during arsenic trioxide-induced apoptosis. Cancer Biol Ther 4:459–467
Ola-Davies O, Ajani OS (2016) Semen characteristics and sperm morphology of Pistia stratiotes Linn. (Araceae) protected male albino rats (Wistar strain) exposed to sodium arsenite. J Complement Integr Med 13(3):289–294
Papandreou MA, Tsachaki M, Efthimiopoulos S, Cordopatis P, Lamari FN, Margarity M (2011) Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav Brain Res 219(2):197–204
Pari L, Latha M (2004) Protective role of Scorparia dulcis plant extract on brain antioxidant status and lipid peroxidation in STZ diabetic male wistar rats. BMC Complement Altern Med 6:16
Piao F, Li S, Li Q, Ye J, Liu S (2011) Abnormal expression of 8-nitroguanine in the brain of mice exposed to arsenic subchronically. Ind Health 49(2):151–157
Popovic-Milenkovic MT, Tomovic MT, Brankovic SR, Ljujic BT, Jankovic SM (2014) Antioxidant and anxiolytic activities of Crataegusnigra Wald. Et kit. Berries. Acta Pol Pharm 71(2):279–285
Porsolt RD (1981) Behavioral despair. In: Enna SJ, Malick JB, Richelson E (eds) Antidepressants: neurochemical, behavioral and clinical perspectives. Raven Press, New York, pp 121–139
Samad N, Parveen T, Haider S, Haleem DJ (2005) Attenuation of restraint induced behavioral deficits by buspirone and propranolol in rats. J Coll Physicians Surg Pak 15(12):795–798
Samad N, Batool F, Haleem DJ (2007) Neurochemical and behavioral effects of 8-OH-DPAT following exposure to restraint stress in rats. Pharmacol Rep 59:173–180
Samad N, Haleem DJ (2014) Haloperidol-induced extra pyramidal symptoms attenuated by imipramine in rats. Pak J Pharm Sci 27(5 Spec no):1497–1501
Samad N, Saleem A (2018) Administration of Allium cepa L. bulb attenuates stress-produced anxiety and depression and improves memory in male mice. Metab Brain Dis 33(1):271–281
Samad N, Saleem A, Yasmin F, Shehzad MA (2018) Quercetin protects against stress-induced anxiety- and depression-like behavior and improves memory in male mice. Physiol Res 67(5):795–808
Sarkaki A, Fathimoghaddam H, Mansouri SM, Korrani MS, Saki G, Farbood Y (2014) Gallic acid improves cognitive, hippocampal long-term potentiation deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Pak J Biol Sci 17(8):978–990
Saygin M, Ozturk O, Ozmen O, Ilhan I, Gonca T, Gumral N, Orhan H, Aslankoc R (2016) The impact of methotrexate on lung inflammatory and apoptotic pathway biomarkers-the role of gallic acid. Biomed Pharmacother 84:1689–1696
Sen D, Biswas PS (2012) Arsenicosis: is it a protective or predisposing factor for mental illness? Iran J Psychiatry 7(4):180–183
Serrano J, Cipak A, Boada J, Gonzalo H, Cacabelos D, Cassanye A, Pamplona R, Zarkovic N, Portero-Otin M (2010) Double-edged sword behaviour of gallic acid and its interaction with peroxidases in human microvascular endothelial cell culture (HMEC-1). Antioxidant and pro-oxidant effects. Acta Biochim Pol 57(2):193–198
Shahrzad S, Aoyagi K, Winter A, Koyama A, Bitsch I (2001) Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. J Nutr 131(4):1207–1210
Shi H, Shi X, Liu KJ (2004) Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem 255(1–2):67–78
Sun BF, Wang QQ, Yu ZJ, Yu Y, Xiao CL, Kang CS, Ge G, Linghu Y, Zhu JD, Li YM, Li QM, Luo SP, Yang D, Li L, Zhang WY, Tian G (2015) Exercise Prevents Memory Impairment Induced by Arsenic Exposure in Mice: Implication of Hippocampal BDNF and CREB. PLoS One 10(9):e0137810
Wang D, Wang X, Liu X, Jiang L, Yang G, Shi X, Zhang C, Piao F (2018) Inhibition of miR-219 alleviates arsenic-induced learning and memory impairments and synaptic damage through up-regulating CaMKII in the hippocampus. Neurochem Res 43(4):948–958
Więdłocha M, Marcinowicz P, Krupa R, Janoska-Jaździk M, Janus M, Dębowska W, Mosiołek A, Waszkiewicz N, Szulc A (2018) Effect of antidepressant treatment on peripheral inflammation markers - a meta-analysis. Prog Neuro-Psychopharmacol Biol Psychiatry 80(Pt C):217–226
Yadav RS, Chandravanshi LP, Shukla RK, Sankhwar ML, Ansari RW, Shukla PK, Pant AB, Khanna VK (2011) Neuroprotective efficacy of curcumin in arsenic induced cholinergic dysfunctions in rats. Neurotoxicology 32(6):760–768
Yadav M, Jindal DK, Dhingra MS, Kumar A, Parle M, Dhingra S (2018) Protective effect of gallic acid in experimental model of ketamine-induced psychosis: possible behavior, biochemical, neurochemical and cellular alterations. Inflammopharmacology 26(2):413–424
Yamanaka K, Hesegwa A, Sawamuna R, Okada S (1991) Cellular response to oxidative damage in lung induced by the administration of dimethylarsinic acid, a major metabolite of inorganic arsenics, in mice. Toxicol Appl Pharmacol 108(2):205–213
Yen YP, Tsai KS, Chen YW, Huang CF, Yang RS, Liu SH (2012) Arsenic induces apoptosis in myoblasts through a reactive oxygen species-induced endoplasmic reticulum stress and mitochondrial dysfunction pathway. Arch Toxicol 86:923–933
Yu NH, Pei H, Huang YP, Li YF (2017) Epigallocatechin-3-Gallate inhibits arsenic-induced inflammation and apoptosis through suppression of oxidative stress in mice. Cell Physiol Biochem 41(5):1788–1800
Zhao J, Khan IA, Fronczek FR (2011) Gallic acid. Acta Crystallogr Sect E: Struct Rep Online 67(Pt 2):o316–o317
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Samad, N., Jabeen, S., Imran, I. et al. Protective effect of gallic acid against arsenic-induced anxiety−/depression- like behaviors and memory impairment in male rats. Metab Brain Dis 34, 1091–1102 (2019). https://doi.org/10.1007/s11011-019-00432-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-019-00432-1