Abstract
To evaluate protection of taurine against arsenic (As)-induced impairment of learning and memory as well as explore its protective mechanism, mice were divided into control, As and taurine protection groups. Mice of As exposure group exposed to drinking water containing 4 ppm As2O3. Mice of taurine protective group received both 4 ppm As2O3 and 150 mg taurine per kilogram. Mice of control group only drank double-distilled water. All animals were treated for 60 days. Morphology of brain was observed by HE staining. Morris water maze (MWM) tests and step-down passive avoidance task were performed to examine cognition function. Moreover, expressions of some genes and proteins related to regulation learning and memory in brain were tested by Real Time RT-PCR and Western Blot. As a result, abnormal morphologic changes in brain tissue and poor performance in cognition functions were observed in As-exposed mice. The expression of TRβ protein, a regulator of CaMK IV gene, significantly decreased in brains of As-exposed mice than in controls. By contrast, impairment in learning and memory, change in brain morphology and disturbance in protein expression were significantly mitigated in mice of taurine protective group. Our results suggest that taurine supplementation protects against neurotoxicity induced by As in mice.
$Huai Guan and Zhewen Qiu as co-first authors are contributed equally to this work.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
- Arsenic trioxide
- Neurotoxicity
- Brain tissue
- Taurine
- Impairment in learning and memory
- Arsenic
- Learning
- Memory
- Protection
1 Introduction
Arsenic (As) is one of the most notorious toxicants known for thousands of years and now still affects more people than any other natural elements. Millions of people are living in areas polluted by high doses of As in more than 20 countries, the major affected areas including Bangladesh, India, China, Taiwan, Mexico, Argentina, Chile, and the USA (Bloom et al. 2014; Tyler and Allan 2014). It has been demonstrated that As is a neural toxicant. Epidemiological investigations in children suggested that chronic As exposure via drinking water was responsible for intellectual impairment (Wasserman et al. 2004). Several researches revealed that As exposure damaged learning and memory abilities as well as other neural behaviors at environmental relevant levels (Baldissarelli et al. 2012; Jing et al. 2012). We previously also found that subchronically exposed to As undermined learning and memory of mice (Wang et al. 2009). These studies indicated that brain is a vital target organ attacked by As exposure.
Long-term potentiation (LTP) and long-term depression (LTD) are the two key models for learning and memory (Malenka and Bear 2004; Mozzachiodi and Byrne 2010; Owen and Brenner 2012). Creb activation is essential to maintaining LTP and LTD which needs be phosphorylated by Ca2+/calmodulin-dependent protein kinase IV (CaMK IV) (Shaywitz and Greenberg 1999). We group had indicated that down-regulating CaMK IV gene and protein was a key link of As inducing learning and memory impairment (Wang et al. 2009; Guan et al. 2016). It has been illustrated that the binding of thyroid hormone receptor (TR) and thyroid hormone (TH) is essential to the activation of CaMK IV. Then this complex binds thyroid hormone-responsive element (TRE) (Li et al. 2004; Liu and Brent 2005; Morte et al. 2010). TH and TR are important regulators of CaMK IV gene.
Imbalance between free radical production and antioxidant leads to oxidative stress, which takes part in the pathogenesis of many disorders. As exposure has been reported to induce overproduction of reactive oxygen species (ROS) in tissues (Chattopadhyay et al. 2002). Increasing of ROS is supposed to be an important mechanism for As neurotoxicity. It was reported that taurine treatment antagonizes toxic effect of ROS caused by some toxic substances including As (Dogru-Abbasoglu et al. 2001, Tabassum et al. 2006). Our group previously found that taurine significantly alleviated As-lead to nucleic acid damage in mice brain (Piao et al. 2009). Therefore, we aim at exploring whether administration of taurine protects against As-induced impairment in learning and memory in mouse. We also determine antagonistic action of taurine on disturbance of As on TR expression in brain and TH level in serum.
In present study, learning and memory functions were examined. Expression of TR in brains was assessed the by qRT-PCR and Western blot. Serum levels of 3,5,3′-triiodothyronine (T3) and thyroxin (T4) were detected by radioimmunoassay (RIA). Moreover, HE staining was performed to observe the morphology of brain tissue.
2 Materials and Methods
2.1 Animals and Treatment
25.6–32.4 g male mice were selected as our experimental animals. They were purchased from Experimental Animal Center, Dalian Medical University. They were raised at condition of 22 ± 3 °C, 12-h light/dark cycle and relative humidity of 55 ± 15%. They were fed with common basal pellet diet (As concentration < 0.7 mg/kg). The animals were randomly divided into three groups. Each group has 12 animals. Group 1 (control group) only drank double-distilled water. Group 2 (As exposure group) exposed to drinking water containing 4 ppm As2O3. Group 3 (taurine protective group) received both 4 ppm As2O3 and 150 mg/kg taurine. Taurine was implemented by gavage twice a week, while As exposure continued for 60 days. After raising for 60 days, all animals underwent behavioral tests. After behavioral tests, all animals were killed. Through centrifugation of whole blood, serum samples were made for detecting THs concentrations. Brain tissues were collected for detection of biochemical indexes. For morphologic research, animals were perfused with saline followed with 4% paraformaldehyde. The animal experiments were carried out according to the guidelines of the committee of Dalian Medical University.
2.2 Behavioral Testing
2.2.1 Step-Down Passive Avoidance Task
We had previously reported the procedure in detail (Guan et al. 2016). In brief, when examining learning ability, error frequency to step down onto a foot-shocks floor within 180 s was the observation index. When examining memory ability, step-down latency was the observation index.
2.2.2 MWM Task
This test is used to examine spatial learning and memory. We had previously reported its methods and procedures in detail (Guan et al. 2016). In brief, our tank is black and round with 100 cm diameter. At the first stage, a hidden platform test was performed. It was used to examine spatial learning ability. Two parameters, escape latency and swimming distance to find hidden platform were the observation indexes. At the second stage, a probe test was performed. It was used to examine spatial memory ability. Two parameters, swimming time and distance in target quadrant were the observation indexes.
2.3 HE Staining
According to standard protocols, the fixed brain tissue was made into sections of 5 μm thickness. Then, they were stained with HE. Finally, they were observed under light microscope.
2.4 Real Time RT-PCR
Trizol® reagent (Takara, China) was used to extract gene sample according to the instructions. Transcriptor First Strand cDNA Synthesis Kit (Roche, \USA) was used to perform RT reactions. TP800 System and SYBR Green PCR kit (Takara, Japan) were used to carry out Real time RT-PCR. 95″C 5 min, followed by 95″C for 30 s, 40 cycles, then 55″C 30 s, 72″C 30 s were used as reaction conditions. The primers are as followed: TRα, GAC AAG GCC ACC GGT TAT CAC TAC, CAG CAG CTG TCA TAC TTG CAG; TRβ, AGC CAG AAC CCA CGG ATG AG, CGA TGG GTG CTT GTC CAAT G; β-actin, CAT CCG TAA AGA CCT CTA TGC CAA C, ATG GAG CCA CCG ATC CAC A.
2.5 Western Blot
Total proteins were extracted from cerebrum tissues with lysis buffer. BCA method was used to qualify protein concentration. SDS-polyacrylamide gel electrophoresis was carried out with same gram of loading sample protein, and the protein samples were transferred to a nitrocellulose membrane. After blocking with 10% milk for 30 min, the blots were incubated with (TRβ1, TRβ2 1:800; TRα1 1:1000; CaMK IV 1:1000; β-actin 1:350) primary antibodies, respectively. The blots were treated with horseradish peroxidase-conjugated secondary antibodies, and then detected by Bio-Rad ChemiDoc™ MP imaging system (Bio-Rad, CA, USA), and then qualified with the Gel-Pro software.
2.6 RIA
T3 and T4 concentrations in serum were detected with RIA kit (Santa, USA) according to the manufacturer’s instructions.
2.7 Statistical Analysis
SPSS 17.0 for Windows was chose to analyze our data. Data were expressed as the mean ± SD. And analyzed by One-way ANOVA. p-value <0.05 was defined statistically significant.
3 Results
3.1 Brain Morphology
Abnormal morphological changes of mice brain of the three groups were examined by HE staining. Taking the frontal lobe as the example of cerebral tissue, in control mice, neuron cells were large and full. Their nuclei were large with karyolemma and nucleolus were clear (Fig. 1Aa). In the group exposed to 4 mg/L As2O3 alone, there were white and bright areas (white arrows) around nuclei in many neuron cells, suggesting cellular edema (Fig. 1Bb). In addition, there were metamorphotic nuclei (yellow arrows) in many neuron cells (Fig. 1Bb). These findings indicated that As exposure damaged cerebral morphology of mouse. In the group exposed to 4 mg/L As2O3 with taurine, there were also edema neurons and metamorphotic nuclei (Fig. 1Cc), but these changes were slighter than those in the group exposed to As alone. It indicate that treatment with taurine mitigated As-induced dam morphological changes in mouse cerebral cortex.
Protective effects of taurine on As-induced morphological abnormity in mouse cerebrum. Group 1 (control group) orally received double distilled water. Group 2 (As exposure group) exposed to drinking water containing 4 ppm As2O3. Group 3 (protective group) received both As2O3 and taurine. All animals were treated for 60 days. Then, the brain was removed and made into sections. HE staining and light microscope were used to research brain morphology. Morphology of frontal lobe was taken as the example of cerebral cortex. (A and a) control group; (B and b) As exposure group; (C and c) protective group. Black arrow: normal neuron; white arrow: edematous neuron; yellow arrow: metamorphotic nucleus. Scale bars, 50 μm
Taking the CA3 area as the example of cerebral hippocampal tissue, in control group, pyramidal cells were large and closed to each other in multilayer. Neuropil was dense and stained uniformly (Fig. 2Aa). In the mice of group exposed to 4 mg/L As2O3 alone, many pyramidal cells showed reduction in cell body and the number of pyramidal cells was also reduced. In addition, there was some unoccupied space in the area of neuropil, even meshy neuropil (Fig. 2Bb). These findings indicate that As exposure induces abnormal morphological changes of mouse hippocampus. In the group exposed to 4 mg/L As2O3 with taurine, there were also the above changes (Fig. 2Cc), but they were slighter than those in the group exposed to As alone, indicating antagonistic action of taurine.
Protective effects of taurine on As-induced morphological abnormity in mouse hippocampus. Group 1 (control group) orally received double distilled water. Group 2 (As exposure group) exposed to drinking water containing 4 ppm As2O3. Group 3 (protective group) received both As2O3 and taurine. All animals were treated for 60 days. Then, the brain was removed and made into sections. HE staining and light microscope were used to research brain morphology. Morphology of CA3 region was taken as the example of hippocampus. (A and a) control group; (B and b) As exposure group; (C and c) protective group. PY pyramidal cell layer, NP neuropil. Scale bars, 50 μm
3.2 Behavioral Tests
3.2.1 Step-Down Passive Avoidance test
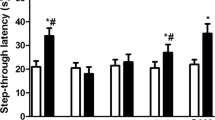
In this test, average error frequency of group treated with 4 mg/L As2O3 alone was markedly higher than that of control group (Fig. 3a). Average latency of group treated with 4 mg/L As2O3 alone was markedly longer than that of control group (Fig. 3b). It suggested that As damaged state-dependent learning and memory. However, average error frequency of group treated with 4 mg/L As2O3 and taurine was similar to that of control group and significantly fewer than that of group exposed to 4 mg/L As2O3 alone (Fig. 3a). Average latency of group treated with 4 mg/L As2O3 and taurine was similar to that of control group and significantly longer than that of group exposed to 4 mg/L As2O3 alone (Fig. 3b). It suggested that taurine significantly alleviates As-induced impairment in state-dependent learning and memory.
Protective effects of taurine on As-induced impairment in state-dependent learning and memory in mice. Group 1 (control group) only received ddH2O. Group 2 (As exposure group) exposed to drinking water containing 4 ppm As2O3. Group 3 (protective group) received both As2O3 and taurine. All animals were treated for 60 days. (a) Error frequency of step-down from a platform after foot-stock (times); (b) Step-down latency from the platform (seconds). a p < 0.05, vs. control group; b p < 0.05, vs. As exposure group
3.2.2 MWM Tests
Hidden platform test was used to examine spatial learning ability of mouse. In this test, mice in each group showed a progressive reduction in swimming distance and escape latency during 5 training days, confirming that mice in each group learned to find the hidden platform across the training stage (Fig. 4a–c). Further analysis showed that on the first training day there was no significant difference in swimming distance or escape latency among mice of three groups (p > 0.05). On the 2nd, 3rd, 4th and 5th day of training, there was a significant difference in swimming distance or escape latency of mice between control and As exposure groups, the latter being longer than the former (all p < 0.05) (Fig. 4a–c). These results suggested that subchronic As intoxication damaged spatial learning ability of mouse. However, 2–5 days after training, the swimming distance and escape latency of mice in protective group were similar to those of control group (p > 0.05) and were markedly shorter than those in As exposure group (p < 0.05) (Fig. 4a–c). The above findings suggested that taurine reverses the impairment in spatial learning in As-intoxicate mice.
Protective effects of taurine on As-induced impairment in spatial learning ability in mice. Group 1 (control group) orally received double distilled water. Group 2 (As exposure group) exposed to drinking water containing 4 ppm As2O3. Group 3 (protective group) received both As2O3 and taurine. All animals were treated for 60 days. Then, MWM tests were used to examine the spatial learning and memory functions. Data were expressed as mean ± SD (12 animals per group). (a) Swimming trace of mice in MWM device; (b) Swimming distance in hidden platform test (cm); (c) Escape latency in hidden platform test (s). a p < 0.05, vs. control group; b p < 0.05, vs. As exposure group
Probe test was used to examine spatial memory ability of mouse. In this test, similar trends were observed. Average time in target quadrant of As exposure group was significantly shorter than that of control group (p < 0.05) (Fig. 5a, b). Compared with control group, average swimming distance in target quadrant of As exposure group was markedly shorter t (Fig. 5a, c). It indicated that As induced impairment in memory retention in mouse. However, the two indexes of protective group were both similar to those of control group and both significantly longer than those of As exposure group (Fig. 5a–c). The above results suggested that taurine prevents As-induced impairment in memory retention in mouse.
Protective effects of taurine on As-induced impairment in spatial memory ability in mice. Group 1 (control group) orally received double distilled water. Group 2 (As exposure group) exposed to drinking water containing 4 ppm As2O3. Group 3 (protective group) received both As2O3 and taurine. All the animals were treated for 60 days. Then, MWM tests were used to examine the spatial learning and memory functions. Data were expressed as mean ± SD (12 animals for each group). (a) Swimming trace of mice in MWM device; (b) Time in target quadrant in probe test(s); (c) Distance in target quadrant in probe test (cm). a p < 0.05, vs. control group; b p < 0.05, vs. As exposure group
3.3 TR Expression in Mouse Brain
Figure 6a showed that mRNA expression of TRβ in brain of As-exposed mice was markedly decreased compared with that of control group. Moreover, expression of TRβ mRNA in brain of As exposure group was also markedly lower than that of protective group. Expression of TRβ mRNA in brain of protective group was similar to that of control group. It indicated that taurine alleviates down-regulation of TRβ mRNA induced by As in mouse brain. Among three groups, there was no significant difference in TRα mRNA expression in mouse brain.
Expression of TR gene (a) and protein (b) in mouse brain. Group 1 (control group) received ddH2O. Group 2 (As exposure group) exposed to drinking water containing 4 ppm As2O3. Group 3 (protective group) received both As2O3 and taurine. All animals were treated for 60 days. Then, they were examined learning and memory capacities. Finally, they were examined some biochemical indexes. Real time RT-PCR and western blot were used to determine mRNA and protein, respectively. Data were expressed as mean ± SD (12 animals for each group). a p < 0.05, vs. control group; b p < 0.05, vs. As exposure group
As shown in Fig. 6b, protein expression of TRβ1 in brain of As-exposed mice was markedly lower than that of control mice. Moreover, expression of TRβ1 protein in brain of As exposure group was also significantly lower than that of protective group (p < 0.05). Expression of TRβ1 protein in brain of protective group was significantly lower than that of control group, but significantly higher than that of As exposure group. It indicated that taurine alleviates down-regulation of TRβ protein induced by As in mouse brain. There was no significant difference in TRα1 or TRβ2 protein level in mouse brain among three groups.
3.4 T3 and T4 Concentrations in Serum
RIA was used to determine T3 and T4 concentrations in mouse serum. As shown in Fig. 7, T3 levels in serum of control, As exposure and protective groups were (1.94 ± 0.06), (1.96 ± 0.07) and (1.97 ± 0.07) mmol/L, respectively. T4 levels in serum of control, As exposure and protective groups were (60.31 ± 7.12), (59.23 ± 6.41) and (60.54 ± 6.84) mmol/L, respectively. Among three groups, there was no significant difference in T3 or T4 level.
T3 (a) and T4 (b) concentrations in mouse serum. Group 1 (control group) received ddH2O. Group 2 (As exposure group) exposed to drinking water containing As2O3. Group 3 (protective group) received both As2O3 and taurine. All animals were treated for 60 days. Then, they were examined learning and memory capacities. Finally, they were examined some biochemical indexes. RIA was used to determine T3 and T4 concentrations in mouse serum. Data were expressed as mean ± SD (12 animals for each group)
4 Discussion
Epidemiological researches showed that As undermined learning (Rodríguez et al. 2003), memory and attention in humans (Tsai et al. 2003). Experimental studies proofed that As exposure impaired learning acquisition (Nagaraja and Desiraju 1994), locomotor behavior and spatial learning paradigm (Nagaraja and Desiraju 1994, Rodríguez et al. 2002). In the present study, we observed that, in step-down passive avoidance test, mouse of As exposure group showed higher error frequency and shorter latency than control. In MWM tests, mouse of As exposure group showed longer swimming distance and longer escape latency to find hidden platform. These findings indicated that As damaged state-dependent and spatial learning and memory functions in mice. Taurine intervention nearly reversed impairment in learning and memory in As exposure mice, which was reflected by the better performance of mice exposed to As with taurine than the mice exposed to As alone, as well as the similar performance of them to the controls. It suggests that taurine can antagonize neurotoxicity induced by As in some way, thereby protects neurological function of As-exposed mice.
Learning and memory are important cognitive functions. Hippocampus and cerebral cortex are proved main functional areas of them. In this study, we observed morphology of hippocampus and cerebral cortex to explore effects of As on mouse brain. Our results showed that, in cerebral cortex, As exposure induced neuron edema and nuclei abnormality. In hippocampal tissue, As resulted in shrinkage and reduction of pyramidal cells, as well as meshy neuropil. These findings suggested that As exposure induced abnormal morphology in mouse brain. However, taurine intervention significantly alleviated the above changes induced by As. It suggests that taurine protects against As-induced pathological damage in mouse brain.
It has been documented that LTP and LTD are two key models of learning and memory (Malenka and Bear 2004). The formation of them requires activation of CREB (Ahn et al. 1999; Casu et al. 2005; El Hajj et al. 2014). And signaling by CaMK IV cascade are involved in CREB activation (Lee et al. 2009). Our previous studies documented that subchronic As exposure depressed gene expressions of CaMK IV, CREB and other memory proteins in mouse brain (Wang et al. 2009). It hinted that down-regulated CaMK IV expression in brain induced by As might be associated with deficit of cognitive function.
It has been documented that binding with TH and TR are needed for transcriptional activation of CaMK IV (Li et al. 2004, Liu and Brent 2005, Morte et al. 2010). TR isoforms include TRα1, TRβ1 and TRβ2. They can bind to TH and target DNA sequence TREs in the CaMK IV gene 5′-flanking region (Liu and Brent 2005) and then activated CaMK IV gene. Therefore, we investigated effects of As on TR gene and protein expression in brain as well as TH level in serum. We also investigated protective effects of taurine as well as potential mechanism. Our study showed that As exposure markedly decreased TRβ mRNA expression in mouse brain. The result was further proved by Western blot examination. However, the decreased TRβ was significantly up-regulated in mouse of taurine protective group. It hinted that taurine supplementation significantly alleviated effect induced by As. On other hand, there was no significant difference in serum T3 or T4 level and TRα mRNA expression among three groups. These results hinted that As exposure disturbed expression of TRβ in brain tissue of mice. Moreover, it is also suggested that taurine protect brain against toxic effects induced by As. Taurine is a intracellular free b-amino acid. It exists in most mammalian tissues. It has many cytoprotective properties including anti-oxidation, osmoregulation, membrane stabilizatio. (Hansen 2001; Schaffer et al. 2003; Redmond et al. 1996). Taurine has been documented a protective agent which can antagonize oxidative stress-induced pathologies. It was reported that taurine might up-regulate anti-oxidant defences, form chloramines with HOCl, or bind free metal ions so as to scavenge oxygen free radicals. Thus, it play roles of protecting cells (Hansen 2001; Schaffer et al. 2003; Redmond et al. 1996). Therefore, protective effects of taurine against toxic effects of As may be attributed to its ability by its direct as well as indirect antioxidant activities.
5 Conclusion
In this study, As exposure induced an impairment in learning and memory, abnormal morphologic changes and down-regulated expression of TRβ in mouse brain. However, taurine supplementation significantly alleviated the toxic effects of As. Our results indicate that taurine protects against neurotoxicity induced by As. Meanwhile, it is also suggested that taurine may be potentially used as a therapeutic/protective agent against As-induced neurotoxicity. Further studies are needed to focus on determining exact molecular mechanisms of these protective effects.
References
Ahn S, Ginty DD, Linden D (1999) A late phase of cerebellar long-term depression requires activation of CaMKIV and CREB. Neuron 23:559–568
Baldissarelli LA, Capiotti KM, Bogo MR, Ghisleni G, Bonan CD (2012) Arsenic alters behavioral parameters and brain ectonucleotidases activities in zebrafish (Danio rerio). Comp Biochem Physiol C Toxicol Pharmacol 155:566–572
Bloom MS, Surdu S, Neamtiu IA, Gurzau ES (2014) Maternal arsenic exposure and birth outcomes: a comprehensive review of the epidemiologic literature focused on drinking water. Int J Hyg Environ Health 217:709–719
Casu MA, Pisu C, Sanna A, Tambaro S, Spada GP, Mongeau R, Pani L (2005) Effect of D9-tetrahydrocannabinol on phosphorylated CREB in rat cerellum: an imminohistochemical study. Brain Res 1048:41–47
Chattopadhyay S, Bhaumik S, Purkayastha M, Basu S, Nag Chaudhuri A, Das Gupta S (2002) Apoptosis and necrosis in developing brain cells due to arsenic toxicity and protection with antioxidants. Toxicol Lett 136:65–76
Dogru-Abbasoglu S, Kanbagli O, Balkan J, Cevikbas U, Aykac-Toke G, Uysal M (2001) The protective effect of taurine against thioacetamide hepatotoxicity of rats. Hum Exp Toxicol 20:23–27
El Hajj CS, Dreumont N, Willekens J, Canabady-Rochelle L, Jeannesson E, Alberto JM, Daval JL, Guéant JL, Leininger-Muller B (2014) Early methyl donor deficiency alters cAMP signaling pathway and neurosteroidogenesis in the cerebellum of female rat pups. Am J Physiol Endocrinol Metab 307:1009–1019
Guan H, Li S, Guo Y, Liu X, Yang Y, Guo J, Li S, Zhang C, Shang L, Piao F (2016) Subchronic exposure to arsenic represses the TH/TRβ1-CaMK IV signaling pathway in mouse cerebellum. Int J Mol Sci 17:157
Hansen SH (2001) The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev 17:330–346
Jing J, Zheng G, Liu M, Shen X, Zhao F, Wang J, Zhang J, Huang G, Dai P, Chen Y, Chen J, Luo W (2012) Changes in the synaptic structure of hippocampal neurons and impairment of spatial memory in a rat model caused by chronic arsenite exposure. Neurotoxicology 33:1230–1238
Lee KH, Chatila TA, Ram RA, Thompson RF (2009) Impaired memory of eyeblink conditioning in CaMKIV KO mice. Behav Neurosci 123:438–442
Li D, Yamada T, Wang F, Vulin AI, Samuels HH (2004) Novel roles of retinoid X receptor (RXR) and RXR ligand in dynamicallymodulating the activity of the thyroid hormone receptor/RXR heterodimer. J Biol Chem 279:7427–7437
Liu YY, Brent GA (2005) Thyroidhormone-dependent gene expression in differentiated embryonic stem cells and embryonal carcinoma cells: identificationof novel thyroid hormone target genes by deoxyribonucleic acid microarray analysis. Endocrinology 146:776–783
Malenka RC, Bear MF (2004) LTP and LTD: an embarrassment of riches. Neuron 44:5–21
Morte B, Díez D, Ausó E, Belinchón MM, Gil-Ibáñez P, Grijota-Martínez C, Navarro D, de Escobar GM, Berbel P, Bernal J (2010) Thyroid hormone regulation of gene expression in the developing rat fetal cerebral cortex: prominent role of the Ca2+/calmodulin-dependent protein kinase IV pathway. Endocrinology 151:810–820
Mozzachiodi R, Byrne JH (2010) More than synaptic plasticity: role of nonsynaptic plasticity in learning and memory. Trends Neurosci 33:17–26
Nagaraja TN, Desiraju T (1994) Effects on operant learning and brain acetylcholine esterase activity in rats following chronic inorganic arsenic intake. Hum Exp Toxicol 13:353–356
Owen GR, Brenner EA (2012) Mapping molecular memory: navigating the cellular pathways of learning. Cell Mol Neurobiol 32:919–941
Piao FY, Yang G, Li QJ, Ye JX, Sun XC, MaO N (2009) Taurine and vitamin C influence 8-nitroguanine brain expression following sub-chronic arsenic exposure in the mouse. Neural Regen Res 4:838–842
Redmond HP, Wang JH, Bouchier-Hayes D (1996) Taurine attenuates nitric oxide- and reactive oxygen intermediate-dependent hepatocyte injury. Arch Surg 131:1280–1208
Rodríguez VM, Carrizales L, Mendoza MS, Fajardo OR, Giordano M (2002) Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol Teratol 24:743–750
Rodríguez VM, Jiménez-Capdeville ME, Giordano M (2003) The effects of arsenic exposure on the nervous system. Toxicol Lett 145:1–18
Schaffer S, Azuma J, Takahashi K, Mozaffari M (2003) Why is taurine cytoprotective? Adv Exp Med Biol 526:307–321
Shaywitz AJ, Greenberg ME (1999) CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68:821–861
Tabassum H, Rehman H, Banerjee BD, Raisuddin S, Parvez S (2006) Attenuation of tamoxifen-induced hepatotoxicity by taurine in mice. Clin Chim Acta 370:129–136
Tsai SY, Chou HY, The HW, Chen CM, Chen CJ (2003) The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology 24:747–753
Tyler CR, Allan AM (2014) The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep 1:132–147
Wang Y, Li S, Piao F, Hong Y, Liu P, Zhao Y (2009) Arsenic down-regulates the expression of CaMK IV, an important gene related to cerebellar LTD in mice. Neurotoxicol Teratol 33:318–322
Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van Geen A, Slavkovich V, LoIacono NJ, Cheng Z, Hussain I, Momotaj H, Graziano JH (2004) Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect 112:1329–1333
Acknowledgments
This work was supported by National Natural Science Foundation of China (grant numbers 30571584, 81102160, 31400452) and Special Financial Grant from the China Postdoctoral Science Foundation (grant number 2014T70969).
Conflicts of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media B.V.
About this paper
Cite this paper
Guan, H. et al. (2017). Protection of Taurine Against Impairment in Learning and Memory in Mice Exposed to Arsenic. In: Lee, DH., Schaffer, S.W., Park, E., Kim, H.W. (eds) Taurine 10. Advances in Experimental Medicine and Biology, vol 975. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-1079-2_23
Download citation
DOI: https://doi.org/10.1007/978-94-024-1079-2_23
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-1077-8
Online ISBN: 978-94-024-1079-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)