Abstract
The present study investigated the therapeutic effect of curcumin on bleomycin (BLM)-induced alterations in glycoprotein components in the fibrotic lungs. Analysis of the bronchoalveolar lavage fluid (BALF) demonstrated increased fibronectin content at 3, 5, 7, and 14 days after BLM administration. Similarly, lung tissue fibronectin content revealed a progressive increase at various times (days 3, 5, 7, 14, and 28) during the development of lung fibrosis. In addition, alveolar macrophage release of fibronectin was also elevated in BLM-treated rats. Analysis of carbohydrate moieties of glycoproteins revealed an increase in total hexose, fucose, sialic acid and hexosamine levels at 7, 14, and 28 days after BLM treatment. Furthermore, the activities of lung glycosidases such as N-acetyl-β-d-glucosaminidase, β-glucosidase, β-galactosidase, and β-fucosidase in the fibrotic rats were elevated. Importantly, curcumin significantly inhibited the BLM-induced increases in BALF and lung fibronectin levels. Treatment of BLM rats with curcumin dramatically suppressed alveolar macrophage release of fibronectin. Curcumin also inhibited the increases in complex carbohydrates and glycosidases in the fibrotic lungs. These findings suggest that BLM-induced lung fibrosis is associated with accumulation of glycoproteins, and curcumin has the ability to suppress the enhanced deposition of glycoproteins in the fibrotic lung.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bleomycin (BLM)-induced lung injury is characterized by the migration of blood-borne proinflammatory cells into the alveolar space and the migration of fibroblasts at sites of injury followed by parenchymal injury and fibrosis of the alveolar structure [1]. Pathologic examinations of the fibrotic lung demonstrate that the lesions are rich in extracellular matrix (ECM) proteins within the interstitial and intraluminal compartments of lung [2]. The lung connective tissue is composed of collagens, elastin, proteoglycans, and non-collagenous glycoproteins [2]. Together, these molecules associate to form supramolecular complexes that surround most cells in the body. Under normal conditions, these components are synthesized and destroyed under precise temporal and spatial patterns, resulting in the maintenance of normal lung structure and function. However, changes in ECM composition and deposition occur in fibrotic lung diseases [2]. Increased deposition of collagen has been demonstrated in BLM-induced pulmonary fibrosis [3]. Increased amounts of proteoglycans were also observed in fibrotic lungs [4,5,6]. However, there is little information on glycoproteins present in lung structures and its role in lung fibrosis. Since glycoproteins possess a variety of biologic properties: maintaining the structural stability of collagen fibrils [7], providing structural support to organs, contributing to the viscosity of tissue fluids, presence on cell surfaces and in the ECM, antigenicity [8], delayed clearance from plasma, and resistance to certain proteolytic enzymes [9], these properties may nevertheless play a role in the pathogenesis of pulmonary fibrosis. Further, their prominent position on the cell membrane may permit glycoproteins to effectively participate in cell–cell and cell–extracellular matrix interaction, including adhesion, migration, and immune recognition during lung injury and repair in fibrosis.
Recent progress in therapies targeting tissue remodeling in idiopathic pulmonary fibrosis (IPF) have only limited efficacy, and these treatments often have devastating side effects, and delay rather than reduce or reverse lung remodeling [10]. Therefore, new therapeutic approaches are needed, and targeting the ECM proteins may be one such approach [11,12,13]. Previous studies of the beneficial effects of curcumin, a yellow curry pigment from turmeric (Curcuma longa), have focused on the suppression of lung inflammation and cytokine release in bleomycin-induced lung injury in rats [14]. Other studies have also demonstrated that curcumin exhibits a variety of potent beneficial effects such as antioxidant, anti‐inflammatory, and anti‐fibrotic [15,16,17,18,19,20]. These observations led us to postulate whether this protective activity of curcumin extends to modulation of ECM turnover and deposition as well. The objective of the present study was to characterize the nature of changes in glycoprotein metabolism during the development of BLM-induced pulmonary fibrosis, and the therapeutic effect of curcumin on BLM-induced alterations in glycoprotein metabolism.
Methods
Experimental design
Healthy male Wistar rats weighing 325–350 g were obtained from the animal house facilities of Central Leather Research Institute, and allowed to acclimatize for one week before experiments. The animals were housed in temperature and humidity controlled cages with 12 h light/dark cycles and had access to water and laboratory rat chow ad libitum. All experiments were carried out according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 86-23, revised 1996). The animal ethical committee of CLRI approved the project proposal and experiments were conducted following the rules of the ethical committee.
Rats were divided into four groups. The first group (SAL) consisted of saline-instilled rats, which received a single intratracheal (i.t.) dose of 0.4 ml of sterile physiological saline. The second group (CUR) received 300 mg/kg of curcumin. The third group (BLM) received a single i.t. dose of bleomycin (0.75 U/100 g body weight in sterile physiological saline). The final group (CUR + BLM) received 300 mg/kg of curcumin 10 days before BLM and daily thereafter throughout the experimental period (28-day period). Saline and BLM were instilled to rats through the endotracheal catheter (a non-toxic polyethylene tubing, inner diameter: 1.67 mm; outer diameter: 2.42 mm, length: 70 mm) using a sterile 1 ml syringe under sodium pentobarbital anesthesia (35 mg/kg) followed by 3 ml of air to distribute the drugs equally. Curcumin, prepared fresh every day, was suspended in 1% gum acacia and administered by gastric intubation. All animals received humane care during the experimental time period.
Collection of bronchoalveolar lavage fluid (BALF)
Bronchoalveolar lavage was performed at days 3, 5, 7, 14, and 28 post-BLM administration. Briefly, the animals were anesthetized with an intraperitoneal dose of sodium pentobarbital. A polyethylene catheter was placed in the trachea and secured in place. Rats were exsanguinated via the abdominal aorta, and an incision was made in the diaphragm (to allow the lungs to expand during lavage procedures). The lungs were lavaged 5 times with 5 ml/wash of calcium and magnesium-free phosphate-buffered saline, pH 7.4, prewarmed at 37 °C. The BALF was centrifuged at 300×g for 10 min at 4 °C, and the cell-free supernatant was aliquoted and stored at − 70 °C.
Preparation of alveolar macrophage (AM)-conditioned media
AM culture was performed as follows. Briefly, the BALF from each rat was centrifuged, cells were separated from the supernatant, and cell number and cell viability were determined by hemocytometer and trypan blue exclusion, respectively. Cell differentials were done on cytocentrifuge preparations, which were fixed in methanol and stained with Diff-Quik (Sigma, St. Louis, MO, USA), and they were resuspended in RPMI-1640 media supplemented with 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% heat-inactivated fetal bovine serum (GIBCO, Grand Island, NY, USA). The cells were seeded (1 × 106 AM/ml) to a 24-well tissue culture plate and allowed to adhere for 2 h at 37 °C in a humidified atmosphere in 95% O2:5% CO2, after which, the non-adherent cells were removed by washing thrice with RPMI media. The AM-enriched monolayers were incubated in fresh RPMI media for an additional 24 h. The macrophage-conditioned media was collected, centrifuged, and the supernatants were stored in aliquots at − 70 °C for fibronectin measurements.
To assess changes in glycoprotein metabolism during early and late stages of bleomycin-induced lung injury, we examined bronchoalveolar lavage fluid and alveolar macrophage fibronectin levels, and lung glycosidase activities at 3, 5, 7, 14, and 28 days post bleomycin instillation. However, lung glycoprotein constituents were analyzed at 7, 14, and 28 days post bleomycin instillation.
Extraction of fibronectin from lung tissue
Fibronectin from lung tissue was extracted by the method of Bray et al. [21]. Briefly, lung tissues (~ 0.5 g) were homogenized in 50 mM sodium phosphate buffer, pH 6.0, containing 2 M urea, 2 mM phenylmethylsulfonylfluoride, and 5 mg/ml of heparin. The homogenate was stirred (using a magnetic stirrer) for 4 h and centrifuged at 8000 g for 20 min. and the clear supernatant was collected and stored at − 20 °C.
Fibronectin analysis
Rat fibronectin analyses were determined by ELISA according to method of Gomez-Lechon and Castell [22]. In brief, cell-free BALF, lung tissue supernatants, and AM-conditioned media were diluted with sodium bicarbonate buffer (50 mM, pH 9.6), and 100 μl of either diluted samples or standards were added to the appropriate wells in a 96-well microtiter plate. Standards consisted of rat plasma fibronectin in the range of 100–1000 ng/ml, whereas diluent buffer served as controls. The samples were incubated for 1 h at 37 °C in a humidified chamber. At the end of incubation, the wells were washed with PBS containing 0.1% Tween 20 (PBS-T) and incubated with goat anti-rat fibronectin (Calbiochem, San Diego, CA, USA) for I h at 37 °C. The plate was washed three times with PBS-T and incubated with rabbit anti-goat IgG conjugated with peroxidase for 1 h at 37 °C. Finally, the plate was washed three times with PBS-T, and the color was developed by adding O-phenylenediamine (0.1 mg/ml in citrate–phosphate buffer, pH 5.0) and 0.003% H2O2. The plate was incubated for 30 min at 37 °C, and the reaction was stopped by the addition of 4 N H2SO4. The optical density was read at 492 nm, and the fibronectin was quantitated from the standard curve.
Extraction and estimation of lung glycoproteins
After lung lavage was performed, lungs were dissected, rinsed in ice-cold 0.15 M sodium chloride to remove blood and non-pulmonary tissues, weighed and finely minced with scissors. Lungs were homogenized in 0.15 M sodium chloride using a Polytron homogenizer in the cold room at 4 °C. The homogenates were kept for shaking in a mechanical shaker at 4 °C for 48 h and then allowed to settle for 3 h at 4 °C. Supernatants were separated and stored and the residue was re-extracted with 0.15 M sodium chloride. Pooled supernatants were then dialyzed against 0.15 M sodium chloride for 48 h at 4 °C and used for the estimation of salt soluble carbohydrate constituents of glycoproteins.
Estimation of hexosamine
Hexosamine content in lung tissue was determined following the procedure of Boas et al. [23]. Since neutral sugars are present in the tissue homogenates, hexosamines were separated from neutral sugars by adsorption and elution from ion-exchange resin (Dowex 50-X 4, 200–400 mesh, H+ form). Prior to the separation of hexosamines from ion-exchange columns, hexosamines were liberated by acid for 8 h in sealed tubes by keeping them in a boiling water bath. The acid from the hydrolysate was completely removed in a rotary evaporator at 50 °C prior to placing the samples on ion-exchange column. The ion-exchange resin was washed, prior to use, with 2 N sodium hydroxide followed by distilled water, 3 N hydrochloric acid, distilled water and finally transferred into the column. The sample was loaded on to the top of the column and washed with distilled water to remove unwanted materials. Hexosamines were then eluted from the column with 2 N hydrochloric acid and the elute was collected, evaporated to dryness at 50 °C in a rotary evaporator. Thus the residue obtained free from acid was dissolved in known amount of water. About 1 ml of this solution was mixed with 1 ml of acetylacetone (2% solution in 1 N sodium carbonate) in a screw- capped tube and placed in a boiling water bath for 45 min. After cooling the tubes in a water bath at room temperature, 2.5 ml of ethanol was added and mixed thoroughly. To this 1 ml of Ehrlich’s reagent (2.67% solution w/v of p-dimethylaminobenzaldehyde in 1:1 mixture of ethanol and concentrated hydrochloric acid) was added, mixed thoroughly and made up to 10 ml with ethanol. The readings were recorded at 530 nm using Shimadzu UV-260 spectrophotometer. Readings were taken for both water blank and glucosamine standard simultaneously.
Fucose analysis
Lung fucose content was measured by the method of Dische and Shettles [24]. Aliquots of tissue homogenate (1 ml) was added to 4.5 ml of cold-sulfuric acid reagent (a mixture of 6 volume of concentrated sulfuric acid and 1 volume of distilled water, and mixed vigorously). The samples were brought to room temperature by placing them under tap water and then placed in a vigorously boiling water bath (sample tubes were capped) for 3 min and cooled to room temperature under tap water. Then 0.1 ml of cysteine reagent (3% w/v, cysteine hydrochloride in water) was added with immediate mixing and the intensity of the color was determined at 396 nm and at 427 nm in a Shimadzu spectrophotometer. Blanks were prepared with water and unknown samples were determined from the standard curve constructed with fucose. Fucose content was determined by subtracting the absorbance difference of the sample between 396 and 427 nm without cysteine from the absorbance of the same sample between 396 and 427 nm with cysteine.
Sialic acid analysis
Lung sialic acid content was measured using thiobarbituric acid (TBA) as described by Warren [25]. Lung homogenates were hydrolyzed with 0.1 N sulfuric acid at 80 °C for 1 h in a sealed tube. Aliquots containing 2–18 mg of sialic acid in a sample (0.2 ml) were mixed with 0.1 ml of periodate solution (sodium metaperiodate, 0.2 M, in 9 M phosphoric acid) and kept at room temperature for 20 min. One ml of the arsenite solution (sodium arsenite, 10% in a solution of 0.5 M sodium sulfate–0.1 N sulfuric acid) was added. The samples were shaken until the yellow-brown color disappeared. Then 3 ml of the TBA reagent (TBA, 0.6% in 0.5 M sodium sulfate) was added and the contents were mixed thoroughly. The sample tubes were capped and heated in a vigorously boiling water bath for 15 min and cooled under tap water for 5 min. Then 4.3 ml of cyclohexanone was added and mixed vigorously to extract the red color into the organic phase and then centrifuged for 3 min at 1000×g. The clear top organic phase was transferred to a cuvette and the absorbance was measured at 549 nm in a Shimadzu UV-260 spectrophotometer. Blanks were prepared with water. Standard curve was constructed using N-acetylneuraminic acid.
Total hexose analysis
Lung content of total hexose was measured following the protocol of Dubois et al. [26] using phenol as the specific organic color-developing reagent. To 2 ml of an aqueous solution containing 10–70 μg of sugar, 0.05 ml of the phenol reagent (80% by weight was prepared by adding 20 g of distilled water to 80 g redistilled phenol) was added. Then 5 ml of concentrated sulfuric acid was added rapidly and the sample was brought to room temperature. The intensity of the color was measured at 490 nm using Shimadzu spectrophotometer. Blanks were prepared by substituting distilled water for the sugar solution. The amount of sugar was estimated from the standard curve constructed with glucose.
Assay of N-acetyl-β-d-glucosaminidase (NAG)
The NAG activity in BALF was measured by the method of Moore and Morris [27]. Known aliquots of the lung homogenates were added to 0.5 ml of incubation buffer containing 2 mM p-nitrophenyl-β-N-acetylglucosaminide (dissolved in 0.1 M citrate buffer, pH 4.5). At the end of incubation, reaction was stopped by the addition of 4 ml of 0.2 M glycine-sodium hydroxide buffer, pH 11.7 containing 2 M sodium dodecyl sulfate and the contents were centrifuged. To the supernatants, 0.5 M sodium hydroxide was added and the absorbance was measured at 410 nm. The activity of NAG is expressed as μM p-nitrophenol liberated/h/ mg protein.
Assay of β-glucosidase and β-galactosidase
Lung tissue β-glucosidase and β-galactosidase levels were measured following the method of Conchie et al. [28]. Except for the differences in substrates used viz. p-nitrophenyl β-d-glucoside for β-glucosidase and p-nitrophenyl β-d-galactoside for β-galactosidase and the buffer viz. 0.2 M phosphate–0.1 M citrate buffer (pH 5.0), the assay method was essentially the same as that of N-acetyl-β-d-glucosaminidase assay. The enzyme activities were expressed as μM p-nitrophenol liberated/h/100 mg protein.
β-fucosidase assay
The activity of lung β-fucosidase was assayed by the method of Bosmann and Hemsworth [29]. Known aliquots of lung homogenate were incubated with 6 μM of substrate (p-nitrophenyl β-N-fucopyranoside, final volume was 1.025 ml, 0.05 M citrate adjusted to pH 4.3) for 1 h at 37 °C. The reaction was terminated by the addition of 2 ml of 0.4 M glycine-sodium hydroxide buffer, pH 10.5. The reaction mixture was centrifuged at 5000×g for 10 min, and the absorbance of the released p-nitrophenol in the supernatant measured at 410 nm. Enzyme activity was calculated from a standard curve and p-nitrophenol served as the standard. Results are expressed as μM p-nitrophenol liberated/h/100 mg protein.
Chemicals
Tissue culture media and reagents were purchased from HiMedia Laboratories, Mumbai, India, and GIBCO, Grand Island, NY, USA. Unless otherwise stated most of the reagents and chemicals were purchased from Sigma chemicals, St Louis, MO, USA. All other reagents were of analytical grade and commercially available.
Statistical analysis
Results are expressed as mean ± standard deviation of six separate experiments. Statistical treatment of data was accomplished using a one-way analysis of variance (ANOVA) for multiple comparisons followed by post hoc Bonferroni test (Graphpad Prism, version 3.01).
Results
Fibronectin levels in BALF and lung tissue of normal and fibrotic rats were examined by ELISA, and the results are summarized in Fig. 1a and b. There were significant (p < 0.001) increases in fibronectin concentrations in the BALF and lungs of fibrotic rats compared with that of control groups. BALF fibronectin showed a 2.8-fold increase at day 3, a 5.5-fold increase at day 5, a 13-fold increase at day 7, and a 2.5-fold increase at day 14 after BLM instillation. However, fibronectin content in the BALF returned to control levels at day 28 post-BLM injections. Similarly, lung fibronectin revealed a 1.8-fold increase at day 3, a 2.6-fold increase at day 5, a 3.5-fold increase at day 7, a 2.0-fold increase at day 14, and a 1.5-fold increase at day 28 after BLM administration. Interestingly, curcumin inhibited BLM-induced increases in the BALF and lung fibronectin levels. As shown in Fig. 2, there was a dramatic increase in AM fibronectin release at various times during the development of BLM-induced pulmonary fibrosis. The marked increase in AM fibronectin release induced by BLM was abrogated by curcumin.
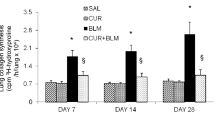
BLM-induced lung injury is characterized by increased production of collagen [3] and proteoglycans [5]. To determine if BLM-induced pulmonary damage is associated with increases in complex carbohydrates, lung levels of total hexose (Fig. 3a), sialic acid (Fig. 3b), fucose (Fig. 4a), and hexosamine (Fig. 4b) were measured. The results showed that there were significant (p < 0.001) increases in the composition of various complex carbohydrates of fibrotic lungs at days 7, 14, and 28 after BLM administration when compared to control rats. The time course studies demonstrated that fibrotic lungs contained higher amounts of glycoproteins at 14 days compared to 7 and 28 days after BLM administration. Treatment of BLM rats with curcumin significantly (p < 0.001) abrogated BLM-induced increases in lung complex carbohydrates.
Protective effects of curcumin on bleomycin-induced changes in total hexose (a) and sialic acid (b) in lung tissues. Results are mean ± S.D. of six rats in each group. *Significantly (p < 0.001) higher than all groups; §significantly (p < 0.001) lower than bleomycin rats. †significantly (p < 0.05) higher than control groups
Protective effects of curcumin on bleomycin-induced changes in fucose (a) and hexosamine (b) in lung tissues. Results are mean ± S.D. of six rats in each group. *Significantly (p < 0.001) higher than all groups; §significantly (p < 0.001) lower than bleomycin rats. †Significantly (p < 0.05) higher than control groups
The results of this study also reveal that there is a significant (p < 0.001) increase in the activities of enzymes involved in the metabolism of complex carbohydrates. In BLM rats, the levels of N-acetyl-β-d-glucosaminidase (Fig. 5a), β-glucosidase (Fig. 5b), β-galactosidase (Fig. 6a), and β-fucosidase (Fig. 6b) were progressively increased from day 3 to day 14 after BLM administration, whereas curcumin treatment ameliorated the BLM-induced increases in these enzymes in fibrotic lungs.
Protective effects of curcumin on bleomycin-induced changes in aN-acetyl-β-d-glucosaminidase and b β-glucosidase in lung tissue. Results are mean ± S.D. of six rats in each group. *Significantly (p < 0.001) higher than all groups; §significantly (p < 0.001) lower than bleomycin rats. †significantly (p < 0.05) higher than control groups
Protective effects of curcumin on bleomycin-induced changes in a β-galactosidase and b β-fucosidase in lung tissue. Results are mean ± S.D. of six rats in each group. *Significantly (p < 0.001) higher than all groups; §significantly (p < 0.001) lower than bleomycin rats. †Significantly (p < 0.05) higher than control groups
Discussion
The major findings of the present study were (i) fibronectin levels in both the BALF and lung were significantly elevated in BLM rats in comparison with control groups, (ii) alveolar macrophage release of fibronectin was higher in BLM rats, (iii) the composition of glycoproteins and the activities of enzymes involved in the metabolism of glycoproteins were increased in BLM-lungs compared to normal lungs, and (iv) curcumin inhibited BLM-induced increases in glycoprotein metabolism.
BLM-induced inflammatory response, a crucial part in the initiation and progression of lung fibrosis, is characterized by activation of inflammatory cells, including macrophages, elevated production of inflammatory mediators, cytokines, and growth factors. Alveolar macrophages have been strongly implicated in orchestrating the pulmonary inflammatory response, and increased synthesis of fibronectin by AM is a part of the inflammatory activity in interstitial lung diseases [30]. Therefore, increased levels of fibronectin in the BALF and lung tissue may result from an exaggerated production of fibronectin by alveolar macrophage in the fibrotic lungs. Consistent with this possibility, upregulation of macrophage production of fibronectin was observed in BLM rats when compared to controls. The present results on BALF fibronectin content are consistent with the results of Hernnas et al. [31], who reported a similar increase in BALF fibronectin levels in BLM-induced pulmonary fibrosis in the rat; however, AM fibronectin release was not addressed in their study. Pathologic accumulations of fibronectin have also been demonstrated in a wide variety of lung diseases, including pulmonary fibrosis [32]. Increased AM fibronectin release has also been demonstrated in dust-induced interstitial lung disease in rats [33], and in rats exposed to cadmium chloride [34]. Increased expression of fibronectin may have pathological implications in the development of pulmonary fibrosis [35]. Fibronectin, a 440 kD large (dimmer) glycoprotein, is chemotactic for fibroblasts, endothelial cells and monocytes [36, 37] in the injured lung tissue, and is capable of interacting with a variety of cell types and the ECM [38], and acting together with other growth factors, it can stimulate fibroblast to proliferate [39, 40], resulting in local accumulation of collagen. Thus, persistent increases in AM fibronectin release could influence excessive tissue repair and scar formation.
Curcumin inhibition of AM fibronectin release and reduced accumulation of fibronectin in the BALF and fibrotic lung reflects a decrease in the activity of inflammatory cells in BLM-treated rats. Curcumin-mediated inhibition of BLM-induced inflammation is also evident by the decrease in BALF biomarkers and reduction in total cell counts in the BALF [14]. Thus, curcumin suppression of fibronectin expression in BLM-injured lungs may prevent recruitment of cells to sites of tissue injury and inflammation, and inhibit cell attachment to ECM proteins, local cell proliferation and collagen accumulation. The remarkable reduction of fibronectin levels in curcumin-treated BLM rats may be due to a reduction in local production by AM. It is also possible that curcumin may inhibit fibronectin production by fibroblasts [41] and epithelial cells [42], leading to reduced accumulation of fibronectin in the injured lung. Decreased levels of fibronectin in the BALF and lung tissue reflect the ability of curcumin to inhibit both the early inflammatory and the late fibrotic phase in BLM-injured lungs.
The results of this study also demonstrate that the levels of various complex carbohydrates in lung structures were higher in fibrotic rats when compared with control rats. Although one study [43] has shown an increase in hexosamine content in BLM-injured rat lungs, there is no information in the literature about changes in other carbohydrates in BLM-induced fibrotic lungs. Interestingly, the present study observed increases in total hexose, fucose and sialic acid, and hexosamine contents of fibrotic lungs. The data also suggest that the levels of complex carbohydrates changed markedly with the development of pulmonary fibrosis. The peak increase in the levels of these sugars was evident at day 14 after BLM compared to day 7 or day 28. These results suggest that metabolism of glycoproteins is a dynamic process, changing during the course of lung fibrosis. Increased levels of various complex carbohydrates have also been demonstrated in cyclophosphamide-induced lung fibrosis [44], in inflammatory conditions [45, 46], malignancy [47], in lung cancers [48] and in hepatic fibrosis [49]
The increase in glycoproteins is probably due to increased turnover, as the levels of various glycosidases were also increased in fibrotic lungs. BLM administration resulted in a time-dependent increase in lung glycosidase activities: the maximal increase in various glycosidases was observed at day 7 after BLM administration; however, the activities of these enzymes were comparable to normal levels at day 28 post-BLM injection. Increased levels of BALF N-acetyl-β-d-glucosaminidase activity were also seen in BLM rats, suggesting that these enzymes are produced in large amounts by BLM-activated inflammatory cells. Similar increases in lung lavage fluid glycosidases have been reported in smoking baboons [50]. BLM-induced inflammatory responses have the potential to destroy pulmonary tissue through alveolar macrophage release of lysosomal glycosidases. Given its presence on cell surfaces and in the ECM, structural degradation of glycoproteins and proteoglycans by glycosidases could contribute to pulmonary inflammation through the release of matrix bound proinflammatory cytokines and growth factors [51, 52]. Thus, abnormal accumulation of complex carbohydrates in fibrotic lungs may result not only from changes in synthesis but also in degradation.
The present results also demonstrate that curcumin was not only able to inhibit fibronectin accumulation but can also prevent increases in complex carbohydrates and glycosidases in BLM-treated rats. Curcumin-mediated decrease in glycosidases in fibrotic lungs is consistent with its anti-inflammatory activity [53, 54]. Interestingly, curcumin has been reported to reduce the release of lysosomal glycosidases in myocardial infarction [54]. Curcumin inhibition of lysosomal enzyme secretion may be due its membrane-stabilizing properties [55, 56]. Inhibition of BLM-induced inflammation by curcumin is sufficient to suppress elaboration of inflammatory mediators and proteolytic enzymes. Thus, curcumin-mediated reduction in glycosidases could prevent structural degradation of glycoproteins and proteoglycans, thereby attenuating pulmonary inflammation through inhibition of release of matrix bound proinflammatory cytokines and growth factors, which in turn could prevent ECM synthesis and deposition.
References
Hay JS, Shahzeidi S, Laurent GJ (1991) Mechanisms of bleomycin-induced lung damage. Arch Toxicol 65:81–94
Crouch E (1990) Pathobiology of pulmonary fibrosis. Am J Physiol 259:L159–184
Laurent GJ, McAnulty RJ (1983) Protein metabolism during bleomycin induced pulmonary fibrosis in rabbits. In vivo evidence for collagen accumulation because of increased synthesis and decreased degradation of the newly synthesized collagen. Am Rev Respir Dis 128:82–88
Bensadoun ES, Burke AK, Hogg JC, Roberts CR (1996) Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med 154:1819–1828
Venkatesan N, Ebihara T, Roughley PJ, Ludwig MS (2000) Alterations in large and small proteoglycans in bleomycin-induced pulmonary fibrosis in rats. Am J Respir Crit Care Med 161:2066–2073
Westergren-Thorsson G, Hernnäs J, Särnstrand B, Oldberg A, Heinegård D, Malmström A (1993) Altered expression of small proteoglycans, collagen, and transforming growth factor-beta 1 in developing bleomycin-induced pulmonary fibrosis in rats. J Clin Invest 92:632–637
Jackson DS, Bentley JP (1968). In: Treatise on collagen (ed. Gould BS) 2, 189–214. Academic Press, London.
Roberts L, Roberts B, Moczar E (1968) Structural glycoproteins of connective tissue chemical and immunological properties. Bibl Paediat Suppl Ann Paediat 86:309–317
Eylar EH (1965) On the biologic role of glycoproteins. J Theor Biol 10:89–113
Kropski JA, Blackwell TS (2019) Progress in understanding and treating idiopathic pulmonary fibrosis. Annu Rev Med 70:211–224
Collins BF, Raghu G (2019) Antifibrotic therapy for fibrotic lung disease beyond idiopathic pulmonary fibrosis. Eur Respir Rev 28:190022
Tomos IP, Tzouvelekis A, Aidinis V, Manali ED, Bouros E, Bouros D, Papiris SA (2017) Extracellular matrix remodeling in idiopathic pulmonary fibrosis. It is the 'bed' that counts and not 'the sleepers'. Expert Rev Respir Med 11:299–309
Hewlett JC, Kropski JA, Blackwell TS (2018) Idiopathic pulmonary fibrosis: Epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol 71–72:112–127
Punithavathi D, Venkatesan N, Babu M (2000) Curcumin inhibition of bleomycin-induced pulmonary fibrosis in rats. Br J Pharmacol 131:169–172
Venkatesan N (1998) Curcumin attenuation of acute adriamycin myocardial toxicity in rats. Br J Pharmacol 124:425–427
Smith MR, Gangireddy SR, Narala VR, Hogaboam CM, Standiford TJ, Christensen PJ et al (2010) Curcumin inhibits fibrosis-related effects in IPF fibroblasts and in mice following bleomycin-induced lung injury. Am J Physiol (Lung Cell Mol Physiol) 298:L616–L625
Gouda MM, Bhandary YP (2018) Curcumin down-regulates IL-17A mediated p53-fibrinolytic system in bleomycin induced acute lung injury in vivo. J Cell Biochem 119:7285–7299
Amini P, Saffar H, Nourani MR, Motevaseli E, Najafi M, Ali Taheri R (2018) Qazvini A (2018) Curcumin mitigates radiation-induced lung pneumonitis and fibrosis in rats. Int J Mol Cell Med 7:212–219
Ghosh SS, Massey HD, Krieg R, Fazelbboy ZA, Ghosh DA, Fakhry SI et al (2009) Curcumin ameliorates renal failure in 5/6 nephrectomized rats: role of inflammation. Am J Physiol Renal Physiol 296:F1146–F1157
Ghosh SS, Salloum FN, Abbate A, Krieg R, Sica DA, Gehr TW et al (2010) Curcumin prevents cardiac remodeling secondary to chronic renal failure through deactivation of hypertrophic signaling in rats. Am J Physiol Heart Circ Physiol 299:H975–H984
Bray BA, Mandl I, Turino GM (1981) Heparin facilitates the extraction of tissue fibronectin. Science 214:793–795
Gomez-Lechon MJ, Castell JV (1985) Enzyme-linked immunosorbent assay to quantify fibronectin. Anal Biochem 145:1–8
Boas NF (1953) Method for the determination of hexosamines in tissues. J Biol Chem 204:553–563
Dische Z, Shettles LB (1966) Complex carbohydrates. Methods Enzymol 8:11–13
Warren L (1966) Complex carbohydrates. Methods Enzymol 8:16–18
Dubois M, Gilles K, Hemilton JK, Rebers PA, Smith FA (1966) Complex carbohydrates. Methods Enzymol 8:93–95
Moore JC, Morris JW (1982) A simple automated colorimetric method for determination of N-acetyl-β-D-glucosaminidase. Ann Clin Biochem 19:157–159
Conchie J, Gelman AL, Levvy GA (1967) Inhibition of glycosidases by aldonolactones of corresponding configuration. The C-4 and C-6 specificity of β-glucosidase, β-galactosidase. Biochem J 103:609–615
Bosmann HB, Hemsworth BA (1971) Intraneural glycosidases. II. Purification and properties of α-fucosidase, β-fucosidase, α-mannosidase and β-xylosidase of rat cerebral cortex. Biochim Biophys Acta 242:152–171
Rennard SI, Hunninghake GW, Bitterman PB, Crystal RG (1981) Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci USA 78:7147–7151
Hernnas J, Nettlebladt O, Bjermer L, Sarnstrand B, Malmstrom A, Hallgren R (1992) Alveolar accumulation of fibronectin and hyaluronan precedes bleomycin-induced pulmonary fibrosis in the rat. Eur Respir J 5:404–410
Limper AH, Roman J (1992) Fibronectin. A versatile matrix protein with roles in thoracic development, repair and infection. Chest 101:1663–1673
Driscoll KE, Maurer JK, Lindenschmidt RC, Romberger D, Rennard SI, Crosby L (1990) Respiratory tract responses to dust: relationships between dust burden, lung injury, alveolar macrophage fibronectin release, and the development of pulmonary fibrosis. Toxicol Appl Pharmacol 106:88–101
Driscoll KE, Maurer JK, Poynter J, Higgins J, Asquith T, Miller NS (1992) Stimulation of rat alveolar macrophage fibronectin release in a cadmium chloride model of lung injury and fibrosis. Toxicol Appl Pharmacol 116:30–37
Lazenby AJ, Crouch EC, McDonald JA, Kuhn C III (1990) Remodeling of the lung in bleomycin-induced pulmonary fibrosis in the rat. Am Rev Respir Dis 142:206–214
Postlethwaite AE, Keski-Oja J, Balian G, Kang AH (1981) Induction of fibroblast chemotaxis by fibronectin. Localization of the chemotactic region to a 140,000-molecular weight non-gelatin-binding fragment. J Exp Med 153:494–499
Clark RA, DellaPelle P, Manseau E, Lanigan JM, Dvorak HF, Colvin RB (1982) Blood vessel fibronectin increases in conjuction with endothelial cell proliferation and capillary ingrowth during wound healing. J Invest Dermatol 9:269–280
Saba TM, Jaffe E (1980) Plasma fibronectin (opsonic glycoprotein): Its synthesis by vascular endothelial cells and role in cardiopulmonary integrity after trauma as related to reticuloendothelial function. Am J Med 68:577–594
Bitterman PB, Rennard SI, Adelberg S, Crystal RG (1983) Role of fibronectin as a growth factor for fibroblasts. J Cell Biol 97:1925–1932
Jetten AM, Shirley JE, Stoner G (1986) Regulation of proliferation and differentiation of respiratory tract epithelial cells by TGF-β. Exp Cell Res 167:539–549
Mosher DF, Furcht LT (1981) Fibronectin: review of its structure and possible function. J Invest Dermatol 77:75–78
Shoji S, Rickard KA, Ertl RF, Robbins RA, Linder J, Rennard SI (1989) Bronchial epithelial cells produce lung fibroblast chemotactic factor: fibronectin. Am J Respir Cell Mol Biol 1:13–20
Chandrasekaran L, Seethalakshmi S, Chandrakasan G, Dhar SC (1987) Alterations in lung and skin compositions of rat in bleomycin-induced fibrosis. Biochem Med Metab Biol 38:205–212
Venkatesan N, Punithavathi D, Chandrakasan G (1998) Glycoprotein composition in cyclophosphamide induced lung fibrosis. Biochim Biophys Acta 1407:125–134
Ravichandran LV, Puvanakrishnan R (1991) Glycoprotein metabolism in isoproterenol induced myocardial infarction in rats. Biochem Arch 7:249–260
Kesava Reddy G, Dhar SC (1988) Studies on carbohydrate moieties of glycoproteins in established adjuvant induced arthritis. Agents Actions 25:63–70
Warren L, Buck GA, Tuszynski GP (1978) Glycopeptide changes and malignant transformation: A possible role for carbohydrate in malignant behaviour. Biochim Biophys Acta 516:97–127
Bryant ML, Stoner GD, Metzger RP (1974) Protein-bound carbohydrate content of normal and tumorous lung tissue. Biochim Biophys Acta 343:226–231
George J, Chandrakasan G (1996) Glycoprotein metabolism in dimethylnitrosamine induced hepatic fibrosis in rats. Int J Biochem Cell Biol 28:353–361
Radhakrishnamurthy B, Smart F, Berenson GS, Rogers WR (1983) The composition of glycoproteins and activities of glycosidases in bronchoalveolar lavages from smoking baboons. Lung 161:165–172
Imai K, Hiramatsu A, Fukushima D, Pierschbacher MD, Okada Y (1997) Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem J 322:809–814
Taipale J, Keski-oja J (1997) Growth factors in the extracellular matrix. FASEB J 11:51–59
Satoskar RR, Shah SJ, Shenoy SG (1986) Evaluation of anti-inflammatory property of curcumin (diferuloylmethane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol 24:651–654
Srimal RC, Dhawan BN (1973) Pharmacology of diferuloylmethane (curcumin), a non-steroidal anti-inflammatory agent. J Pharm Pharmacol 25:447–452
Nirmala C, Puvanakrishnan R (1996) Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol Cell Biochem 159:85–93
Srivastava V, Srimal KC (1985) Modification of certain inflammation induced biochemical changes by curcumin. Ind J Med Res 81:215–223
Acknowledgements
The authors would like to thank the Director, CLRI for his interest and permission to publish this work and Mr V. Elango for his help in animal experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All animals were treated with humane conditions and institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Durairaj, P., Venkatesan, S., Narayanan, V. et al. Protective effects of curcumin on bleomycin-induced changes in lung glycoproteins. Mol Cell Biochem 469, 159–167 (2020). https://doi.org/10.1007/s11010-020-03737-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03737-3