Abstract
In this study, we investigated the effects of astragaloside IV (As-IV) on pulmonary fibrosis and its mechanisms of action. Sprague-Dawley rats were used in a model of pulmonary fibrosis induced by an intratracheal instillation of bleomycin (BLM). Rats were intraperitoneally injected with As-IV (10, 20, 50 mg/kg) daily for 28 days, while the rats in control and BLM groups were injected with a saline solution. The effects of As-IV treatment on pulmonary injury were evaluated with the lung wet/dry weight ratios, cell counts, and histopathologic. Oxidative stress was evaluated by detecting the levels of malondialdehyde (MDA), superoxide dismutase (SOD), total antioxidant capacity (T-AOC), and reactive oxygen species (ROS) in lung tissue. Inflammation was assessed by measuring the levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 in bronchoalveolar lavage fluid (BALF). The results indicated that As-IV treatment remarkably ameliorated BLM-induced pulmonary fibrosis and attenuated BLM-induced oxidative stress and inflammation. Our findings indicate that As-IV-mediated suppression of fibroproliferation may contribute to the anti-fibrotic effect against BLM-induced pulmonary fibrosis. Its mechanisms of action are associated with inhibiting oxidative stress and inflammatory response. In summary, our study suggests a therapeutic potential of As-IV in the treatment of pulmonary fibrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Pulmonary fibrosis, which is characterized by accumulation of extracellular matrix and loss of pulmonary function, is the most common form of interstitial lung disease [1, 2]. The studies found that a number of key factors have been implicated in the pathogenesis of pulmonary fibrosis, such as inflammation, apoptosis, oxidative stress, and hyperplasia [3–6]. Inflammation is the initial response following lung injury. In the lower airways, the inflammatory cells accumulate and release some pro-inflammatory cytokines, growth factors, and so on, which regulate the proliferation and secretary activity of alveolar fibroblasts [7, 8]. There is increasing evidence that that oxidative stress is involved in the pathogenesis of pulmonary fibrosis. Markers of oxidative stress have been identified in patients and aberrant antioxidant activity has been demonstrated to exacerbate pulmonary fibrosis in animal models [9, 10]. Therefore, anti-inflammation and antioxidant treatment strategies are being developed to treat pulmonary fibrosis.
Astragaloside IV (As-IV; 3-O-β-D-xylopyranosyl-6-O-β-D-glucopyranosyl cycloastragenol) is a natural saponin purified from Astragalus membranaceus with antioxidant, anti-inflammatory, and anti-apoptotic effects [11–14]. It has demonstrated the anti-inflammatory effects of As-IV through inhibition of NF-kB-mediated inflammatory genes expression [15]. Moreover, As-IV exhibits antioxidant effects through the inhibition of reactive oxygen species (ROS) production, the reduction of lipid peroxidation, and the stimulation of antioxidant enzymes [16]. As we mentioned before, anti-inflammation and antioxidant treatment strategies are being developed to treat pulmonary fibrosis. Therefore, these researches suggest As-IV may have a protective effect on fibrotic diseases. Its mechanisms of action may be associated with inhibiting oxidative stress and inflammatory response. However, there is a lack of data regarding the effects of As-IV on fibrotic lung disease.
Therefore, this study intends to investigate the effects of As-IV on pulmonary fibrosis in a bleomycin (BLM)-induced pulmonary fibrosis rat model. The underlying regulatory mechanisms associated with the potential anti-inflammatory and antioxidant effects of As-IV were also investigated.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (around 250 g, 8 weeks old) were obtained from the Experimental Animal Center of Suzhou Aiermaite Technology Co. Ltd. (SPF grade, Certificate No. SCXK20140007). The rats were maintained at a constant room temperature (22 ± 2 °C) and humidity (50 ± 10 %) with 12 h light/dark cycle and free access to water and food.
Establishment of BLM-Induced Pulmonary Fibrosis in Rats and As-IV Treatment
The rats were randomly divided into five groups (n = 10): control, BLM, BLM + As-IV10, BLM + As-IV20, BLM + As-IV50. Induction of pulmonary fibrosis with BLM was conducted according to a previously described method [17]. Briefly, all rats were anesthetized with 10 % chloral hydrate (3.5 ml/kg body weight; Sinopharm, Shanghai, China). Then, the rats were administered a single intratracheal instillation of BLM dissolved in saline (5 mg/kg body weight; Melone Pharmaceutical Co., Ltd, Dalian, China), while an equal volume of saline was injected into the rats from the control group.
The rats from the BLM + As-IV groups received an intraperitoneal injection of As-IV (99.2 % purity; National Institute for the Control of Pharmaceutical and Biological Products, Shanghai, China) at a dose of 10, 20, and 50 mg/kg bw, respectively, every day for a total of 28 days, and the rats in control and BLM groups were injected with a saline solution. On day 28 after BLM induction, the rats were sacrificed by intraperitoneal injection of 10 % chloral hydrate (5 ml/kg body weight). The bronchoalveolar lavage fluids (BALFs) were collected and the lungs were excised for further analysis.
Lung Wet/Dry Weight Ratios
The lungs of the rats were removed and weighed immediately, and following drying in an incubator at 60 °C for 72 h to obtain the dry weight. The pulmonary edema was calculated as a wet/dry weight ratio.
Cell Counting in Bronchoalveolar Lavage Fluid (BALF)
The BALF was centrifuged at 1000×g for 10 min at 4 °C, and the supernatant was stored immediately at −80 °C until analysis. The sediment cells were resuspended in 50 μl PBS and 10 μl of the cell suspension was made into the smear on a glass slide. The cell smear was air-dried, fixed in methanol for 15 min, and stained with Giemsa solution (Jiancheng Bioengineering Institute, Nanjing, China). Then, total cells, neutrophils, macrophages, and lymphocytes were counted with a hemocytometer (Hausser Scientific, Horsham, PA, USA).
BALF Biochemical Analysis
The levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 in BALF were measured by Enzyme-Linked Immunosorbent Assay (ELISA) kits (Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the manufacturer’s instructions.
Measurement of Oxidative Stress
The lung homogenates were centrifuged for 30 min at 3000×g at 4 °C. The serum levels of malondialdehyde (MDA), superoxide dismutase (SOD), total antioxidant capacity (T-AOC), and reactive oxygen species (ROS) in lung tissue were measured according to the instructions of detection kits (Jiancheng Bioengineering Institute, Nanjing, China).
Histopathological Examination
The lungs were fixed, paraffin embedded, sectioned into 5-μm slices, and then stained with hematoxylin and eosin (H&E), and examined under a light microscope (DP73; Olympus).
Statistical Analysis
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) was used to perform all statistical analysis. The present data were expressed as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to compare differences among multiple groups, followed by Bonferroni post hoc test for comparisons between two groups. P < 0.05 was considered to indicate a statistically significant difference.
RESULTS
Wet/Dry Weight Ratio
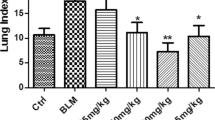
The lung wet/dry ratio was evaluated to indicate the pulmonary edema (Fig. 1). Compared to the control group, the lung wet/dry ratio significantly increased in the BLM-induced pulmonary fibrosis models (from 4.21 ± 0.13 to 6.62 ± 0.23, P < 0.05), and As-IV treatment significantly decreased the lung wet/dry ratios in BLM-induced models (from 6.62 ± 0.23 to 4.93 ± 0.17, P < 0.05).
As-IV Mitigated the Inflammatory Responses in BLM-Induced Pulmonary Fibrosis
To determine the effect of As-IV on BLM-induced pulmonary inflammation responses in rats, the inflammatory cell counts in BALF were assayed. As shown in Table 1, the number of total cells, neutrophils, macrophages, and lymphocytes in BALF were significantly increased after BLM injection. As-IV administration dose-dependently reduced the increases of inflammatory cell counts in BALF in BLM-stimulated rats. In addition, the levels of TNF-α, IL-1β, and IL-6 in BALF were subsequently detected. It was noted that As-IV significantly prevented BLM-induced elevation of TNF-α, IL-1β, and IL-6 in a dose-dependent manner (Fig. 2).
As-IV Attenuated Oxidative Stress in BLM-Induced Pulmonary Fibrosis
Oxidative stress was evaluated by detecting the levels of MDA, SOD, T-AOC, and ROS in pulmonary tissue (Fig. 3). In the BLM group, MDA and ROS levels were significantly elevated while SOD and T-AOC levels were reduced. In contrast, treatment with As-IV remarkably decreased the levels of MDA and ROS, simultaneously increased the levels of SOD and T-AOC.
Histopathological Evaluation (HE)
The HE images of lung tissue are shown in Fig. 4. The results shown that the control group displayed normal structure and no inflammatory or fibrotic lesions (Fig. 4a). In BLM group, the lung structure was severely damaged and the lung interstitium was evidently thickened (Fig. 4b). On the contrary, these pathological changes were attenuated by the administration of As-IV (Fig. 4c–e).
DISCUSSION
BLM-induced pulmonary fibrosis is one of the most commonly used experimental models in the study of pulmonary fibrosis. In the present study, we established a rat model to investigate the protective effects of As-IV against pulmonary fibrosis induced by intratracheal instillation of BLM. Our findings indicated that administration of As-IV markedly ameliorated BLM-induced pulmonary edema, oxidative stress, and inflammatory responses. Given these results, we demonstrated that As-IV treatment significantly ameliorates BLM-induced pulmonary fibrosis. Our data reveal that As-IV has potential for treatments of BLM-induced pulmonary fibrosis.
Previous researches have shown that inflammation and oxidative stress are both involved in the pathogenesis of pulmonary fibrosis [18–21]. Following BLM administration, inflammatory responses were stimulated. Our data showed that total cells, macrophages, and lymphocytes in BALF were significantly raised after BLM injection. These inflammatory cells stimulate the proliferation of fibroblasts and the activation of myofibroblasts gradually resulting in pulmonary fibrosis [22]. We also found that As-IV reduced these cells counts in BALF in a dose-dependent manner. In addition, the levels of TNF-α, IL-1β, and IL-6 in BALF were enhanced following BLM treatment. TNF-α, IL-1β, and IL-6 play important roles in pulmonary fibrosis, which induces stimulates the proliferation of fibroblasts, increasing collagen aggregation and synthesis of other cytokines [23, 24]. Our study noted that As-IV significantly prevented BLM-induced elevation of TNF-α, IL-1β, and IL-6 in a dose-dependent manner. Accumulating evidence has indicated that oxidative stress is a possible molecular mechanism underlying fibrosis in a variety of organs [25], and it is an imbalance between the generation of ROS and the capacity to detoxify these intermediates [26]. Accordingly, in the present study, oxidative stress was evaluated by detecting MDA, SOD, T-AOC, and ROS levels in lung tissue. MDA level reflects the degree of organic lipid peroxidation, which indicated the severity of damage to cell membranes [27]. SOD is an important component of the antioxidant enzymatic defense system and T-AOC reflects the overall cellular endogenous antioxidative capability [28]. It was shown that in the BLM group, MDA and ROS levels was significantly elevated while SOD and T-AOC levels were reduced. In contrast, treatment with As-IV remarkably decreased the levels of MDA and ROS, and simultaneously increased the levels of SOD and T-AOC. Those results suggested that As-IV could significantly decrease BLM-induced inflammation and oxidative stress, which might be important protective mechanisms against pulmonary fibrosis induced by BLM.
There are some limitations of this study. Further studies are required to investigate alternative and additional mechanisms.
CONCLUSIONS
The present study has shown that As-IV inhibited BLM-induced pulmonary fibrosis in rats. This protection is associated with anti-inflammatory and antioxidative properties of As-IV. The present findings suggest a potential therapeutic usefulness of As-IV on pulmonary fibrosis.
References
Crestani, B., S. Marchand-Adam, A. Fabre, M. Dehoux, and P. Soler. 2007. Mechanisms in pulmonary fibrosis. La Revue du Praticien 57: 2222–2226.
Fernandez, I.E., and O. Eickelberg. 2012. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet 380: 680–688.
Wynn, T.A. 2008. Cellular and molecular mechanisms of fibrosis. Journal of Pathology 214: 199–210.
Sisson, T.H., M. Mendez, K. Choi, et al. 2010. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine 181: 254–263.
Raghu, G., H.R. Collard, J.J. Egan, et al. 2011. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American Journal of Respiratory and Critical Care Medicine 183: 788–824.
Noble, P.W., C.E. Barkauskas, and D. Jiang. 2012. Pulmonary fibrosis: patterns and perpetrators. Journal of Clinical Investigation 122: 2756–2762.
Zhao, L., X. Wang, Q. Chang, et al. 2010. Neferine, a bisbenzylisoquinline alkaloid attenuates bleomycin-induced pulmonary fibrosis. European Journal of Pharmacology 627: 304–312.
Sener, G., N. Topaloglu, A.O. Sehirli, F. Ercan, and N. Gedik. 2007. Resveratrol alleviates bleomycin-induced lung injury in rats. Pulmonary Pharmacology & Therapeutics 20: 642–649.
Kliment, C.R., and T.D. Oury. 2010. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radical Biology and Medicine 49: 707–717.
Cheresh, P., S.J. Kim, S. Tulasiram, and D.W. Kamp. 2013. Oxidative stress and pulmonary fibrosis. Biochimica et Biophysica Acta 1832: 1028–1040.
Wang, H., Y. Zhang, T. Xia, et al. 2013. Synergistic promotion of blood vessel regeneration by astragaloside IV and ferulic acid from electrospun fibrous mats. Molecular Pharmaceutics 10: 2394–2403.
Qiu, L.H., X.J. Xie, and B.Q. Zhang. 2010. Astragaloside IV improves homocysteine-induced acute phase endothelial dysfunction via antioxidation. Biological and Pharmaceutical Bulletin 33: 641–646.
Xu, W., X. Shao, L. Tian, et al. 2014. Astragaloside IV ameliorates renal fibrosis via the inhibition of mitogen-activated protein kinases and antiapoptosis in vivo and in vitro. Journal of Pharmacology and Experimental Therapeutics 350: 552–562.
Gui, D., J. Huang, Y. Guo, et al. 2013. Astragaloside IV ameliorates renal injury in streptozotocin-induced diabetic rats through inhibiting NF-kB-mediated inflammatory genes expression. Cytokine 61: 970–977.
Zhang, W.J., P. Hufnagl, B.R. Binder, and J. Wojta. 2013. Anti-inflammatory activity of astragaloside IV is mediated by inhibition of NF-kB activation and adhesion molecule expression. Thrombosis and Haemostasis 90: 904–914.
Ko, J.K., F.Y. Lam, and A.P. Cheung. 2005. Amelioration of experimental colitis by Astragalus membranaceus through anti-oxidation and inhibition of adhesion molecule synthesis. World Journal of Gastroenterology 11: 5787–5794.
Chen, X.H., R.S. Sun, J.M. Hu, et al. 2009. Inhibitory effect of emodin on bleomycin-induced pulmonary fibrosis in mice. Clinical and Experimental Pharmacology and Physiology 36: 146–153.
Kinnula, V.L., C.L. Fattman, R.J. Tan, and T.D. Oury. 2005. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. American Journal of Respiratory and Critical Care Medicine 172: 417–422.
Walters, D.M., H.Y. Cho, and S.R. Kleeberger. 2008. Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: a potential role for Nrf2. Antioxidants and Redox Signaling 10: 321–332.
Homer, R.J., J.A. Elias, C.G. Lee, and E. Herzog. 2011. Modern concepts on the role of inflammation in pulmonary fibrosis. Archives of Pathology and Laboratory Medicine 135: 780–788.
Bringardner, B.D., C.P. Baran, T.D. Eubank, and C.B. Marsh. 2008. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxidants and Redox Signaling 10: 287–301.
Mouratis, M.A., and V. Aidinis. 2011. Modeling pulmonary fibrosis with bleomycin. Current Opinion in Pulmonary Medicine 17: 355–361.
Wynn, T.A. 2011. Integrating mechanisms of pulmonary fibrosis. Journal of Experimental Medicine 208: 1339–1350.
Denis, M. 1992. Interleukin-6 in mouse hypersensitivity pneumonitis: changes in lung free cells following depletion of endogenous IL-6 or direct administration of IL-6. Journal of Leukocyte Biology 52: 197–201.
Caspary, W.J., D.A. Lanzo, and C. Niziak. 1982. Effect of deoxyribonucleic acid on the production of reduced oxygen by bleomycin and iron. Biochemistry 21: 334–338.
Todd, N.W., I.G. Luzina, and S.P. Atamas. 2012. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis & Tissue Repair 5: 11.
Del Rio, D., A.J. Stewart, and N. Pellegrini. 2005. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutrition, Metabolism, and Cardiovascular Diseases 15: 316–328.
Dominici, M., K. Le Blanc, I. Mueller, et al. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Statements
All animal use procedures were approved by the Committee on the Ethics of Animal Experiments of Yantai Hospital of Traditional Chinese Medicine. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Conflict of Interest
The author declares no conflict of interest.
Additional information
Wei-Na Yu and Li-Feng Sun contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yu, WN., Sun, LF. & Yang, H. Inhibitory Effects of Astragaloside IV on Bleomycin-Induced Pulmonary Fibrosis in Rats Via Attenuation of Oxidative Stress and Inflammation. Inflammation 39, 1835–1841 (2016). https://doi.org/10.1007/s10753-016-0420-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0420-5