Abstract
Adequate placental angiogenesis is critical for the establishment of the placental circulation and thus for normal feto-placental growth and development. Fatty acid-binding protein-4 (FABP4) plays a pro-angiogenic role in endothelial cells; however, very little information is available in placental first trimester trophoblast cells. Here we report that exogenously added FABP4 (exo-FABP4) stimulated tube formation (as a measure of in vitro angiogenesis) in HTR8/SVneo trophoblastic cells. HTR-8/SVneo cells were incubated in the presence of exogenously added FABP4 at different concentrations and time points. Cellular growth, proliferation, in vitro tube formation, expression of growth stimulatory-, fatty acid transporters, and angiogenic genes were investigated. Internalization of exo-FABP4 was carried out using immunocytochemistry. Radioactive fatty acid uptake was determined in the presence and absence of FABP4 metabolic inhibitor. Exo-FABP4 (10–100 ng/ml) stimulated proliferation of HTR8/SVneo cells as compared to control. Exo-FABP4 dose dependently increased growth and viability of the cells to the similar extent as done by 50 µM of arachidonic acid. Exo-FABP4-induced tube formation and proliferation were significantly inhibited by FABP4 (BMS309403) inhibitor. Exo-FABP4 stimulated the expression of growth stimulatory genes such as tissue inhibitor of matrix metalloproteinases-1 (TIMP1), insulin-like growth factor 1 (IGF1), and also prokineticin 2 (PROK2), the pro-angiogenic mediators in these cells. In addition, expressions of genes associated with proliferation and differentiation such as sonic hedgehog (SHH) and WNT1 inducible signalling pathway protein 1 (WISP1) were significantly expressed when cells were exposed to exo-FABP4. Our findings reveal a pro-angiogenic role of FABP4 in first trimester placental trophoblast cells and its regulation may have impact in placental physiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatty acid-binding proteins (FABPs), ubiquitously expressed in tissues including placenta, may play a role in intracellular fatty acid transport and metabolism [1]. Several of the FABPs have been shown to deliver their ligands to nuclear transcription factors, thus modulating gene expression in a tissue-specific manner. FABP4, also known as adipocyte FABP (A-FABP) or aP2, is a member of the FABP family [1]. FABP4 is an intracellular fatty acid transporter, and the role of circulating FABP4 is not well understood. FABP4 was shown to be involved in vascular endothelial growth factor (VEGF)-mediated angiogenesis in endothelial cells [2]. FABP4 plays a pro-angiogenic role in endothelial cells by promoting cell proliferation, migration, survival, lipid accumulation, and morphogenesis [3]. Recent studies also demonstrated FABP4 as a novel target of VEGF and its receptors (VEGF/VEGFR2) pathway [4,5,6]. In these cells, FABP4 expression is induced by pro-angiogenic stimuli, such as VEGF and basic fibroblast growth factor (bFGF) [4]. The VEGF-mediated expression of FABP4 was inhibited by siRNA-mediated knockdown of VEGFR2, whereas the VEGFR1 agonists and placental growth factors (PIGFs) had no such effect. We showed earlier that FABP4 was most significantly up-regulated by VEGF in first trimester trophoblast cells, HTR8/SVneo [7,8,9,10]. We also demonstrated that leptin, docosahexaenoic acid, 22:6n−3 (DHA), and c9, t11-conjugated linoleic acid (c9, t11-CLA) stimulated FABP4 synthesis with concomitant enhanced tube formation in these cells [7,8,9,10]. FABP4 expression is also induced by hypoxia that is essential for lipid accumulation in placental last trimester under increased lipid loads [11, 12]. One of the metabolic changes in cells often observed during angiogenesis is their increased synthesis and transport of lipids, and lipid droplet formation. It is also well known that cells alter their metabolism to suit their needs for promoting angiogenesis that includes cell growth and proliferation, and relevant gene expression. Our previous data showed that fatty acids favoured energy-intensive tube formation process in the first trimester trophoblast cells [7, 10, 13], suggesting that lipid chaperones may therefore be involved in these processes. Several data indicate that FABP4 has a role in activation of mitogenic pathways and expression of key mediators of angiogenesis [4]. In endothelial cells, inhibition of FABP4 blocks most of the VEGF effects [14]. The Delta-like ligand (DLL) 4-NOTCH directly regulates FABP4 gene expression, by binding of NICD (Notch Intracellular Domain) to specific regions of the FABP4 promoter in endothelial cells. The FABP4 response to VEGF is dependent on the NOTCH pathway, as inhibition of DLL4 binding to NOTCH and inhibition of NOTCH cleavage lead to FABP4 reduction in response to VEGF [14]. The relationship between FABP4-mediated angiogenesis and different angiogenic factors (VEGF, leptin, ANGPTL4) and dietary fatty acids needs further investigation in placental trophoblasts. Recent data demonstrated that maternal serum FABP4 is independently associated with the subsequent development of preeclampsia [15, 16]. Elevated maternal serum FABP4 levels may also play a role in the pathogenesis of preeclampsia through pathways related to insulin resistance, inflammation, and abnormal lipid metabolism [12, 16]. Metabolic and Transport activities of long chain fatty acids carriers such as FABP4 are crucial for placental development. FABP4 is also important in early placental development since emerging evidences are demonstrating its involvement in angiogenesis processes in various cancer and endothelial cells [4,5,6, 14]. Previous data from our lab reported that VEGF and DHA stimulate tube formation of HTR8/SVneo cells with concomitant increased expression of FABP4. However, very little information is available whether the exogenous FABP4 affects first trimester placental trophoblasts function. In this purview, this study examined whether metabolic activities of FABP4 are involved with the tube formation of the HTR8/SVneo cells.
This paper therefore aimed to investigate the effects of exogenously added FABP4 on cellular proliferation and angiogenesis-associated activities of the placental first trimester trophoblasts by determining the cell viability, proliferation, and tube formation and expression of angiogenesis-NOTCH signalling mediators in the HTR8/SVneo cells. This report demonstrates for the first time that exogenously added FABP4 stimulates cellular growth and proliferation and in vitro tube formation and directly regulates expression of genes relevant to growth, angiogenesis, and cellular differentiation processes.
Materials and methods
Materials
HTR8/SVneo trophoblast cell line was kindly provided by Dr. C.H. Graham, Queen’s University, Canada. Recombinant human FABP4 was procured from Cayman, USA. Lactate dehydrogenase (LDH) assay kit was obtained from Roche, Germany. Radioactive [3H] thymidine 250 µCi was obtained from Perkin Elmer, USA. [1-14C]Oleic Acid (OA) (specific activity 54.6 mCi/mmol) was obtained from American Radiolabelled Chemical, USA. Matrigel was procured from BD Biosciences, USA. FABP4 inhibitor (BMS309403) was obtained from Calbiochem, UK. p38 MAPK inhibitor (SB203580) was obtained from Cell Signaling Technology, Inc., USA. Trypsin–EDTA solution, oleic acid, penicillin–streptomycin solution, RPMI 1640, and all other chemicals and solvents were obtained from Sigma-Aldrich Norway AS, Norway.
Cell culture
The HTR8/SVneo cells were cultured in RPMI-1640 medium as described before [7]. Human vascular endothelial cells, EA.hy926 was procured from ATCC, USA. These cells were cultured in DMEM (12-709F-Lonza) along with other supplements as per the supplier’s instruction.
Cell proliferation assay
HTR8/SVneo cell proliferation was measured by the incorporation of a radiolabeled DNA precursor, [3H] thymidine, into the replication strands of DNA produced during cell division. Cells were seeded overnight in 96-well plate in the presence of 5% FCS-RPMI. Next day cells were starved at least 4 h with 1% FCS-RPMI followed by incubation with assay media for 24 h. Radioactive [3H] thymidine was added as 0.3 µCi per 100 μl @50 µl per well. Cells were trypsinized with 100 µl of 0.2% trypsin for at least 30 min at 37 °C, 5% CO2 to ensure homogenous cells suspension of the well by pipetting several times. Cells were harvested using Unifilter 96 (Laborel, Pakard,) and transferred to unifilter 96 GF/B plate with 1 µM pore size (Perkin Elmer #6005177). Microsint LSC cocktail (20 μl/well, Perkin Elmer #6013611) was added to each of the 96-well plate. The incorporated radioactivity [3H] was counted on a liquid scintillation counter (Topcount; Packard Instrument, Meriden, CT). Count per minute (cpm) is compared with case and control after deducting blank value, as described previously [17].
Cytotoxicity measurement of different agents on HTR8/SVneo cells
The cytotoxicity effects of FABP4 and MAPK inhibitors, FABP4 protein, and fatty acids (100 μM) on HTR8/SVneo cells were determined by measuring LDH releases after incubating these cells for 24 h [13].
Cellular growth and viability assay
Cell viability and growth was performed as described before [10]. Cells were incubated with different reagents for 24 h. The dye 3-(4,-dimethylthiazol-2-yl)-2,-diphenyl tetrazoliumbromide (MTT) was used to detect number of viable cells. The absorbance (OD) was read at 562 nm and calculated between control and treatment after adjusting blank value
Immunocytochemistry and fluorescence microscopy
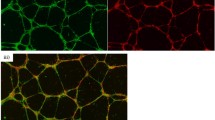
HTR8/SVneo cells were seeded on 18-mm-round cover glasses (VWR #631-1580, UK) in 12-well plate with or without FABP4 protein in the presence or absence of BMS309403. Cells were fixed using 4% paraformaldehyde and permeabilized in 0.2% Triton X-100 in PBS for 10 min. The samples were then blocked with 1% bovine serum albumin in 0.1% Tween-20 in PBS (PBST) for 1 h. Cells were incubated overnight at 4 °C with an anti-FABP4 antibody (AFABP Cat# sc 18661, Santa Cruz) and further incubated with an Alexa Fluor® 488 anti-goat antibody (Cat# A11055, Life technology) for 1 h in the dark. Cells were washed three times with PBST followed by four times with PBS and incubated with the nuclear stain DAPI (4,6-diamidino-2-phenylindole, dilactate, Sigma #F6057) for 5 min. The images were acquired using a confocal microscope LSM-700 laser scanning microscope (Carl Zeiss, Germany), at constant laser intensity and photomultiplier tube settings. Regions of interest (ROIs) were designated over definite numbers of cells nuclei (to normalize the region selection) in maximal projection images. Mean fluorescence intensity analysis was performed using a custom MATLAB script as described earlier [18].
Measurement of radiolabelled oleic acid uptake by HTR8/SVneo cells
The radiolabelled fatty acid uptake was carried out as described before [13]. The cells were pre-incubated with and without exo-FABP4 for 3 h followed by 30 min incubation with [1-14C]oleic acid. The radioactivity was determined using a scintillation counter. Soluble protein content was measured by colorimetric estimation derived on the biuret reaction using bicinchoninic acid (Uptima). Fatty acid uptake was expressed as picomoles (pmol) of fatty acid taken up per µg of cellular protein.
Gene expression analysis
Treated cells were lysed and total RNA was isolated as described previously [7, 10]. cDNAs were synthesized from RNA and real-time PCR was performed using TaqMan assays (Table 1) on ABI7900HT real-time PCR System. Gene expression was measured by estimating the level of mRNA expressed after normalized with TBP. The relative RNA expression levels were calculated using the comparative Ct method.
Analysis of gene expression pathways by real-time PCR array
HTR8/SVneo cells were pre-incubated with or without exo-FABP4 (100 ng/ml) for 24 h. Cells were lysed and total RNA was purified as per the instruction provided by the manufacturer (Qiagen, Cat# 74104). RNA sample were treated with genomic DNA elimination (GE) mixture and converted to cDNA using the RT2 First Strand Kit (Qiagen Cat# 330401). The RT2 profiler PCR array of human NOTCH signalling pathway (Qiagen Cat# PAHS-059YA) and human angiogenesis pathway (Qiagen Cat# PAHS-024ZA) were used to evaluate differential gene expression after HTR8/SVneo exposed to exogenous FABP4. Real-time PCR was performed using SYBR green/ROX PCR master mix (Qiagen Cat# 330520) in ABI 7900HT according to the instruction of the supplier. Levels of mRNA are expressed by fold differences over control.
Tube formation assay
Tube formation assay was performed in HTR8/SVneo cells as described before [7, 10]. The cells were seeded (7 × 104cells/well/96 well plate) on matrigel (growth factor reduced) coated wells and inhibitor were added in the presence or absence of exo-FABP4 to the cells. The wells were captured at least five different fields per well after 8 h by an inverted microscope at ×4 magnification (Nikon TS100F, Japan). Extreme edges were excluded due to gel meniscus formation. Mean capillary tube length was quantified by Image J as pixel and expressed as a unit tube length in centimetre. Experimental groups were compared statistically by considering tube length per field per images derived from independent experiments (n = 3) performed in duplicates.
Gene silencing assay
Cells (HTR8/SVneo and EA.hy926) were transfected (104cells/well/96 well) with siRNA for 48 h in antibiotic free media. Smart pool contains predesigned siRNAs targeting cyclophilin B (Cat# D-001820) as positive control, scramble siRNA (Cat# D-001810) as non-target control, and FABP4 siRNA (cat# L-008853) were obtained from Dharmacon, USA. Forward transfection approach was employed using 25 nM siRNA and 0.5 μl of Dharmafect reagent/well as per the instruction of the manufacturer. Knockdown efficiency was measured by mRNA expression levels of the respective genes after normalized with TBP as endogenous control as described before.
Statistics and data analysis
All the values are presented as mean and standard errors of mean (SEM). The significance was calculated using Student’s t test for non-parametric values. Majority of the data are obtained from three independent experiments performed with many parallels. p value of <0.05 was considered statistically significant.
Results
Effects of exogenously added FABP4 (exo-FABP4) on cell proliferation of the trophoblast cells, HTR8/SVneo
In order to investigate, whether exogenous FABP4 has any effects on proliferation of HTR8/SVneo cells, proliferation was measured by analysing [3H] thymidine incorporation of active DNA synthesis of the growing cells. Compared to control, [3H] thymidine incorporation was significantly higher when HTR8/SVneo cells were pre-stimulated with exo-FABP4. More specifically, exo-FABP4 (100 ng/ml) increased [3H] thymidine incorporation by 17% (control vs. exo-FABP4: 56650 ± 818.2, n = 3 vs. 66,220 ± 885.4, n = 3, cpm) as compared to control (Fig. 1). Moreover, exo-FABP4 stimulated cell proliferation across the doses of 10–100 ng/ml as compared to control (p < 0.05). To investigate further, if exo-FABP4-induced cell proliferation mediated through its metabolic activities, HTR8/SVneo cells were co-incubated with FABP4 protein and its inhibitor (BMS309403). Exo-FABP4 (100 ng/ml)-induced cellular proliferation was inhibited significantly by 22% (exo-FABP4 vs. exo-FABP4 + BMS309403: 66,220 ± 885.4, n = 3 vs. 53,930 ± 273.0, n = 3, p < 0.05) as compared to control inhibition (control vs. control + BMS309403: 56,650 ± 818, n = 3 vs. 55,880 ± 1440, n = 3, p > 0.05). Thus, cell proliferation was unaffected at basal level (control), while exo-FABP4-induced cell proliferation was significantly inhibited in the presence FABP4 inhibitor, i.e. BMS309403 (Fig. 1).
Effects of exogenous-FABP4 on proliferation of the trophoblast cells. HTR8/SVneo was investigated by radioactive thymidine incorporation assay. Cells were stimulated with exogenous FABP4 (10–100 ng/ml) in the presence or absence of FABP4 inhibitor, BMS309403 (50 μM). After 24 h, the cells were pulsed with [3H] thymidine and incubated for another 12 h before the cells were harvested, and [3H] thymidine incorporation was determined as described in the methods. Experiment was performed repeatedly (n = 3) in parallel of eight samples for each treatment. Data are shown as mean ± SEM. *p value <0.05 versus control # p value <0.05 versus inhibitor (BMS309403); cpm counts per minute
Effects of exogenously added FABP4 (exo-FABP4) on cell growth and viability of the trophoblast cells, HTR8/SVneo
To assess the effect of exo-FABP4 on cell growth and viability, we performed dose–response experiments using MTT assay. We previously reported that arachidonic acid (50 μM) is a growth stimulator of HTR8/SVneo cells and therefore served as a positive control of the assay. Exo-FABP4 at 1–100 ng/ml levels increased cellular viability and growth in HTR8/SVneo cells (Fig. 2a). Growth stimulation was observed dose dependently up to 50 ng/ml and effects were comparable with arachidonic acid induction (Fig. 2a). In order to investigate whether exo-FABP4 induced cell growth and viability are affected by metabolic inhibitors of FABP4 and MAPK, cell growth and viability was measured in the presence of BMS309403) and SB203580. Compared to control, exo-FABP4 (100 ng/ml) increased cellular growth and viability by 31% (control vs. exo-FABP4: 99.28 ± 1.638, n = 3 vs. 131.2 ± 0.7846, n = 3. Exo-FABP4-induced cellular proliferation was significantly inhibited by both SB203580 and BMS309403, respectively (p < 0.05) (Fig. 2b).
Effect of exo-FABP4 on growth and viability of the HTR8/SVneo cells. a Cells (5000 cells/well/96 plate) are pre-stimulated with increasing levels of FABP4 (1–100 ng/ml) and arachidonic acid (50 μM) for 24 h. *p < 0.05, where data are significantly different from control. b Effects of FABP4 (BMS309403) and MAPK (SB203580) inhibitors on FABP4-stimulated cell growth. Cells were stimulated with exogenous FABP4 (100 ng/ml) in the presence or absence of BMS309403 (50 μM) and SB203580 (5 μM) for 24 h. Data are shown as mean ± SEM from three independent experiments *(p < 0.05) vs. exo-FABP4
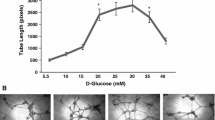
Effect of exo-FABP4 on tube-forming ability of the HTR8/SVneo cells
Exo-FABP4 protein dose dependently stimulated tube formation of HTR8/SVneo cells (Fig. 3a, b). Compared to control, exo-FABP4 (10-100 ng/ml) significantly increased tube formation in HTR8/SVneo cells (p < 0.05). Increasing levels of FABP4 beyond 10 ng/ml, dose dependently stimulated tube formation (control: 136.6 ± 13.43 cm, n = 3 vs. FABP4 10 ng/ml: 218.3 ± 7.295 cm, n = 3, p < 0.05 and exo-FABP4 100 ng/ml: 231.4 ± 5.599, n = 3, p < 0.05) (Fig. 3b). To investigate the metabolic roles of exo-FABP4 on tube formation and angiogenesis like activities, a series of cellular growth inhibitors such as BMS309403 and SB203580 were used. We previously demonstrated that 50 μM of BMS309403 and 5 μM of SB203580 showed maximum inhibitory effects at basal levels of tube formation in HTR8/SVneo cells without producing any cytotoxic effects on these cells [19]. Figure 3c shows the effect of these inhibitors on basal and exo-FABP4-induced tube formation of HTR8/SVneo cells. Tube formation was blocked in the presence of BMS309403 and SB203580 at basal level by 30 and 39% (100.0 ± 9.23 vs. 69.67 ± 4.37, n = 3 and 61.00 ± 1.73, n = 3, p < 0.05) as compared to 46 and 63% (100.0 ± 9.23 vs. 54.33 ± 3.52, n = 3 and 37.67 ± 2.02, n = 3 p < 0.001) with FABP4 induction. Compared to basal level (without FABP4 induction), a net inhibition under induced state was comparatively higher with MAPK inhibitor (24%) compared to FABP4 inhibitor (16%).
Effect of exo-FABP4 on tube-forming abilities of the HTR8/SVneo cells. Tube formation assay (in vitro angiogenesis) was performed in matrigel as described in “Materials and method” section. a Representative images of tubules formed in the presence of FABP4 after 8-h culture on matrigel. b Capillary tube length was quantified by Image J as described in “Materials and method”. Scale was set with white bar as 100 μM and expressed as a unit tube length in cm. c Effects of exo-FABP4 induced tube formation in the presence of FABP4 (BMS309403) and MAPK (SB203580) inhibitors in HTR8/SVneo cells. Cells were stimulated with or without exogenous FABP4 (100 ng/ml) in the presence of BMS309403 (50 μM) and SB203580 (5 μM) for 8 h. Capillary tube length was measured and expressed as the percentage of control. Data are shown as mean ± SEM. *p < 0.05, **p < 0.001 where data are significantly different from control
Effect of exo-FABP4 and FABP4 inhibitor on oleic acid uptake in HTR8/SVneo cells
To investigate whether FABPs plays any roles in cellular fatty acid uptake, we examined the effects of exo-FABP4 on [1-14C]Oleic Acid,18:1n-9 (OA) uptake by first trimester trophoblast cells. The presence of exo-FABP4 did not affect any significant changes in the uptake of this fatty acid by these cells as compared with control. Uptakes of [1-14C]OA were 47 ± 2.5 and 50 ± 0.32 pmol/μg protein in the absence and presence of exo-FABP4, respectively, p > 0.05.
Effects of exo-FABP4 on expression of growth and angiogenesis pathway mediators
In order to investigate, whether exo-FABP4-stimulated tube formation is associated with increased expression of signalling mediators of growth and angiogenesis processes, a strategy to measure the levels of gene expression was considered. In this study, we observed that exo-FABP4 (100 ng/ml) significantly increased the mRNA expression of specific tissue inhibitor of matrix metalloproteinases-1 (TIMP1/TBP mRNA fold expression: exo-FABP4 (100 ng/ml) vs. control: 3.05 ± 0.18 vs. 1.00 ± 0.38, n = 3, p < 0.05) in HTR8/SVneo cells. However, this induction was insignificant with 10 ng/ml of exo-FABP4. Therefore, 100 ng/ml FABP4 was used for stimulating cells in most of the assays. Induction of exo-FABP4 on TIMP1 was specific since expression of other matrix metalloproteinases that are expressed in these cells such as TIMP2, MMP9, and MMP2 remained unaffected under these conditions (data not shown).
Gene expression analysis is widely used as a powerful tool for investigating the transcriptional behaviour of cellular system to classify cellular response under certain environmental milieu. In this case, RT2 profiler PCR arrays were used to evaluate differential mRNA expression after HTR8/SVneo incubated without or with FABP4 (100 ng/ml). As shown in Tables 2 and 3, exo-FABP4- and control-treated HTR8/SVneo cells confirmed alteration in significant number of genes that are mediators (>threefold) of angiogenesis and NOTCH signalling pathways. Human angiogenesis array shows that insulin-like growth factor 1 (IGF1) and prokineticin 2 (PROK 2) were significantly up-regulated by 30- and 15-fold, whereas CCL11 and CXCL9 were down-regulated by five- and threefold, respectively (Table 2; Fig. 4). In another array, exo-FABP4 significantly increased mRNA expression of sonic hedgehog, SHH, (16-fold), transducing-like enhancer of split 1, TLE1 (fivefold), and WNT1 inducible signalling pathway protein 1, WISP1 (fivefold) and decreased loricrin, LOR (fourfold), HEY2, hairy/enhancer-of-split related with YRPW motif 2 (threefold) genes that are directly or indirectly involved with NOTCH signalling mediators (Table 3; Fig. 5).
Effect of exo-FABP4 on the mRNA expression of angiogenesis pathway mediators in HTR8/SVneo cells. Gene expression was analysed after measuring mRNA expression levels. Relative mRNA expression was measured after the cells were incubated with or without FABP4 (100 ng/ml) for 24 h. Experimental procedure and data analysis are described in the “Methodology” section. Data are presented by 3-D bar diagram. Each bar shows mRNA fold expressions as up (light grey) or down (dark grey) induced by FABP4 over control expression after normalized with housekeeping genes. Cut-off levels of >threefold expression were considered for analysis
Effect of exo-FABP4 on the mRNA expression of NOTCH signalling pathway mediators in HTR8/SVneo cells. Experimental condition and data analysis were performed similarly as described in Fig. 5. Bar shows mRNA fold expression as up-regulated (light grey) or down-regulated (dark grey) induced by exo-FABP4 over control expression after normalized with endogenous control housekeeping genes. Expression differences between control and exo-FABP4 (100 ng/ml) with more than threefold were considered for analysis
Internalization of exo-FABP4 by HTR8/SVneo cells
In order to investigate whether exo-FABP4 was internalized into HTR8/SVneo cells, we incubated cells with exo-FABP4 for 10 min in the absence and presence of FABP4 inhibitor (BMS309403). Cellular FABP4 was then quantified by measuring the intensity of the FABP4 signal per unit area. During this short exposure, no significant difference in the cellular pool of FABP4 positive cells was observed in the presence or absence of exo-FABP4. In addition, we did not observe any significant reduction of FABP4 expression (signal/unit area) in the presence of metabolic FABP4 inhibitor, BMS309403 (Fig. 6a, b).
Effect of exo-FABP4 on the expression of intracellular FABP4 protein in the presence or absence of BMS309403 in HTR8/SVneo cells. a Cells (1.2 × 104) were stimulated with exogenous FABP4 (100 ng/ml) for 10 min in the presence or absence of BMS309403 (50 μM); Cells were fixed with 4% PFA and immunostained with FABP4 antibody 1:200 followed by flurostaining with Alexa Fluor® 488. Cells were stained with DAPI. Image was captured at ×4 using confocal microscope. LSM700. b Mean fluorescence intensity per unit areas of FABP4 staining in the presence and absence of BMS309403 was analysed statistically
Fatty acid transporter expression and silencing of FABP4 gene in HTR8/SVneo cells
In order to investigate the mechanism of FABP4 action on growth and angiogenic activities of the trophoblast cells, functional inhibitors and gene silencing approaches were adopted. Silencing of FABP4 gene was compared in two different cells, i.e. trophoblast (HTR8/SVneo) and human vascular endothelial cells (EA.hy926). Silencing of cyclophilin B (positive control siRNA) resulted 88% decreased in the mRNA expression of normalized cyclophilin B as compared to control siRNA in HTR8/SVneo cells (Supplementary Fig. 1A) confirmed that gene silencing assay is working. Under similar transfection condition, expression of FABP4 mRNA was not silenced in HTR8/SVneo cells (Supplementary Fig. 1B). Again, expression of FABP4 mRNA was silenced up to 92% as compared to control siRNA in EA.hy926 (Supplementary Fig. 1C). Thus, unlike in EA.hy926, FABP4 mRNA expression could not be silenced in HTR8/SVneo cells.
To investigate whether exo-FABP4 causes any direct effects on the levels of gene expression, mRNA expression of fatty acid metabolic transporters was examined. Compared to control, exo-FABP4 (100 ng/ml) per se did not make any significant changes in the mRNA expression of fatty acid-binding proteins/fatty acid transporters (FABP1, FABP3, FABP4, FABP5, FABPpm, FATP1, FATP2, FATP3, FATP4, and FATP6) in HTR8/SVneo cells (data not shown).
Discussion
In addition to its roles in fatty acid transport and metabolism, several evidences indicating FABP4 is involved in angiogenesis mostly in endothelial and some cancer cells [2, 8, 20]. FABP4 is highly expressed in adipocytes, macrophages, endothelial cells, and human placental trophoblasts [4, 19, 21]. Earlier, we demonstrated that VEGF and DHA stimulated tube formation in HTR8/SVneo cells concomitant with an increased expression of FABP4 mRNA [7]. Consequently, we proposed that FABP4 might play a role as an important mediator of angiogenesis in HTR8/SVneo cells [8, 13, 22]. In this paper, we demonstrated that exo-FABP4 stimulated cell growth, proliferation, and tube formation (as in vitro measure of angiogenesis) in HTR8/SVneo cells. BMS309403, a functional inhibitor of FABP4, strongly abolished the stimulatory activity of exo-FABP4 on tube formation and cellular proliferation suggesting that the metabolic activity of FABP4 may be involved in the process. In addition, MAPK inhibitor also inhibited the exo-FABP4-mediated tube formation. MAPK pathway is known as the primary regulators of cell proliferation and differentiation. FABP4-induced muscle cell proliferation was reported to be mediated primarily through a MAPK-dependent pathway [23]. Growth-promoting stimulus of the exo-FABP4 was also observed with selective stimulation of tissue inhibitors of metalloproteinase-1 (TIMP1) expression. Earlier we reported that TIMP1 expression was stimulated by DHA in HTR8/SVneo cells. It is known that TIMPs regulate extracellular matrix turnover. TIMP-1 mRNA is unique since its expression is limited to the stage at which epithelial proliferation is high [24]. It is possible that exo-FABP4 in these cells contribute to epithelial proliferation of the trophoblastic cells and may regulate extracellular matrix turnover.
It is intriguing that the mRNA expression of FABP4 in these cells was not silenced markedly by siRNA as has been the case with other cells such as mesangial cells [25]. Present data demonstrate the difficulty in silencing FABP4 gene in HTR8/SVneo cells using this method. Unlike protein level, FABP4 mRNA expression is lower in HTR8/SVneo cells [19]. The difference in the efficiency of FABP4 gene silencing could be due to lower FABP4 mRNA expression in HTR8/SVneo cells as compared to endothelial cells, Ea.Hy926, as evidenced by their differential Ct values under these conditions. Therefore, metabolic FABP4 inhibitor was used in this study instead of FABP4 knockout cells.
It seems that physiologically higher concentrations of FABP4 are required to produce significant effects in vitro. However, levels of recombinant FABP4 used in other in vitro studies were ~15–100 times higher than the levels used in our study. Lamounier-Zepter et al. [26] used ~1500 ng/ml of FABP4 to show an effect in cardiomyocytes, and Lu et al. [27] used 2500–10,000 ng/ml of FABP4.
The mechanisms by which exo-FABP4 affecting the gene expression, cell growth, proliferation, and tube formation in these cells are not yet fully understood. Our study did not confirm that exo-FABP4 was internalized into the cell as the cellular pool of FABP4 was not significantly increased in these cells after exo-FABP4 exposure. However, further work with different exposure time points and concentrations is required for definitive conclusions. Recent data show that exo-FABP4 per se activates gene expression in HEPG2 cells [28]. We also found that exo-FABP4 stimulated gene expression through the activation of intermediate signalling molecules associated with growth stimulation and angiogenesis pathway (TIMP1, IGF1, PROK2, SHH, and WISP1). Recent data highlight that prokineticins are the key control points in regulating physiological function of the reproductive processes. PROK1 acts as an angiogenic growth factor, also known as endocrine gland-derived vascular endothelial factor (EG-VEGF) which is an important regulator for embryo implantation and placental development. Dysregulation of PROK activities is linked with inadequate trophoblast invasion and angiogenesis during placentation [29]. It is not known whether exo-FABP4 mediates its effects in similar way as with other cells. Further studies are required to confirm exo-FABP4’s participation to intracellular signalling process of the trophoblast cells. In endothelial cells, exo-FABP4 affected the angiogenesis process by interacting extracellular membrane protein cytokeratin1 [30]. This sort of FABP4-cytokeratin interaction was reported in many cell types [30]. Exo-FABP4 forms protein complex with CK1 in HepG2 cells that do not express FABP4, supporting the importance of the interaction of circulating FABP4 with peripheral tissues.
In conclusion, our results demonstrate that exo-FABP4 induces proliferation in vitro, mainly via PROK2 and IGF1 expression in these cells. Furthermore, the data from this study suggest that like other adipokines such as leptin, FABP4 may also be involved in early placentation process.
References
Duttaroy AK (2009) Transport of fatty acids across the human placenta: a review. Prog Lipid Res 48:52–61. doi:10.1016/j.plipres.2008.11.001
Ghelfi E, Yu CW, Elmasri H, Terwelp M, Lee CG, Bhandari V, Comhair SA, Erzurum SC, Hotamisligil GS, Elias JA, Cataltepe S (2013) Fatty acid binding protein 4 regulates VEGF-induced airway angiogenesis and inflammation in a transgenic mouse model: implications for asthma. Am J Pathol 182:1425–1433. doi:10.1016/j.ajpath.2012.12.009
Yu CW, Liang X, Lipsky S, Karaaslan C, Kozakewich H, Hotamisligil GS, Bischoff J, Cataltepe S (2016) Dual role of fatty acid-binding protein 5 on endothelial cell fate: a potential link between lipid metabolism and angiogenic responses. Angiogenesis 19:95–106. doi:10.1007/s10456-015-9491-4
Elmasri H, Karaaslan C, Teper Y, Ghelfi E, Weng M, Ince TA, Kozakewich H, Bischoff J, Cataltepe S (2009) Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J 23:3865–3873. doi:10.1096/fj.09-134882
Elmasri H, Ghelfi E, Yu CW, Traphagen S, Cernadas M, Cao H, Shi GP, Plutzky J, Sahin M, Hotamisligil G, Cataltepe S (2012) Endothelial cell-fatty acid binding protein 4 promotes angiogenesis: role of stem cell factor/c-kit pathway. Angiogenesis. doi:10.1007/s10456-012-9274-0
Cataltepe O, Arikan MC, Ghelfi E, Karaaslan C, Ozsurekci Y, Dresser K, Li Y, Smith TW, Cataltepe S (2011) Fatty acid binding protein 4 is expressed in distinct endothelial and non-endothelial cell populations in glioblastoma. Neuropathol Appl Neurobiol. doi:10.1111/j.1365-2990.2011.01237.x
Johnsen GM, Basak S, Weedon-Fekjaer MS, Staff AC, Duttaroy AK (2011) Docosahexaenoic acid stimulates tube formation in first trimester trophoblast cells, HTR8/SVneo. Placenta 32:626–632. doi:10.1016/j.placenta.2011.06.009
Basak S, Das MK, Duttaroy AK (2013) Fatty acid-induced angiogenesis in first trimester placental trophoblast cells: possible roles of cellular fatty acid-binding proteins. Life Sci 93:755–762. doi:10.1016/j.lfs.2013.09.024
Basak S, Duttaroy AK (2012) Leptin induces tube formation in first-trimester extravillous trophoblast cells. Eur J Obstet Gynecol Reprod Biol. doi:10.1016/j.ejogrb.2012.05.033
Basak S, Duttaroy AK (2013) cis-9, trans-11 Conjugated linoleic acid stimulates expression of angiopoietin like-4 in the placental extravillous trophoblast cells. Biochim Biophys Acta 1831:834–843. doi:10.1016/j.bbalip.2013.01.012
Biron-Shental T, Schaiff WT, Rimon E, Shim TL, Nelson DM, Sadovsky Y (2008) Hypoxia enhances the expression of follistatin-like 3 in term human trophoblasts. Placenta 29:51–57. doi:10.1016/j.placenta.2007.09.001
Scifres CM, Chen B, Nelson DM, Sadovsky Y (2011) Fatty acid binding protein 4 regulates intracellular lipid accumulation in human trophoblasts. J Clin Endocrinol Metab 96:E1083–E1091. doi:10.1210/jc.2010-2084
Basak S, Duttaroy AK (2013) Effects of fatty acids on angiogenic activity in the placental extravillious trophoblast cells. Prostaglandins Leukot Essent Fatty Acids 88:155–162. doi:10.1016/j.plefa.2012.10.001
Harjes U, Bridges E, McIntyre A, Fielding BA, Harris AL (2014) Fatty acid-binding protein 4, a point of convergence for angiogenic and metabolic signaling pathways in endothelial cells. J Biol Chem 289:23168–23176. doi:10.1074/jbc.M114.576512
Scifres CM, Catov JM, Simhan H (2012) Maternal serum fatty acid binding protein 4 (FABP4) and the development of preeclampsia. J Clin Endocrinol Metab 97:E349–E356. doi:10.1210/jc.2011-2276
Wotherspoon AC, Young IS, McCance DR, Patterson CC, Maresh MJ, Pearson DW, Walker JD, Holmes VA, Diabetes and Pre-eclampsia Intervention Trial Study Group (2016) Serum fatty acid binding protein 4 (FABP4) predicts pre-eclampsia in women with type 1 diabetes. Diabetes Care 39:1827–1829. doi:10.2337/dc16-0803
Eriksen AB, Indrevaer RL, Holm KL, Landskron J, Blomhoff HK (2012) TLR9-signaling is required for turning retinoic acid into a potent stimulator of RP105 (CD180)-mediated proliferation and IgG synthesis in human memory B cells. Cell Immunol 279:87–95. doi:10.1016/j.cellimm.2012.09.003
Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN (2006) Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res 66:7843–7848. doi:10.1158/0008-5472.CAN-06-1010
Pandya AD, Das MK, Sarkar A, Vilasagaram S, Basak S, Duttaroy AK (2016) Tube formation in the first trimester placental trophoblast cells: differential effects of angiogenic growth factors and fatty acids. Cell Biol Int 40:652–661. doi:10.1002/cbin.10601
Elmasri H, Ghelfi E, Yu CW, Traphagen S, Cernadas M, Cao H, Shi GP, Plutzky J, Sahin M, Hotamisligil G, Cataltepe S (2012) Endothelial cell-fatty acid binding protein 4 promotes angiogenesis: role of stem cell factor/c-kit pathway. Angiogenesis 15:457–468. doi:10.1007/s10456-012-9274-0
Thompson BR, Mazurkiewicz-Munoz AM, Suttles J, Carter-Su C, Bernlohr DA (2009) Interaction of adipocyte fatty acid-binding protein (AFABP) and JAK2: AFABP/aP2 as a regulator of JAK2 signaling. J Biol Chem 284:13473–13480. doi:10.1074/jbc.M900075200
Basak S, Das MK, Srinivas V, Duttaroy AK (2015) The interplay between glucose and fatty acids on tube formation and fatty acid uptake in the first trimester trophoblast cells, HTR8/SVneo. Mol Cell Biochem 401:11–19. doi:10.1007/s11010-014-2287-9
Girona J, Rosales R, Plana N, Saavedra P, Masana L, Vallve JC (2013) FABP4 induces vascular smooth muscle cell proliferation and migration through a MAPK-dependent pathway. PLoS ONE 8:e81914. doi:10.1371/journal.pone.0081914
Fata JE, Leco KJ, Moorehead RA, Martin DC, Khokha R (1999) Timp-1 is important for epithelial proliferation and branching morphogenesis during mouse mammary development. Dev Biol 211:238–254. doi:10.1006/dbio.1999.9313
Yao F, Li Z, Ehara T, Yang L, Wang D, Feng L, Zhang Y, Wang K, Shi Y, Duan H, Zhang L (2015) Fatty acid-binding protein 4 mediates apoptosis via endoplasmic reticulum stress in mesangial cells of diabetic nephropathy. Mol Cell Endocrinol 411:232–242. doi:10.1016/j.mce.2015.05.003
Lamounier-Zepter V, Look C, Schunck WH, Schlottmann I, Woischwill C, Bornstein SR, Xu A, Morano I (2015) Interaction of epoxyeicosatrienoic acids and adipocyte fatty acid-binding protein in the modulation of cardiomyocyte contractility. Int J Obes (Lond) 39:755–761. doi:10.1038/ijo.2014.193
Lu L, Wang YN, Sun WH, Liu ZH, Zhang Q, Pu LJ, Yang K, Wang LJ, Zhu ZB, Meng H, Yang P, Du R, Chen QJ, Wang LS, Yu H, Shen WF (2013) Two-dimensional fluorescence in-gel electrophoresis of coronary restenosis tissues in minipigs: increased adipocyte fatty acid binding protein induces reactive oxygen species-mediated growth and migration in smooth muscle cells. Arterioscler Thromb Vasc Biol 33:572–580. doi:10.1161/ATVBAHA.112.301016
Bosquet A, Guaita-Esteruelas S, Saavedra P, Rodriguez-Calvo R, Heras M, Girona J, Masana L (2016) Exogenous FABP4 induces endoplasmic reticulum stress in HepG2 liver cells. Atherosclerosis 249:191–199. doi:10.1016/j.atherosclerosis.2016.04.012
Traboulsi W, Brouillet S, Sergent F, Boufettal H, Samouh N, Aboussaouira T, Hoffmann P, Feige JJ, Benharouga M, Alfaidy N (2015) Prokineticins in central and peripheral control of human reproduction. Horm Mol Biol Clin Investig 24:73–81. doi:10.1515/hmbci-2015-0040
Saavedra P, Girona J, Bosquet A, Guaita S, Canela N, Aragones G, Heras M, Masana L (2015) New insights into circulating FABP4: interaction with cytokeratin 1 on endothelial cell membranes. Biochim Biophys Acta 1853:2966–2974. doi:10.1016/j.bbamcr.2015.09.002
Acknowledgements
We are grateful to Aud Jørgensen for her technical assistance. We are grateful to Camilla Solberg for her help in thymidine assay. The authors convey their thanks to Srinivas Vilasagaram for his assistance in siRNA experiments. This study was supported by the grant from the Thune Holst Foundation and HRD fellowship, Department of Health Research (Dr. Sanjay Basak), Government of India.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (PPTX 108 kb)
Supplementary Fig. 1. Silencing of FABP4 mRNA in the first trimester trophoblast cell line, HTR8/SVneo and endothelial cell line, EA.hy926 Cells (104 cells/well/96 well) were transfected with scramble siRNA (control) and target siRNAs (FABP4 or Cyclophilin B) at final concentration of 25 nM for 48 h. Knockdown efficiency was measured by mRNA expression levels of FABP4 or Cyclophilin after normalized with TBP (endogenous control) in both HTR8/SVneo and EA.hy926 cells . A, B Fold mRNA expression of cyclophilin B (positive control), and FABP4 are presented for HTR8/SVneo cells and C FABP4 level for EA.hy926 cells after silencing of the respective genes for 48 h
Rights and permissions
About this article
Cite this article
Basak, S., Sarkar, A., Mathapati, S. et al. Cellular growth and tube formation of HTR8/SVneo trophoblast: effects of exogenously added fatty acid-binding protein-4 and its inhibitor. Mol Cell Biochem 437, 55–64 (2018). https://doi.org/10.1007/s11010-017-3095-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3095-9