Abstract
Placental growth factor (PlGF) is an important angiogenic factor which has an emerging role in the clinical management of suspected preeclampsia. The role of PlGF in normal placental development is not completely understood and it is uncertain whether PlGF influences trophoblast and endothelial cell interactions central to uterine spiral artery remodelling, especially in variable oxygen conditions. A two-cell model of endovascular invasion was used. Tissue culture plates were coated with Matrigel™, on which fluorescent-labelled uterine microvascular endothelial cells (1 × 105/well) and HTR8/SVNeo cells were co-cultured (1 × 105/well) for 20 h. Co-cultures were treated with recombinant human PlGF (rhPlGF) (10 or 100 ng/mL) and incubated at either 21% O2 or 2% O2. Images were captured by fluorescence microscopy and analysed using ImageJ (n = 7). Data was analysed using SPSSv24. Treatment with rhPlGF did not improve integration in co-cultures irrespective of oxygen conditions but increased proliferation in 2% O2 of both trophoblast and endothelial cells. Expression of angiogenic factors VEGF, sFLT-1, PlGF and CXCL12 in both co-cultures and in isolated trophoblast cells was not altered by rhPlGF treatment. Expression of TLR-3 mRNA in co-cultures was increased by rhPlGF 100 ng/mL at 21% O2 (p = 0.03). PlGF contributes to trophoblast and endothelial cell proliferation in the setting of physiological hypoxia but does not influence trophoblast and endothelial cell interactions in an in vitro model of spiral artery remodelling. Upregulation of TLR-3 expression in co-cultures may indicate a role for PlGF in the placental inflammatory response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Changes in the circulating angiogenic factor placental growth factor (PlGF) are a marker of placental dysfunction. The measurement of PlGF concentrations and its partner molecule soluble fms-like tyrosine kinase-1 (sFLT-1) has an increasingly important role in the diagnosis of suspected preeclampsia and in predicting preeclampsia [2, 3]. Placental growth factor is decreased in early gestation of pregnant women with placental dysfunction but it is uncertain whether decreased PlGF is a cause or a consequence of abnormal placentation [4, 5].
The understanding of the role of PlGF in placental development remains limited. Placental growth factor increases proliferation [4] and reduces apoptosis of trophoblast cells [1] but PlGF appears redundant as PlGF knockout mice are fertile and their pups have similar growth potential compared with wild-type mice [6]. Detailed histological studies of implantation sites of PlGF knockout mice however demonstrate abnormal placental vasculature. There is decreased branching in the anti-mesometrial (foetoplacental) vessels and increased lacunarity, indicating a lack of uniformity of vessel formation [7]. Uteroplacental vessels also display decreased branching but decidual invasion is not influenced [7].
From the second trimester of human pregnancy, placental expression of PlGF progressively increases, [8] correlating with the period of myometrial spiral artery remodelling in the ‘second wave’ of invasion. However, there have been conflicting reports as to whether PlGF actually contributes to trophoblast invasion into endothelial structures [9,10,11]. Trophoblasts develop invasive characteristics in response to increased oxygen tension, and PlGF expression in trophoblasts also increases with improved placental oxygenation [12], but it is uncertain whether these two events are directly linked.
Trophoblast invasion is mediated by a multitude of factors including the chemokine C-X-C motif chemokine-12 (CXCL12) and its receptor C-X-C motif receptor-4 (CXCR4) [13]. Trophoblast-derived CXCL12 can increase the invasiveness of trophoblasts by upregulating MMP-9 and MMP-2 [14]. Clinical evidence also exists supporting a pathogenic role of CXCL12 in poor placentation due to its effect on the expression of pro-angiogenic factors [15] as well as the observation that it is increased in the placentas of women with preeclampsia [16]. CXCL12 increases VEGF expression in ovine trophoectoderm cells [15] and attracts uterine natural killer (uNK) cells which are known to produce PlGF [17].
Toll-like receptors (TLRs) act as part of the innate immune system against non-self-antigens and are likely to play an important role in the maternal response to semi-allogeneic trophoblast cells [18]. Toll-like receptor-3 (TLR-3) has been demonstrated in other tissues to promote the expression of CXCL12 [19] and subsequent recruitment of endothelial progenitor cells. The effect of PlGF on trophoblast expression of both CXCL12 and TLR in conjunction with the potential role in trophoblast-endothelial cell interactions has not previously been analysed.
This study aimed to determine the effect of PlGF in an in vitro model of human trophoblast invasion into endothelial cell networks in varying oxygen tensions to clarify the role of PlGF in development of the placenta. If PlGF influences the trophoblast invasion required for spiral arteriole remodelling, perhaps replacement of PlGF in women with low PlGF in early gestation may mitigate or prevent placental dysfunction. This study uses co-culture which models the process of pseudovasculogenesis whereby extravillous trophoblast cells adhere to maternal spiral uterine arteriole endothelial cells, a vital step in the development of uteroplacental circulation [20]. This process usually occurs in an environment of physiological hypoxia (2% O2), [21] so testing in hypoxia was also undertaken. The effect of PlGF on CXCL12 and TLR expression in trophoblast–endothelial cell co-cultures was also examined.
Methods
Cell Culture
Human uterine myometrial microvascular endothelial cells (UtMVEC) (Lonza, Switzerland) were cultured in EGM-2-MV medium (Lonza, Switzerland) and HTR-8/SVneo cells (HTR-8 cells) were cultured in Roswell Park Memorial Institute 1640 media (Sigma-Aldrich, Australia) supplemented with 5% foetal bovine serum (CellSera, Australia). Cells (passages 4–9) were grown to 80% confluence before use in co-culture. Testing for mycoplasma was performed on each passage before use. HTR-8 cells were chosen as this cell line mimics the invasive characteristics of the first trimester extravillous trophoblast [22].
Co-culture on Matrigel
Co-culture of UtMVEC and HTR-8 cells was performed as described in the published literature [23]. Twenty-four-well tissue culture plates (Corning Incorporated, NY) were coated with 300 μL of undiluted Matrigel (Discovery Labware, MA) and allowed to gelatinise at 37 °C for 30 min. The UtMVEC were dyed with red fluorescent cell membrane labeller (PKH26, Sigma-Aldrich, Australia) and seeded into each well (1 × 105/well). Capillary networks were allowed to form for at least 4 h at 37 °C. The HTR-8 cells (1 × 105/well), dyed with green fluorescent cell membrane labeller (PKH67, Sigma Aldrich, Australia), were then added and co-cultured for 20 h.
Co-cultures were incubated with recombinant human placental growth factor (rhPlGF) (264-PGB-010 R&D Systems, MN) at 10 ng/mL or 100 ng/mL. The PlGF was added simultaneously with HTR-8 cells—and incubated at 21% (‘normoxic’) or 2% O2 (‘hypoxic’) with 5% CO2 at 37 °C and 95% humidity for 20 h. Hypoxic cultures were performed to replicate physiological oxygen levels in early placentation [24].
A minimum concentration of 10 ng/mL was chosen based on previous studies which had shown maximal effect on cell proliferation at concentrations between 5 and 25 ng/mL [9, 25]. Labelled HTR-8 cells alone were also seeded on Matrigel-coated wells and incubated in similar conditions. The experiment was repeated 7 times with different passages of both UtMVEC and HTR-8 cells. Single–cell culture experiments were also performed comparing 21% and 2% O2. Previous work has demonstrated that HTR-8 cells develop an invasive phenotype when grown on Matrigel [26, 27].
Image Analysis and Quantification
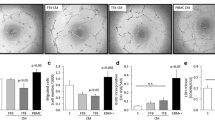
Images were captured using fluorescence microscopy with red and green fluorescence filter sets (Olympus IX71) and analysed using ImageJ (National Institutes of Health, USA). The ratio of trophoblast migration to endothelial cell networks was calculated by dividing the total trophoblast network area by the total endothelial cell network area, and used to quantify cell integration as previously published [23]. Trophoblast network length was calculated using the Angiogenesis Analyzer macro for ImageJ. The ratio for treatment wells was compared with the control well for each individual experiment with the control well set as 1.0 (Fig. 1).
Representative images of co-cultures. Cell integration was determined by calculating the ratio of total trophoblast network area ((i) green fluorescent-labelled cells) to total endothelial network area ((ii) red fluorescent-labelled cells). Cell integration of experimental wells was expressed as a ratio relative to control wells (n = 7). The combined images (iii) indicate co-localised cells in yellow but this overlay image was not used for quantification
Quantitative PCR of Co-cultures
Cells were recovered from the Matrigel with a cell recovery solution (Discovery Labware, MA). Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Germany) treated with deoxyribonuclease 1 (DNAse1) using a kit (Sigma-Aldrich, Australia) and reverse transcribed to cDNA using the iScript cDNA synthesis kit (Bio-Rad, Australia) as per manufacturer’s instructions. Quantitative PCR of mRNA targets listed in Table 1 was performed using iQ SYBR Green Supermix (Bio-Rad, Australia). All samples were assayed in triplicate in a Bio-Rad CFX96 Optic Module under the following conditions: 95 °C for 3 min, 95 °C for 30 s, variable annealing temperature for 30 s and 72 °C for 30 s for 40 cycles followed by a melt curve.
ELISA of Conditioned Media
Angiogenic factor and CXCL12 expression in conditioned media were analysed with commercially available ELISA kits—human VEGF (DVE00), PlGF (DPG00), sFLT-1 (DVR100C) and CXCL12/SDF-1α (DSA00) (R&D Systems, MN, USA). Assays with an intra-assay coefficient of variation < 10% and inter-assay of < 15% were deemed acceptable. All samples were assayed in duplicate. Intra-assay coefficient of variation was determined using two standards assayed in quadruplicate. Samples were re-assayed if duplicates differed by more than 10%.
Statistical Analysis
The control (untreated) group was set as 1.0 and the differences expressed as a fold change (median and interquartile range (IQR) reported).
As data was generally non-parametric, the Mann-Whitney U or Kruskal-Wallis test was performed and post hoc analysis performed using Dunn’s test with correction for multiple comparisons. Results are expressed as median (IQR). Statistics were performed using GraphPad Prism 7.02 (GraphPad Software, La Jolla, CA, USA). Statistical significance was determined if p < 0.05.
Results
Cellular Effects of Hypoxia
There was decreased integration when co-cultures were undertaken in hypoxic conditions compared with normoxic conditions [1 (21% O2) vs 0.91 (2% O2) (0.82–1.03), p = 0.04]. Most of the effect was a result of trophoblast changes. There was a reduction in the surface area covered by the trophoblast cells within co-cultures under hypoxic conditions compared with normoxia [1 (21% O2) vs 0.80 (2% O2) (0.75–0.90), p = 0.02] but no difference in surface area covered by endothelial cells [p = 0.2].
Cellular Effects of rhPlGF
The addition of rhPlGF (10 ng/mL and 100 ng/mL) to co-cultures did not affect the integration of cells when compared with control (untreated) co-cultures. This was the case if experiments were undertaken in either normoxic [10 ng/mL, p = 0.8; 100 ng/mL, p = 1)] or hypoxic conditions [10 ng/mL, p = 0.09; 100 ng/mL, p = 0.3].
Addition of rhPlGF at 10 ng/mL and 100 ng/mL in normoxic conditions did not alter cell surface area of either the endothelial or trophoblast cells [HTR-8 p = 0.9; UtMVEC p = 0.1]. However, in hypoxic conditions, the addition of both concentrations of rhPlGF resulted in an increase in both trophoblast and endothelial cell surface areas. Trophoblast cell surface area significantly increased [1 (control) vs 1.27 (rhPlGF 10 ng/mL) (1.15–1.38), p = 0.006; 1 (control) vs 1.09 (rhPlGF 100 ng/mL) (1.05–1.23), p = 0.04] (Fig. 2). Similarly, the endothelial cell surface area increased with rhPlGF treatment [1 (control) vs 1.22 (rhPlGF 10 ng/mL) (1.14–1.66), p = 0.001; 1 (control) vs 1.23 (rhPlGF 100 ng/mL) (1.08–1.35), p = 0.01] (Fig. 2). There was no difference in the effect of rhPlGF at low or high concentration in either the trophoblast [rhPlGF 10 ng/mL vs rhPlGF 100 ng/mL, p = 1] or endothelial cells [rhPlGF 10 ng/mL vs rhPlGF 100 ng/mL, p = 1].
Cell proliferation induced by rhPlGF. Representative images of trophoblast (panel A, green) and endothelial cells (panel B, red) in co-culture treated with rhPlGF in 2% O2 demonstrating increased cell proliferation. (A + B-i) Untreated/control. (A + B-ii) rhPlGF 10 ng/mL. (A + B-iii) rhPlGF 100 ng/mL. (Panel C) Surface area of trophoblast and endothelial cells in co-culture with rhPlGF treatment in 2% O2. Median (IQR), *p = 0.04, **p = 0.01, ***p = 0.006, ****p = 0.0001 (Kruskal-Wallis test, Dunn’s multiple comparisons test), n = 7
Trophoblast network formation was unaffected by rhPlGF treatment in normoxic conditions [p = 0.9]. In hypoxia however, network length was significantly increased when rhPlGF was added at a concentration of 100 ng/mL [1 vs 1.17 (1.03–2.22), p = 0.03] (Fig. 3).
(Panel A) Trophoblast network formation on Matrigel. Untreated/control (i) and with addition of rhPlGF 10 ng/mL (ii) and rhPlGF 100 ng/mL (iii). (Panel B) Trophoblast network formation on Matrigel with rhPlGF treatment in 2% O2. Median (IQR), *p = 0.03 (Kruskal-Wallis test, Dunn’s multiple comparisons test), n = 9. PlGF = placental growth factor
Angiogenic Factors
There was no effect of treatment with rhPlGF 10 ng/mL or 100 ng/mL irrespective of oxygen conditions on VEGF [21% O2p = 0.5; 2% O2p = 0.8] and sFLT-1 [21% O2p = 0.8; 2% O2p = 1] (ELISA) in the conditioned media of co-cultures. The findings in trophoblast cell cultures were similar [VEGF 21% O2p = 0.6; 2% O2p = 0.9; sFLT-1 21% O2p = 0.05; 2% O2p = 0.8]. Similarly, there was no change in mRNA expression of PlGF, VEGF and sFLT-1 in co-cultures treated with rhPlGF in both 21% and 2% O2.
There was a reduction in the PlGF in the conditioned media of the co-cultured cells grown in hypoxic conditions compared with normoxia (p = 0.048). However, there was no difference in mRNA expression of PlGF between normoxic and hypoxic environments (p = 0.3). When trophoblast cells were cultured alone, the PlGF in conditioned media was unchanged by oxygen environment (p = 0.4) (Table 2).
Conditioned media of co-cultures grown in hypoxia compared with normoxia contained increased VEGF (p = 0.002). The VEGF mRNA expression of co-cultured cells was also increased in a hypoxic environment (p = 0.004). The VEGF in conditioned media was also increased in trophoblast cells alone in hypoxia compared with normoxic conditions (p = 0.0002) (Table 2).
We demonstrated there was no difference in sFLT-1 concentration in the conditioned media of co-cultures grown in hypoxia compared with normoxia, although this has been previously published by others [29]. Despite the lack of change in sflt-1 in conditioned media, there was an increase in the mRNA expression of sFLT-1 in the co-cultured UtMVEC and HTR-8 cells during this short 20 h incubation. However, hypoxia decreased sFLT-1 in conditioned media of trophoblast cells alone (Table 2).
CXCL12 and TLR
Concentrations of CXCL12 in the conditioned media of both co-cultured and trophoblast cells were not influenced by oxygen environment (co-cultures p = 0.5; trophoblast p = 0.7) or rhPlGF treatment (co-cultures p = 0.6; trophoblast p = 1). Expression of TLR-3 mRNA in both co-cultures (p = 1) and trophoblast cells (p = 1) was not influenced by oxygen environment. Expression of TLR-4 mRNA in co-cultures was suppressed by hypoxia when compared with normoxia [1 vs 0.58 (0.37–1.06), p = 0.048]. In trophoblast cells alone, oxygen conditions had no effect on TLR-4 mRNA expression (p = 0.2).
Co-cultures in normoxic conditions treated with rhPlGF 100 ng/mL had increased mRNA expression of TLR-3 [1 vs 1.3 (1.0–1.4), p = 0.03] (Fig. 4). In hypoxic conditions, rhPlGF at either concentration did not influence TLR-3 mRNA expression (Fig. 4). The expression of TLR-3 in trophoblast cells alone was not influenced by rhPlGF treatment [p = 0.2]. The mRNA expression of TLR-4 in co-cultures or trophoblast was not influenced by the addition of rhPlGF irrespective of oxygen conditions [co-cultures 21% O2p = 0.9; 21% O2p = 0.9; trophoblast 21% O2p = 0.6 2% O2p = 0.9].
Discussion
Co-cultures of UtMVEC and HTR-8 cells with rhPlGF increased the cell surface areas of both endothelial and trophoblast cell networks in physiological (2%) hypoxia. However, rhPlGF treatment did not alter the integration of the cells in either normoxia or hypoxia. Although there was no effect of rhPlGF on the expression of other angiogenic factors or CXCL12, the addition of rhPlGF increased the expression of TLR-3 in co-cultures.
There is much evidence suggesting reduced PlGF is associated with impaired placental development [30,31,32] and these experiments demonstrate that replacing the PlGF could improve the area of placentation but not improve the integration of trophoblast and endothelial cells required for uterine spiral artery remodelling. Athanassiades et al. demonstrated that PlGF-1 in combination with heparan sulfate proteoglycans increased extravillous trophoblast cell proliferation but did not have any effect on cell migration or invasion [9]. In keeping with this, our study showed that the addition of rhPlGF to co-cultures incubated in physiological hypoxia increased both endothelial and trophoblast cell surface areas. Trophoblast cells cultured alone on Matrigel also showed increased network length when treated with rhPlGF in a hypoxic environment. Low oxygen tension increases the proliferative capacity of trophoblasts [33] and thus may explain the specific response to rhPlGF in this setting. Rapid trophoblast cell proliferation is thought to contribute to the development of trophoblast plugs in uterine arteriole lumens, [34] which may protect trophoblasts against early exposure to high oxygen tension, hypothesised to be a cause of early placentation failure [21].
Previous studies have shown inconsistent effects of rhPlGF on trophoblast invasion capability, [9,10,11] and where there was a significant increase in invasion, the magnitude of invasion induced by the PlGF was small (18%) [10]. These studies used Matrigel invasion assays of trophoblast cell lines that assess invasion by calculating the proportion of cells passing through a Matrigel barrier. This may represent the interstitial pathway of trophoblast invasion but not reflect the process of endovascular invasion represented in the co-culture model. Our in vitro model allows for crosstalk between endothelial and trophoblast cells which may alter trophoblast phenotype. That CXCL12, a chemokine known to increase invasiveness of trophoblasts, was not affected by rhPlGF also supports our other finding that PlGF does not influence trophoblast invasion.
The findings in our human cell experiments are supported by data in mice. In a study of implantation sites of pregnant PlGF knockout mice, although there was decreased branching of uteroplacental and foetoplacental vessels, there was no impact of PlGF deficiency on trophoblast invasion into the decidua during early placentation [7]. Thus, although women with preeclampsia and intrauterine growth restriction have decreased peripheral concentrations of PlGF in early pregnancy, increasing concentrations of PlGF are unlikely to influence spiral artery remodelling which is characteristically abnormal in these placental disorders [35, 36].
Exogenous rhPlGF did not affect PlGF mRNA expression suggesting the absence of a feedback mechanism on PlGF expression in placental tissues. Treatment with rhPlGF also did not alter VEGF or sFLT-1 expression, irrespective of oxygen environment. These findings are consistent with that of transgenic mice where overexpression of PlGF in T cells did not change placental VEGF or VEGF/PlGF heterodimers as measured using ELISA [37]. The balance of angiogenic factors VEGF and PlGF is in favour of VEGF in early pregnancy and PlGF in later pregnancy [8]. As for sFLT-1 and PlGF, the peak in sFLT-1 at the end of pregnancy is associated with a concomitant reduction in PlGF. The observation that PlGF administration does not markedly alter the expression of other angiogenic factors indicates that VEGF or sFLT-1 regulation is not directly driven by PlGF even though the differential expression of these factors is coordinated.
This study showed that TLR-3 mRNA expression was stimulated by high concentrations of rhPlGF in the setting of normoxia. In hypoxia, TLR-4 mRNA expression was decreased in co-cultures but not in trophoblast cells, consistent with previous work showing that TLR-4 expression is downregulated by hypoxia in endothelial cells (0% vs 20% O2) [38]. In this study, addition of rhPlGF had no effect on TLR-4 expression even though one other work has shown that TLR-4 activation in first trimester trophoblasts decreases PlGF expression [39]. The significance of these effects of PlGF on TLR-3 and TLR-4 in placental cells is uncertain but PlGF may have as yet unknown influences on the placental immune system. While the role of TLR-3 and TLR-4 in the periphery is predominantly as defence against viral and bacterial infections, respectively, in the placenta, these receptors may influence tolerance of foetus [18].
This work has several limitations. Although this study has a significant number of negative results, these are important to disseminate as they enhance our mechanistic understanding of altering placentation. The process of ‘pseudovasculogenesis’ in early placentation is complex and in vitro models of this process can only provide limited insight. Although this model aims to mimic cell interactions occurring during spiral artery remodelling, the multitude of potentially influential factors in the microenvironment of the developing placenta cannot be replicated and experimental factors such as growth factors in Matrigel and culture media may alter cellular responses to the addition of rhPlGF. In these experiments, the percentage of oxygen deemed ‘normoxic’ against which ‘hypoxic’ cultures were compared, though standard, is not physiological. Experiments were performed under two oxygen conditions in an attempt to replicate the conditions in which the first (hypoxic) and second (normoxic) ‘waves’ of trophoblast invasion occur. Hypoxic cultures were performed at 2% O2 to reflect the physiological oxygen tension of the first trimester as in vivo oxygen tension is measured at approximately 18 mmHg at 8 weeks, equivalent to 2.5% oxygen [24]. The normal oxygen tension of placental tissues once the maternal circulation is established after 10 to 12 weeks’ gestation is 8.5% O2, [24] and as other authors have suggested, the physiologically normoxic environment for incubation of placental tissues should be 5–10% O2 [40]. However, the interpretation of the results in this study with respect to existing literature was possible as previous researchers have generally used 21% O2 as a comparator [40].
In conclusion, this work demonstrates that PlGF promotes cell proliferation but does not influence trophoblast–endothelial cell interactions in vitro in a system designed to mimic factors that are crucial to uterine spiral artery remodelling. Placental growth factor may also directly increase TLR-3 expression in placental cells. The implications of PlGF treatment or remediation in early pregnancy to restore placental function remain uncertain.
References
Arroyo J, Price M, Straszewski-Chavez S, Torry RJ, Mor G, Torry DS. XIAP protein is induced by placenta growth factor (PLGF) and decreased during preeclampsia in trophoblast cells. Syst Biol Reprod Med. 2014;60(5):263–73.
Chappell LC, Duckworth S, Seed PT, Griffin M, Myers J, Mackillop L, et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation. 2013;128(19):2121–31.
Duhig KE, Myers J, Seed PT, Sparkes J, Lowe J, Hunter RM, et al. Placental growth factor testing to assess women with suspected pre-eclampsia: a multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial. Lancet. 2019;393(10183):1807–18.
Torry DS, Mukherjea D, Arroyo J, Torry RJ. Expression and function of placenta growth factor: implications for abnormal placentation. J Soc Gynecol Investig. 2003;10(4):178–88.
Chau K, Hennessy A, Makris A. Placental growth factor and pre-eclampsia. J Hum Hypertens. 2017;31:782.
Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, de Mol M, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7(5):575–83.
Ratsep MT, Carmeliet P, Adams MA, Croy BA. Impact of placental growth factor deficiency on early mouse implant site angiogenesis. Placenta. 2014;35(9):772–5.
Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen--a review. Placenta. 2000;21(Suppl A):S16–24.
Athanassiades A, Lala PK. Role of placenta growth factor (PIGF) in human extravillous trophoblast proliferation, migration and invasiveness. Placenta. 1998;19(7):465–73.
Knuth A, Liu L, Nielsen H, Merril D, Torry DS, Arroyo JA. Placenta growth factor induces invasion and activates p70 during rapamycin treatment in trophoblast cells. Am J Reprod Immunol. 2015;73(4):330–40.
Lash GE, Cartwright JE, Whitley GS, Trew AJ, Baker PN. The effects of angiogenic growth factors on extravillous trophoblast invasion and motility. Placenta. 1999;20(8):661–7.
Gobble RM, Groesch KA, Chang M, Torry RJ, Torry DS. Differential regulation of human PlGF gene expression in trophoblast and nontrophoblast cells by oxygen tension. Placenta. 2009;30(10):869–75.
Wang L, Li X, Zhao Y, Fang C, Lian Y, Gou W, et al. Insights into the mechanism of CXCL12-mediated signaling in trophoblast functions and placental angiogenesis. Acta Biochim Biophys Sin. 2015;47(9):663–72.
Zhou WH, Du MR, Dong L, Yu J, Li DJ. Chemokine CXCL12 promotes the cross-talk between trophoblasts and decidual stromal cells in human first-trimester pregnancy. Hum Reprod. 2008;23(12):2669–79.
Quinn KE, Ashley AK, Reynolds LP, Grazul-Bilska AT, Ashley RL. Activation of the CXCL12/CXCR4 signaling axis may drive vascularization of the ovine placenta. Domest Anim Endocrinol. 2014;47:11–21.
Lei GQ, Wu ZY, Jiang WB, et al. Effect of CXCL12/CXCR4 on migration of decidua-derived mesenchymal stem cells from pregnancies with preeclampsia. Am J Reprod Immunol. 2019:e13180.
Hanna J, Wald O, Goldman-Wohl D, et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16- human natural killer cells. Blood. 2003;102(5):1569–77.
Mor G, Romero R, Aldo PB, Abrahams VM. Is the trophoblast an immune regulator? The role of toll-like receptors during pregnancy. Crit Rev Immunol. 2005;25(5):375–88.
Zhao R, Zhang J, Wang Y, et al. Activation of toll-like receptor 3 promotes pathological corneal neovascularization by enhancement of SDF-1-mediated endothelial progenitor cell recruitment. Exp Eye Res. 2019;178:177–85.
Aldo PB, Krikun G, Visintin I, Lockwood C, Romero R, Mor G. A novel three-dimensional in vitro system to study trophoblast-endothelium cell interactions. Am J Reprod Immunol. 2007;58(2):98–110.
Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157(6):2111–22.
Hannan NJ, Paiva P, Dimitriadis E, Salamonsen LA. Models for study of human embryo implantation: choice of cell lines? Biol Reprod. 2010;82(2):235–45.
Xu B, Charlton F, Makris A, Hennessy A. Antihypertensive drugs methyldopa, labetalol, hydralazine, and clonidine improve trophoblast interaction with endothelial cellular networks in vitro. J Hypertens. 2014;32(5):1075–83 discussion 1083.
Jauniaux E, Watson A, Ozturk O, Quick D, Burton G. In-vivo measurement of intrauterine gases and acid-base values early in human pregnancy. Hum Reprod. 1999;14(11):2901–4.
Desai J, Holt-Shore V, Torry RJ, Caudle MR, Torry DS. Signal transduction and biological function of placenta growth factor in primary human trophoblast. Biol Reprod. 1999;60(4):887–92.
Tarrade A, Goffin F, Munaut C, Lai-Kuen R, Tricottet V, Foidart JM, et al. Effect of matrigel on human extravillous trophoblasts differentiation: modulation of protease pattern gene expression. Biol Reprod. 2002;67(5):1628–37.
Highet AR, Zhang VJ, Heinemann GK, Roberts CT. Use of Matrigel in culture affects cell phenotype and gene expression in the first trimester trophoblast cell line HTR8/SVneo. Placenta. 2012;33(7):586–8.
Xu B, Shanmugalingam R, Chau K, Pears S, Hennessy A, Makris A. The effect of acetyl salicylic acid (aspirin) on trophoblast-endothelial interaction in vitro. J Reprod Immunol. 2017;124:54–61.
Munaut C, Lorquet S, Pequeux C, Blacher S, Berndt S, Frankenne F, et al. Hypoxia is responsible for soluble vascular endothelial growth factor receptor-1 (VEGFR-1) but not for soluble endoglin induction in villous trophoblast. Hum Reprod. 2008;23(6):1407–15.
Powers RW, Roberts JM, Plymire DA, Pucci D, Datwyler SA, Laird DM, et al. Low placental growth factor across pregnancy identifies a subset of women with preterm preeclampsia: type 1 versus type 2 preeclampsia? Hypertension. 2012;60(1):239–46.
Andraweera PH, Dekker GA, Laurence JA, Roberts CT. Placental expression of VEGF family mRNA in adverse pregnancy outcomes. Placenta. 2012;33(6):467–72.
Maebayashi Asanuma A, Yamamoto T, Azuma H, Kato E, Yamamoto N, Murase T, et al. Expression of placenta growth factor, soluble fms-like tyrosine kinase-1, metal-responsive transcription factor-1, heme oxygenase 1 and hypoxia inducible factor-1alpha mRNAs in pre-eclampsia placenta and the effect of pre-eclampsia sera on their expression of choriocarcinoma cells. J Obstet Gynaecol Res. 2014;40(10):2095–103.
Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277(5332):1669–72.
Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27(9–10):939–58.
Falco ML, Sivanathan J, Laoreti A, Thilaganathan B, Khalil A. Placental histopathology associated with pre-eclampsia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;50(3):295–301.
Ogge G, Chaiworapongsa T, Romero R, Hussein Y, Kusanovic JP, Yeo L, et al. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia. J Perinat Med. 2011;39(6):641–52.
Kang MC, Park SJ, Kim HJ, et al. Gestational loss and growth restriction by angiogenic defects in placental growth factor transgenic mice. Arterioscler Thromb Vasc Biol. 2014;34(10):2276–82.
Ishida I, Kubo H, Suzuki S, et al. Hypoxia diminishes toll-like receptor 4 expression through reactive oxygen species generated by mitochondria in endothelial cells. J Immunol. 2002;169(4):2069–75.
Kato E, Yamamoto T, Chishima F. Effects of cytokines and TLR ligands on the production of PlGF and sVEGFR1 in primary Trophoblasts. Gynecol Obstet Investig. 2017;82(1):39–46.
Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Hum Reprod Update. 2010;16(4):415–31.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chau, K., Xu, B., Hennessy, A. et al. Effect of Placental Growth Factor on Trophoblast–Endothelial Cell Interactions In Vitro. Reprod. Sci. 27, 1285–1292 (2020). https://doi.org/10.1007/s43032-019-00103-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-019-00103-7