Abstract
The aim of this study was to determine the levels of regulatory peptides apelin, glucagon-like peptide (GLP-1) and visfatin in hypercholesterolemic and hyperhomocysteinemic state and to examine their relation with nitric oxide (NO) metabolism. 32 Male guinea pigs were divided into four groups and each group was fed as follows: (a) commercial chow, (b) cholesterol (chol)-rich diet, (c) methionine (meth)-rich diet, and (d) chol + meth-rich diet. Blood samples were drawn at the end of 10 weeks, and abdominal aorta was dissected for histopathological examination. Serum insulin, GLP-1, apelin, visfatin, and nitrotyrosine concentrations were measured by the manufacturer’s kits based on ELISA; asymmetric dimethylarginine (ADMA) and arginine levels were measured by the high performance liquid chromatography. Homocysteine level was measured by the chemiluminescence immunoassay; glucose, total chol and triglyceride levels were measured by the autoanalyzer. The microscopic examination of aorta indicated varying degrees of vascular disturbance in chol- and chol + meth-fed groups. High levels of chol and homocysteine, accompanied with significantly low levels of apelin and GLP-1 were detected in the plasma. Visfatin, ADMA, and nitrotyrosine levels both in chol- and chol + meth-fed groups were significantly higher than those in control animals, whereas arginine and arginine/ADMA ratio were lower. This study indicated that circulating levels of apelin, GLP-1, and visfatin are markedly altered during the development of atherosclerotic changes in close association with chol, homocysteine, NO, and ADMA levels. The measurements of these peptides in serum may help for the diagnosis and follow-up of vascular dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High plasma cholesterol (chol) and homocysteine, the major risk factors for the development of atherosclerosis, are known to cause impairments in endothelial-dependent relaxation which is linked a decrease in the bioavailability of nitric oxide (NO) [1]. NO is a major endothelium-derived relaxing substance synthesized from l-arginine by the activity of NO synthase (NOS). In order to elucidate the mechanisms through which chol and homocysteine exacerbate the development of atherosclerosis, either genetic modifications or dietary regimens enriched with chol and methionine (meth), the precursor of homocysteine, have commonly been used in animal experiments.
Atherosclerotic and inflammatory conditions are always accompanied by irregular synthesis of adipocyte-derived substances. The synthesis of pro-inflammatory mediators by the increased bulk of adipose tissue may further induce inflammatory changes in obesity, and cause a vicious circle for the production of several hormones and adipokines. Apelin is one of these regulatory peptides synthesized in adipocytes as well as other organs, and mainly in endothelial cells [2, 3]. It has been identified as an endogenous ligand of the G-protein-coupled receptor [4]. The effects of apelin have been demonstrated in cardiovascular and immune functions and energy homeostasis [5, 6]. Previous observations indicated that apelin lowers the blood pressure via a NO-dependent mechanism [7].
Visfatin, another novel peptide synthesized in visceral adipocytes, is expressed mostly in macrophage-infiltrating adipose tissue during inflammatory response [8]. Plasma visfatin levels are known to increase progressively with the degree of obesity, and associate with insulin resistance [9]. Significantly elevated visfatin levels were observed in coronary artery disease, suggesting the involvement of visfatin in the pathogenesis of atherosclerosis [10].
Glucagon-like peptide (GLP-1), a member of the proglucagon incretin family, has been shown to play a role in atherosclerotic process through inducing eNOS [11]. GLP-1 receptors are abundantly expressed in endothelial cells, monocyte/macrophages, and smooth muscle cells. Recent studies suggested that the anti-inflammatory, antiproliferative, and vasodilatory properties of GLP-1 signaling may protect the vascular wall against atherogenesis [12]. In contrast, some researchers have reported positive associations of circulating GLP-1 levels and the development atherosclerosis [13, 14].
Although several animal models have been used to investigate mechanisms of homocysteine and/or chol-induced vascular dysfunction, experimental studies related to the role of regulatory peptides in this process are limited. In this study, we used the combination of high dietary chol and meth in order to develop atherosclerotic changes and endothelial dysfunction in guinea pigs, and measured the circulating levels of apelin, GLP-1, and visfatin together with the biochemical parameters of NO metabolism in order to evaluate their involvement with vascular dysfunction.
Materials and methods
Study groups
Male Dunkin–Hartley guinea pigs, 4–6 months old, and weighing 695 ± 38.6 g, were used. The animals were obtained from the Experimental and Medical Research Institute, Istanbul University. They were kept in steel wire cages at room temperature (25 °C) and maintained on a 12-h light/dark cycle. The study protocols were approved by the Animal Care and Use Committee, Istanbul University.
Thirty-two animals were divided into four groups, eight animals in each. Group 1 (control) was fed a commercial laboratory chow. For the other three groups, a diet chow was prepared by the addition of chol (Alfa Aesar A11470) and/or l-methionine (Sigma). Group 2 (chol) received a diet supplemented with 1.5 % (w/w) chol, while group 3 (meth) had a diet containing 2 % (w/w) meth only. Group 4 (chol + meth) was fed with chol (1.5 %) + meth (2 %)-supplemented diet [15, 16]. Food and water were supplied ad libitum.
Methods
At the end of 10 weeks, animals were anesthetized by sodium thiopental following an overnight fasting and blood samples were drawn by the cardiac venipuncture. Aliquots of serum and plasma were stored at −80 °C until studied, and used for the biochemical analyses. Serum insulin and apelin levels were measured by the competitive binding enzyme immunoassay kits (Wuhan EIAab Science, and Novateinbio, Cambridge, USA, respectively), and serum GLP-1 levels were determined by sandwich enzyme-linked immunosorbent method (Wuhan EIAab Science, Wuhan, China).
Glucose, total chol and triglyceride levels were carried out on the same day by using Roche autoanalyzer. Homeostasis model assessment (HOMA-IR) was calculated by the formula of insulin (mU/L) × glucose (mmol/L)/22.5 [17].
Homocysteine concentrations were measured by the chemiluminescence immunoassay using Immulite 2000 XPI (Siemens Medical Solutions Diagnostics, IL, USA).
Serum asymmetric dimethylarginine (ADMA) and l-arginine concentrations were determined using high performance liquid chromatography following pre-column derivation with o-phthalaldehyde [18].
Nitrotyrosine levels were measured by the enzyme-linked immunosorbent assay (Cell Biolabs, Inc.). NO levels were estimated as total nitrite + nitrate using spectrophotometric commercial kit (Oxford Biomedical Research, Oxford, USA).
Histopathological studies
Pieces of abdominal aorta from the control and experimental groups were removed immediately and fixed in 10 % buffered formaldehyde and processed for paraffin sectioning. Sections 5 μm in thickness were stained with haematoxylin and eosin (H&E) using a standard protocol and analyzed by the pathologist on the light microscopy.
Statistical analysis
The data were analyzed using SPSS 15 (SPSS, Chicago, IL, USA). The results were expressed as mean ± SD. One-way analysis of variance followed by Tukey’s post-hoc test was used for equal variances. Kruskal–Wallis variance analysis and a post-hoc analysis using Mann–Whitney U-test were performed for unequal variances. In all cases, a difference was considered significant when p < 0.05. Correlation analyses were carried out by the Pearson test.
Results
The biochemical data are presented in Table 1. Levels of glucose and insulin in chol, meth, and chol + meth groups were not different than those from the control group, and therefore similar HOMA-IR values were obtained in all groups. Serum chol levels were higher in all diet-fed groups than those in control animals. Homocysteine levels were high in chol, meth, and chol + meth groups.
Serum NO levels were slightly lower in chol-fed (18 %) and chol + meth-fed animals (26 %) than in controls. However, the difference with regard to control group was not significant. Nitrotyrosine levels were significantly high in chol- and chol + meth-fed animals (Table 1).
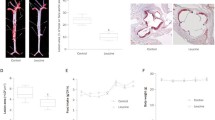
Significantly lower levels of apelin were found in chol, meth, (p < 0.02) and chol + meth groups (p < 0.01) in comparison to controls; apelin levels being markedly lower in the chol + meth group than the other groups (p < 0.05; Fig. 1a).
Serum visfatin levels in chol and chol + meth groups were significantly high compared to the control group (p < 0.05 and <0.01, respectively; Fig. 1b). Serum GLP-1 concentrations were significantly lower in these groups as well as in the meth group than in controls (p < 0.01; Fig. 1c). No difference was noticed between the diet-fed groups.
ADMA levels in all diet-fed groups were significantly high as compared to the control group (p < 0.01). Addition of meth to the high-chol diet caused more drastic increment in ADMA concentrations (Fig. 2a), together with significant decrements in the arginine/ADMA ratio (Fig. 2b).
Correlation analyses
Significant correlations were obtained as follows: apelin positively with GLP-1 (r = 0.44), NO (r = 0.41, p < 0.05); and negatively with visfatin (r = −0.44, p = 0.05), ADMA (r = −0.76), nitrotyrosine (r = −0.55), chol (r = −0.64, p < 0.001); and homocysteine (r = −0.50, p < 0.01).
Visfatin levels were associated negatively with GLP-1 (r = −0.44) and NO (r = −0.41, p < 0.05); and positively with chol (r = 0.65), homocysteine (r = 0.59), and ADMA (r = 0.52, p < 0.01). GLP-1 levels were associated negatively with chol (r = −0.55) and ADMA (r = −0.51, p < 0.01).
ADMA and nitrotyrosine levels were correlated positively (r = 0.73, p < 0.01).
Histopathological findings
Examination of aorta revealed some pathological changes in the diet-fed groups (Fig. 3). Meth feeding caused slight increases in intima-media thickness and muscle cell proliferation. In the chol and chol + meth groups, increased intima-media thickness, smooth muscle cell proliferation, lipid vacuoles, and in some areas fatty streaks resembling chol crystals were seen.
Histopathological examination of the aortic sections from the animals in the study groups (H&E, magnification 200). a Control, b chol increased intima-media thickness (1), smooth muscle cell proliferation (2) and lipid vacuoles with fatty streaks resembling cholesterol crystals (3), c meth only slight increase in intima-media thickness and smooth muscle cell proliferation, and d chol + meth similar to chol group
Discussion
In the present study, we fed the guinea pigs with high meth and chol diet for a 10-week period in order to stimulate hyperhomocysteinemia and hypercholesterolemia. The microscopic examination of aortic sections indicated an early phase of vascular disturbance which was accompanied by significantly high chol and homocysteine levels in the plasma. These alterations were more prominent in the chol + meth-fed group. Meth load is known to cause hypercholesterolemia by stimulating several mechanisms. Firstly, increased meth concentration in liver enhances the bioavailability of methyl groups for the methylation of phosphatidylethanolamine, thereby leading to increases in phosphatidylcholine:phosphatidylethanolamine ratio which has a regulatory function in chol metabolism [19]. More importantly, homocysteine induces 3-hydroxy-3-methylglutaryl coenzyme A reductase, the rate-limiting enzyme in chol biosynthesis, by activating transcription factors [20, 21]. Therefore, chol + meth load would be expected to have a more profound effect on plasma chol. Our findings in the animals fed chol + meth diet is in good agreement with the previous reports.
The decreased activity of NOS and impaired NO bioavailability are prominent events leading to vascular dysfunction [22, 23]. ADMA, an endogenous inhibitor of NOS, is a major determinant of NO production [24]. It has been reported that plasma ADMA levels are increased in the presence of hypercholesterolemia [25, 26]. In our study, markedly elevated ADMA levels in hypercholesterolemic and hyperhomocysteinemic animals confirm the relation of ADMA to the development of endothelial dysfunction. Additionally, plasma arginine/ADMA ratios were found significantly decreased in all diet-fed groups, the degree of decrease being more prominent in the chol + meth group.
A positive correlation between plasma ADMA and homocysteine levels has been well-documented [27]. Reduced dimethylarginine dimethylaminohydrolase (DDAH) activity is considered as the major factor for the elevation of ADMA [28, 29]. In patients with peripheral arterial disease, meth load caused elevations in plasma homocysteine and ADMA levels [30]. Homocysteinylation of lysine residues in DDAH protein due to hyperhomocysteinemia may result in inactivation of the enzyme, thereby leading to increases in circulating ADMA [31, 32]. As summarized in Fig. 4, homocysteine itself not only induces chol synthesis, but also alters ADMA metabolism in the liver. A profound increment in ADMA levels in the chol + meth group is likely to be resulted from dual effect of hyperhomocysteinemia.
Elevated ADMA levels are indicative of decreased NO formation. We measured both NO and nitrotyrosine levels in order to detect the bioavailability of NO. Decreased NO levels accompanying to markedly elevated nitrotyrosine seemed to be due to superoxide radical generation in chol and chol + meth groups. It is known that conditions of oxidative stress promote S-glutathionylation of cysteine residues in endothelial NOS, which causes decreased NO synthesis and increased superoxide generation from the reductase domain of the enzyme [33]. An excess generation of superoxide radical can scavenge NO, thus decreasing its bioavailability and increasing nitrotyrosine formation [34, 35].
One of the main purposes in our study was to see the possible relation of apelin with early vascular lesions. In a previous study, exogenous apelin administration to rats caused elevations in plasma NO concentrations. Also, apelin exerted a hypotensive effect which was abolished by the presence of NOS inhibitor [7]. In our study, plasma apelin levels were significantly decreased in chol- and chol + meth-fed animals. Apelin levels were negatively correlated with those of ADMA, suggesting a possible involvement of this peptide in vascular changes. Several clinical studies have focused on the relation of apelin with hypercholesterolemia and cardiovascular disease [6, 36, 37]. The decrease in apelin levels was thought to be associated with insulin resistance in these patients. Therefore, in our study, we evaluated the HOMA-IR to see whether any changes occurred in glucose homeostasis. Neither glucose nor insulin levels seemed to be affected during atherogenic regiments. Decreased apelin levels were negatively correlated with both chol and homocysteine. Furthermore, serum apelin levels were decreased more drastically when meth was added to the atherogenic diet. To our knowledge, there is no study with regard to the effect of hyperhomocysteinemia on apelin synthesis or secretion. The decrement in apelin levels seems to be related to the ongoing atherogenic process with an additive impact of hyperhomocysteinemia.
Many experimental studies revealed that GLP-1 and related drugs exert protective effects on atherosclerosis, hypertension and cardiac dysfunction [38, 39]. In a mouse model of obesity, GLP-1-based therapy activated several cardioprotective pathways, as well as it prevented obesity-induced insulin resistance and inflammation [40]. In clinical trials, treatment with GLP-1 analogs not only had the ability to reduce blood glucose, but also exerted several cardioprotective effects, by influencing positively some risk factors, and improving endothelial function. GLP-1 analogs increased the eNOS expression [41] and decreased the number of inflammatory cells and ROS production [42]. In our study, GLP-1 levels were decreased in chol- and chol + meth-fed animals. A negative association between GLP-1 and ADMA levels was observed, suggesting a possible involvement of ADMA on GLP-1 secretion.
As a potential inflammatory mediator, visfatin plays a role in chronic inflammation, thus contributes to the pathogenesis of atherosclerosis and cardiovascular disease. A positive association between visfatin levels and coronary atherosclerosis has been observed [43]. Moreover, visfatin impairs microvascular endothelium-dependent relaxation through a mechanism involving NADPH oxidase stimulation [44]. Serum visfatin levels were found markedly elevated in both in hypertensive and prehypertensive patients [45]. Uslu et al. have observed high visfatin levels in type 2 diabetic patients which was associated with hyperhomocysteinemia, suggesting a role of visfatin in endothelial dysfunction [46]. In our study, visfatin levels were significantly elevated both in chol and chol + meth groups, and correlated negatively with GLP-1 levels. Moreover, significant correlations between visfatin levels and the markers of endothelial dysfunction were observed.
The roles of apelin, visfatin, and GLP-1 in cardiovascular dysfunction have been investigated previously in clinical studies and their physiological effects have been noted. In this study, their relation with ADMA metabolism was searched in an experimental model of atherogenesis. Our results indicated that levels of apelin, GLP-1, and visfatin are markedly altered during the development of atherosclerotic changes in close association with chol, homocysteine, NO, and ADMA levels. According to the results of the present study, measurement of these peptides in circulation may help to assess the development of vascular dysfunction in patients with metabolic abnormalities.
References
Zulli A, Widdop RE, Hare DL (2003) High methionine and cholesterol diet abolishes endothelial relaxation. Arterioscler Thromb Vasc Biol 23:1358–1363
Boucher J, Castan-Laurell I, Daviaud D (2005) Adipokine expression profile in adipocytes of different mouse models of obesity. Horm Metab Res 37:761–776
Kleinz MJ, Davenport AP (2004) Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul Pept 118:119–125
Tatemoto K, Hosoya M, Habata Y et al (1998) Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 251:471–476
Carpene C, Dray C, Attane C et al (2007) Expanding role for the apelin/APJ system in physiopathology. J Physiol Biochem 63:359–373
Goetze JP, Rehfeld JF, Carlsen J et al (2006) Apelin: a new plasma marker of cardiopulmonary disease. Regul Pept 133:134–138
Tatemoto K, Takayama K, Zou MX et al (2001) The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept 99:87–92
Saddi-Rosa P, Oliveira CS, Giuffrida FM et al (2010) Visfatin, glucose metabolism and vascular disease: a review of evidence. Diabetol Metab Syndr 2:21
Chen MP, Chung FM, Chang DM (2006) Elevated plasma level of visfatin/pre-B cell colony-stimulating factor in patients with type 2 diabetes. J Clin Endocrinol Metab 91:295–299
Liu SW, Qiao SB, Yuan JS et al (2009) Association of plasma visfatin levels with inflammation, atherosclerosis, and acute coronary syndromes in humans. Clin Endocrinol (Oxf) 71:202–207
Doyle ME, Egan JM (2007) Mechanisms of action of GLP-1 in the pancreas. Pharmacol Ther 113:546–593
Mita T, Watada H (2012) Glucagon like peptide-1 and atherosclerosis. Cardiovasc Hematol Agents Med Chem 10:309–318
Yamaoka-Tojo M, Tojo T, Takahira N et al (2010) Elevated circulating levels of an incretin hormone, glucagon-like peptide-1, are associated with metabolic components in high-risk patients with cardiovascular disease. Cardiovasc Diabetol 9:17
Piotrowski K, Becker M, Zugwurst J et al (2013) Circulating concentrations of GLP-1 are associated with coronary atherosclerosis in humans. Cardiovasc Diabetol 12:117. doi:10.1186/1475-2840-12-117
Coban J, Evran B, Ozkan F et al (2013) Effect of blueberry feeding on lipids and oxidative stress in the serum, liver and aorta of guinea pigs fed on a high-cholesterol diet. Biosci Biotechnol Biochem 77:389–391
Yalcinkaya S, Unlucerci Y, Giris M et al (2009) Oxidative and nitrosative stress and apoptosis in the liver of rats fed on high methionine diet: protective effect of taurine. Nutrition 25:436–444
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Teerlink T (2005) Determination of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine in biological samples by HPLC. Methods Mol Med 108:263–274
Sugiyama K, Kumazawa A, Zhou H et al (1998) Dietary methionine level affects linoleic acid metabolism through phosphatidylethanolamine N-methylation in rats. Lipids 33:235–242
Hirche F, Schröder A, Knoth B et al (2006) Effect of dietary methionine on plasma and liver cholesterol concentrations in rats and expression of hepatic genes involved in cholesterol metabolism. Br J Nutr 95:879–888
Woo CW, Siow YL, Pierce GN et al (2005) Hyperhomocysteinemia induces hepatic cholesterol biosynthesis and lipid accumulation via activation of transcription factors. Am J Physiol Endocrinol Metab 288:E1002–E1010
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352:1685–1695
Li H, Wallerath T, Münzel T et al (2002) Regulation of endothelial type NO synthase expression in pathophysiology and in response to drugs. Nitric Oxide Biol Chem 7:149–164
Szuba A, Podgórski M (2006) Asymmetric dimethylarginine (ADMA) a novel cardiovascular risk factor—evidence from epidemiological and prospective clinical trials. Pharmacol Rep 58:16–20
Böger RH, Maas R, Schulze F et al (2009) Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality—an update on patient populations with a wide range of cardiovascular risk. Pharmacol Res 60:481–487
Landim MBP, Dourado PM, Casella-Filho A et al (2013) High plasma concentrations of asymmetric dimethylarginine inhibit ischemic cardioprotection in hypercholesterolemic rats. Braz J Med Biol Res 46:454–459
Sydow K, Schwedhelm E, Arakawa N et al (2003) ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of l-arginine and B vitamins. Cardiovasc Res 57:244–252
Cooke JP, Ghebremariam YT (2011) DDAH says NO to ADMA. Arterioscler Thromb Vasc Biol 31:1462–1464
Bekpinar S, Develi-Is S, Unlucerci Y et al (2013) Modulation of arginine and asymmetric dimethylarginine concentrations in liver and plasma by exogenous hydrogen sulfide in LPS-induced endotoxemia. Can J Physiol Pharmacol 91:1071–1075
Stühlinger MC, Oka RK, Graf EE et al (2003) Endothelial dysfunction induced by hyperhomocyst(e)inemia: role of asymmetric dimethylarginine. Circulation 108:933–938
Jakubowski H, Zhang L, Bardeguez A et al (2000) Homocysteine thiolactone and protein homocysteinylation in human endothelial cells: implications for atherosclerosis. Circ Res 7:45–51
Knipp M, Braun O, Vasák M (2005) Searching for DDAH inhibitors: S-nitroso-l-homocysteine is a chemical lead. J Am Chem Soc 127:2372–2373
Förstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33:829–837
Gryglewski RJ, Palmer RM, Moncada S (1986) Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320:454–456
Reiter CD, Teng RJ, Beckman JS (2000) Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J Biol Chem 275:32460–32466
Tasci I, Dogru T, Naharci I et al (2007) Plasma apelin is lower in patients with elevated LDL-cholesterol. Exp Clin Endocrinol Diabetes 115:428–432
Karadag S, Ozturk S, Gursu M et al (2014) The relationship between apelin and cardiac parameters in patients on peritoneal dialysis: is there a new cardiac marker? BMC Nephrol 16(15):18. doi:10.1186/1471-2369-15-18
Oyama J, Node K (2014) Incretin therapy and heart failure. Circ J 78:819–824
Avogaro A, Vigili de Kreutzenberg S, Fadini GP (2014) Cardiovascular actions of GLP-1 and incretin-based pharmacotherapy. Curr Diabetes Rep 14:483. doi:10.1007/s11892-014-0483-3
Noyan-Ashraf MH, Shikatani EA, Schuiki I et al (2013) A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation 127:74–85
Ding L, Zhang G (2012) Glucagon-like peptide-1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacol Sin 33:75–81
Shiraki A, Oyama J, Komoda H et al (2012) The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis 221:375–382
Kadoglou NP, Gkontopoulos A, Kapelouzou A et al (2011) Serum levels of vaspin and visfatin in patients with coronary artery disease—Kozani study. Clin Chim Acta 412:48–52
Vallejo S, Romacho T, Angulo J et al (2011) Visfatin impairs endothelium-dependent relaxation in rat and human mesenteric microvessels through nicotinamide phosphoribosyltransferase activity. PLoS ONE 6:e27299
Gunes F, Akbal E, Cakir E et al (2012) Visfatin may be a novel marker for identifying stages of essential hypertension in advanced age patients. Intern Med 51:553–557
Uslu S, Kebapci N, Kara M et al (2012) Relationship between adipocytokines and cardiovascular risk factors in patients with type 2 diabetes mellitus. Exp Ther Med 4:113–120
Acknowledgments
This study was supported by the Research Fund, Istanbul University, Project No. 22342.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kusku-Kiraz, Z., Genc, S., Bekpinar, S. et al. Circulating levels of apelin, glucagon-like peptide and visfatin in hypercholesterolemic–hyperhomocysteinemic guinea-pigs: their relation with NO metabolism. Mol Cell Biochem 400, 69–75 (2015). https://doi.org/10.1007/s11010-014-2263-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2263-4