Abstract
l-arginine (l-Arg) uptake is mediated by members of cationic amino acid transporter (CAT) family and may coincide with the induction of nitric oxide synthases (NOS). The present study was conducted to investigate the extracellular concentrations of l-Arg regulating the CAT-1, CAT-4 and inducible NOS (iNOS) in chick intestinal epithelial cells. The cells were cultured for 4 days in Arg-free Dulbecco’s modified Eagle’s medium containing 10, 100, 200, 400, or 600 μM l-Arg. Cell viability, nitric oxide (NO) concentrations, uptake and metabolism of l-[3H]-Arg as well as expression of CAT-1, CAT-4, and iNOS were determined. Our results showed that l-Arg enhances cell growth with a maximal response at 10–400 μM. Addition of 100, 200, or 400 μM l-Arg increased the l-[3H]-Arg uptake, which was associated with greater conversion of l-[3H]-citrulline and NO production in comparison with 10 μM l-Arg group. Increasing extracellular concentrations of l-Arg from 10 to 400 μM dose dependently increased the levels of CAT-1 mRNA and protein, while no effect on CAT-4 mRNA abundance was found. Furthermore, supplementation of 100, 200, or 400 μM l-Arg upregulated the expression of iNOS mRNA, and the relative protein levels for iNOS in 200 and 400 μM l-Arg groups were higher than those in 10 and 100 μM l-Arg groups. Collectively, we conclude that the CAT-1 isoform plays a role in l-Arg uptake, and l-Arg-mediated elevation of NO via iNOS promotes the growth of chick intestinal epithelial cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The main functions of epithelial cells are to retain most of the gut contents in the inner cavity, to absorb and dispatch selected nutrients, and to produce secretions with protective or signaling properties [1]. Many studies have reported that l-arginine (Arg) plays an important role in intestinal physiology [2], such as cell growth, proliferation, migration, and protein synthesis [3–5]. These studies involving the action of l-Arg mostly focused on mammalian cells. However, mammals and avians have difference in l-Arg requirement. Because most mature mammals are able to synthesize l-Arg to meet their requirements, while uricotelic species like birds cannot synthesize l-Arg due to an incomplete urea cycle [6]. Therefore, chicks have an absolute need for arginine and are highly dependent on additional supplement for this amino acid. In the cell, l-Arg is metabolized by arginases to l-ornithine for polyamine biosynthesis, and by nitric oxide synthases (NOS) to l-citrulline and nitric oxide (NO) [7]. NO is a molecule that has been a versatile regulator of many physiological processes, including vasodilatation, immune responses, neurotransmission, and cell survival [8]. Thus, it seems that l-Arg is nutritionally essential for other important functions instead of just its role in protein deposition.
The cellular uptake of l-Arg is mediated by the cationic amino acid transporter (CAT) family of protein [9]. The uptake of basic amino acids (l-arginine, l-lysine, and l-ornithine) is mediated by the Na+-independent system (y+, y+ L, b0,+, b+) and the Na+-dependent system (B0,+) [9, 10]. System y+, composed of CAT-1, CAT-2A, CAT-2B, CAT-3, and CAT-4, is the principal cationic amino acid transport system for regulating the intracellular supply of l-Arg for NOS. With the exception of the liver, CAT-1 is expressed almost ubiquitously and has the most pronounced trans-stimulation among the CATs [11]. The two CAT-2 variants exhibit a quite distinct pattern. CAT-2A, a low-affinity carrier for cationic amino acids, is most abundant in the liver and also expressed in skeletal muscle and pancreas, whereas a significant expression of CAT-2B is only induced under the treatment of cytokine or lipopolysaccharide [12, 13]. CAT-3 is abundantly expressed in thymus, moderately expressed in testis and mammary gland, and weakly expressed in ovary and stomach [14]. The expression of CAT-4 in the plasma membrane of mammalian cells is not sufficient to mediate amino acid transport [15]; however, information on whether the CAT-4 gene in poultry can be regulated by extracellular l-Arg or not is obscure. Moreover, the homologous rate between CAT-1 and CAT-3 (60 %) is higher than that between CAT-1 and CAT-4 (40 %) [11].
NO is synthesized from l-Arg by three NOS isoforms, namely neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). At the gastrointestinal level, endothelial eNOS and nNOS are expressed basally at the vascular endothelium and the enteric nervous system of the gastrointestinal tract, respectively [16]. The inducible isoform of NOS (iNOS) is expressed in macrophages and neutrophils, but with potent signals for induction, it may also be observed in epithelial cells and neurons [17]. Previous studies found that the expression of the NOS enzymes for the production of NO was often coordinated with CAT-mediated l-Arg uptake [7, 18]. It is often induced together with the iNOS and is generally co-expressed with CAT-1 [11]. It is not known whether l-Arg upregulation of CAT-1 is co-ordinated with iNOS levels to modulate l-Arg uptake and NO production for the survival of chick intestinal epithelial cells (IEC). Thus, we used an established chick primary IEC derived from 14-day-old chick embryo by culturing cells with increasing l-Arg concentrations to investigate the effects of Arg on regulation of CAT-1, CAT-4, and iNOS expression, and l-Arg uptake and metabolism.
Materials and methods
Cell culture and preparation
Chick intestinal epithelial cell, isolated from the intestinal crypts of the 14-day-old chick embryo by collagenase digestion, was obtained by the previous study [19]. All experimental procedures were approved by the Animal Care and Use Committee of Animal Science College of Zhejiang University (Hangzhou, Zhejiang, China). Chick IEC (passage 2–5) were grown in uncoated plastic culture dishes (100 mm) in Dulbecco’s modified Eagle medium (DMEM, high glucose, Gibco-Invitrogen, Cergy Pontoise, France) containing 2 mM Gln, 20 ng/mL epidermal growth factor, 2 µg/mL insulin (Sigma-Aldrich, Saint Louis, Missouri, USA), 5 % fetal bovine serum (FBS, Gibco-Invitrogen, Cergy Pontoise, France), and 100 U/mL penicillin–streptomycin (Jinuo Biomedical Technology Co. Ltd., Hangzhou, China). Medium was changed every 2 days. When grown to 80–85 % confluence, cells were trypsinized and seeded in 96-well culture plates at a density of 1 × 104 per well and maintained at 37 °C in a 5 % CO2 incubator. After an overnight incubation, the cells were starved for 6 h in custom-made Arg-free DMEM (Merck Millipore Beijing Skywing, Beijing, China) and all the following experiments were performed under this treatment. The 5 % FBS in the Arg-free DMEM provided 10 µM l-Arg [5, 20]. Cells were cultured in medium containing 10, 100, 200, 400, and 600 µM l-Arg, and the supplemented medium was changed every 2 days. The optimum time and l-Arg concentration for cell growth were determined by the 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, Saint Louis, Missouri, USA) assay following the designated incubation interval (0, 2, 4, and 6 days); each group included 8 wells.
Detection of NO production by chick IEC
After a 4-day period of culture with 10, 100, 200, and 400 µM l-Arg in 24-well plates (4 wells for each group, 2 × 104 per well), NO concentrations in cells were measured as nitrite using an NO detection kit (Beyotime Biotechnology, Beijing, China) according to the manufacturer’s protocol. Briefly, a standard curve was prepared with standard nitrite solutions in lysis medium. The standard solutions or cytolysates were reacted with nitrate reductase for 30 min in a 96-well plate, and then Griess reagent I and Griess reagent II were added. After a 10-min incubation at room temperature, the absorbance at 540 nm was read in a microplate reader (Bio-tek ELX800, USA). The samples were assayed in duplicate.
l-[3H]-Arg uptake and metabolism by chick IEC
The procedure for l-[3H]-Arg uptake and metabolism was a modification of previous study [21, 22]. After a 4-day period of culture with 10, 100, 200, and 400 µM l-Arg in 6-well plates (1 × 105 per well), cells were washed with the LiCl-Dulbecco (140 mM LiCl, 5 mM KCl, 2 mM Na2HPO4, 1.2 mM MgSO4, 2.5 mM CaCl2, 11 mM glucose, and 10 mM HEPES-Tris solution, 37 °C, pH 7.4) three times. Then cells were incubated with l-[2,3,4,5-3H]-Arg (l-[3H]-Arg, 1 uCi/mL, 59 Ci/mmol, Ruxu Biotechnology, Shanghai, China) in 2 mL LiCl-Dulbecco solution for 15 min. To determine the nonspecific uptake of l-[3H]-Arg, cells incubated with 1 uCi/mL l-[3H]-Arg and 10 mM nonlabeled l-Arg in 2 mL LiCl-Dulbecco solution were placed as the control. The transport was stopped by washing the cells three times with 2 mL ice-cold LiCl-Dulbecco solution. After solubilization of the cells in 400 µL lysis buffer, 50 µL of cytolysates were also taken for measurements of protein content using a BCA protein assay kit according to the instructions of manufacturer (Beyotime Biotechnology, Beijing, China), and 250 µL of cytolysates were added to 15 mL scintillation fluid and inserted into a liquid scintillation counter (Perkin Elmer, Waltham, MA, USA). Specific l-[3H]-Arg uptake was determined by subtracting the total l-[3H]-Arg uptake from the nonspecific l-[3H]-Arg uptake). l-[3H]-Arg was expressed as pmol incorporation of l-[3H]-Arg into protein per mg protein.
The metabolism of l-[3H]-Arg to l-[3H]-ornithine and l-[3H]-citrulline can be quantitated by measuring the appearance of [3H] in l-ornithine and l-citrulline through thin layer chromatography (TLC) as described by previous study [23]. Briefly, 40 µL of the cytolysates from each l-[3H]-Arg uptake experiment was streaked directly on a silica gel plate, and then the plate was exposed to a mobile phase consisting of chloroform/methanol/ammonium hydroxide/distilled water (9:9:4:1 by volume). The distinguish of l-ornithine and l-citrulline was determined by comparison with the retardation factor (Rf) values of the standard l-ornithine and l-citrulline (0.57 for l-ornithine and 0.75 for l-citrulline). Then the spots in TLC plates were scraped into liquid scintillation vials containing 15 ml scintillation fluid, and the counts were measured in the liquid scintillation counter. The radioactivity of the solution was determined by a liquid scintillation counter.
RNA extraction and cDNA synthesis
Total RNA was isolated from the cells in each 6-well plate (1 × 105 per well) using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The RNA quality was checked by 1.5 % agarose gel electrophoresis and it had a ratio of optical density at 260 nm: optical density at 280 nm between 1.8 and 2.0. Reverse transcription was performed from 2 μg of total RNA by M-MLV reverse transcriptase (Takara, Dalian, China) at 42 °C for 60 min with oligo (dT) adaptor primer following the protocol of the manufacturer.
Quantification of mRNA by real-time PCR analysis
Primer pairs for four of the genes have been reported previously and are as follows: CAT-1 [24] (forward 5′-ATGTAGGTTGGGATGGAGCC-3′, reverse 5′-AACGAGTAAGCCAGGAGGGT-3′), CAT-4 [25] (forward 5′-CTCTTGCAGATCCCTCTGGTC-3′, reverse 5′-CAGATGCCGTAGCCAAAGTAG-3′), iNOS [26] (forward 5′-CAGCTGATTGGGTGTGGAT-3′, reverse 5′-TTTCTTTGGCCTACGGGTC-3′), β-actin [26] (forward 5′-GAGAAATTGTGCGTGACATCA-3′, reverse 5′-CCTGAACCTCTCATTGCCA-3′). The abundance of mRNA was determined on a Real-Time PCR system (ABI 7300, Applied Biosystems, Foster City, CA). The PCR reaction used SYBR Premix PCR kit (TaKaRa, Dalian, China) and the program was 95 °C for 10 s, followed by 40 cycles of 95 °C for 10 s, and 60 °C for 31 s. The standard curve was determined using pooled samples. Efficiency of the real-time PCR primers for all the examined genes was calculated from the standard curves. Each sample was performed in duplicate and no template control was included. Specificity of the amplification was verified at the end of PCR run by melting curve analysis. Average gene expression relative to the endogenous control for each sample was calculated using the 2−ΔΔCt method [27]. The calibrator for each studied gene was the average ΔCt value of 10 μM l-Arg group.
Western blot analysis
Cells were cultured for 4 days in the presence of either 10, 100, 200, or 400 μM l-Arg. The cells were then lysed for 30 min at 4 °C in a lysis buffer. The cell lysates were centrifuged (10,000 g for 15 min at 4 °C). Protein concentration in the supernatant fluid was determined as described above. All samples were adjusted to an equal protein concentration (25 μg), then diluted with 5 × loading buffer (Beyotime Biotechnology, Beijing, China), and heated in boiling water for 5 min. Denatured proteins were separated using SDS-polyacrylamide gel electrophoresis (4–10 % gradient gel) and transferred to nitrocellulose (Bio-Rad, Hercules, CA, USA) overnight at 220 mA using the Bio-Rad Transblot apparatus (Bio-Rad, Hercules, CA, USA). Membranes were blocked in 5 % fat-free milk in Tris–Tween-buffered saline (TTBS) for 1 h and then incubated with the following primary antibodies at 4 °C overnight with gentle rocking: CAT-1 (1:200 dilution), iNOS (1:400), and β-actin (1:500) (Abcam Inc., Cambridge, MA, USA). After washing three times with TTBS, the membranes were incubated at room temperature for 2 h with secondary antibodies and the membranes were washed with TTBS, followed by development using the ECL-Plus kit (Beyotime Biotechnology, Beijing, China). The images were detected on Fujifilm LAS-3000 (Tokyo, Japan). All treatments were normalized to β-actin, and all data were expressed as the relative values to 10 μM l-Arg group.
Statistical analysis
Results were analyzed by one-way analysis of variance (ANOVA) using SPSS for Windows, version 16.0 (SPSS Inc., Chicago, IL, USA). Differences among all treatments were separated by the Tukey test for multiple comparisons, and probability values of less than 0.05 were considered as significant. Results are expressed as mean ± SE.
Results
Cell growth responds to l-Arg
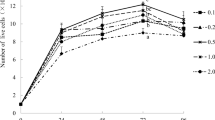
The growth curves of chick IEC treated with l-Arg are illustrated in Fig. 1. On days 0 and 2, the number of cells did not differ among the 10, 100, 200, 400, and 600 µM l-Arg groups. On day 4, the number of cells increased with the extracellular concentrations of l-Arg from 10 to 600 µM (P < 0.01). On day 6, the number of cells in 100, 200, 400, and 600 µM l-Arg groups were also higher (P < 0.01) than those in 10 µM l-Arg group, but no significant differences were found between 100 and 200 µM l-Arg groups, 400 and 600 µM l-Arg groups. The number of cells in 100, 200, 400, and 600 µM l-Arg groups increased (P < 0.01) on day 4 and day 6 compared with those on days 0 and 2.
Effects of l-Arg on NO production in cells
As Fig. 2 shown, NO production was minimal in cells cultured in the presence of 10 µM l-Arg. And increasing extracellular l-Arg concentrations from 10 to 400 µM dose dependently increased (P < 0.01) the concentrations of NO production in cells.
Effects of l-Arg on the concentrations of NO in chick intestinal epithelial cell. Cells were cultured in 24-well plates (4 wells for each group, 2 × 104 per well) supplemented with Arg-free DMEM containing 10, 100, 200, and 400 µM l-Arg for 4 days. Data are expressed as mean ± SE, n = 8. a–d Means with different letters differ (P < 0.01)
Effects of Arg on l-[3H]-Arg uptake and metabolism by chick IEC
Figure 3a reveals that l-[3H]-Arg uptake by chick IEC was significantly increased (P < 0.01) in response to extracellular concentrations of l-Arg. Increasing extracellular concentrations of l-Arg from 10 to 400 μM dose dependently increased (P < 0.01) the conversion of l-[3H]-Arg to l-[3H]-citrulline, and the conversion of l-[3H]-Arg to l-[3H]-ornithine in 10 μM l-Arg group was the lowest (P < 0.01) among other groups, while no significant differences were found among 100–400 μM l-Arg groups (Fig. 3b, c).
Effects of l-Arg on l-[3H]-Arg uptake and metabolism. Cells were cultured in 6-well plates (1 × 105 per well) supplemented with Arg-free DMEM containing 10, 100, 200, and 400 µM l-Arg for 4 days. a Total l-[3H]-Arg taken up by cells for the period of incubation. b l-[3H]-citrulline production from extracellular l-[3H]-Arg. c l-[3H]-ornithine production from extracellular l-[3H]-Arg. Bars represent mean ± SE of 4 experiments made in 4 replicates. a–c Means with different letters differ (P < 0.01)
Effects of l-Arg on CAT-1, CAT-4 and iNOS expression in chick IEC
The mRNA abundances of CAT-1 and CAT-4 are shown in Fig. 4a. There were no obvious changes in CAT-4 mRNA expression in response to the extracellular concentrations of l-Arg from 10 to 400 μM, while expression of CAT-1 in 100, 200, and 400 μM l-Arg groups were 2.12-fold (P < 0.01), 2.45-fold (P < 0.01) and 3.53-fold (P < 0.01) higher, respectively, than that in 10 μM l-Arg group. Similarly, relative protein level for CAT-1 in 100, 200, and 400 μM l-Arg groups were 1.36-fold (P < 0.01), 2.02-fold (P < 0.01), and 2.58-fold (P < 0.01) higher, respectively, than that in 10 μM l-Arg group (Fig. 5). Extracellular concentrations of l-Arg dose dependently upregulated (P < 0.01) the expression of iNOS mRNA as demonstrated by RT-PCR analysis (Fig. 4b), expression of which in 100, 200, and 400 μM l-Arg groups were 2.34-fold (P < 0.01), 2.98-fold (P < 0.01), and 3.67-fold (P < 0.01) higher, respectively, than that in 10 μM l-Arg group. There were no significant differences in iNOS protein abundance between 10 and 100 μM l-Arg groups or 200 and 400 μM l-Arg groups, while relative protein levels for iNOS in 200 and 400 μM l-Arg groups were 1.65-fold (P < 0.01)and 1.73-fold (P < 0.01) higher, respectively, than those in 10 μM l-Arg group (Fig. 6).
Effects of l-Arg on mRNA abundance. Cells were cultured in Arg-free DMEM containing 10, 100, 200, and 400 µM l-Arg for 4 days and total RNA was isolated from cells in each 6-well plate (1 × 105 per well). a mRNA expression of amino acid transporters (CAT-1 and CAT-4). b mRNA expression of iNOS. Data are expressed as mean ± SE, n = 4. a–d Means with different letters differ (P < 0.01)
Effects of l-Arg on the protein abundance of CAT-1 in chick intestinal epithelial cell. Cells were cultured in each 6-well plate (1 × 105 per well) supplementing with Arg-free DMEM containing 10, 100, 200, and 400 µM l-Arg for 4 days. Data are expressed as mean ± SE, n = 4. a–d Means with different letters differ (P < 0.01)
Discussion
Determining changes in cell number in a culture dish over time is the best available method to assess cell growth and viability [28]. By using MTT assay, we found that l-Arg concentrations at 10–400 μM enhanced cell growth. However, when extracellular concentrations of l-Arg in the medium increased from 400 to 600 μM, there was no further increase in cell growth (Fig. 1). Thus, we conducted all subsequent experiments with 10–400 μM l-Arg. Cell growth depends on the balance between protein synthesis and degradation, and l-Arg has been reported to improve cell survival and growth in several mammalian species [29]. Arginase catalyzes the irreversible conversion of arginine into ornithine, and the progressive depletion of arginine from the culture medium could potentially inhibit protein synthesis and cell growth [30]. Alternately, more l-Arg requirement of chick IEC could be that l-Arg is the substrate of the enzyme NOS for the production of NO. Therefore, the essentiality of l-Arg for cell growth was mainly due to its role as a constituent amino acid for protein synthesis and a precursor for NO production in chick IEC where l-Arg synthesis was incapable.
Arginine is a physiological precursor for NO production [31]. Our studies found that increasing extracellular l-Arg concentrations dose dependently increased concentrations of NO production in chick IEC. So, it is speculated that the availability of intracellular l-Arg was a rate-limiting factor in NO production. Meanwhile, the increased NO production was accompanied with the improvement of cell growth. This result was consistent with the previous studies which found that the elevated levels of NO could increase cell viability and inhibit cell apoptosis [32] because physiological levels of NO stimulate mucus secretion which acts as a physical barrier to damage as well as helps in protecting the epithelium from damage caused by acid and pepsin [33, 34], and is responsible for helping to maintain the integrity of the gastric epithelium and the mucus barrier [35].
In the present study, we found that the uptake of l-[3H]-Arg was increased with the increasing concentrations of extracellular l-Arg. Interestingly, the difference in l-[3H]-Arg uptake by chick IEC corresponded to greater l-[3H]-Arg conversation to l-[3H]-citrulline than l-[3H]-ornithine. These findings suggested that increasing extracellular concentrations of l-Arg increased the CAT-1-mediated l-Arg uptake and the intracellular metabolism of l-Arg involved more NOS forming l-citrulline. To the best of our knowledge, this is the first demonstration linking l-Arg uptake with l-Arg metabolism by chick IEC following the exposure of extracellular l-Arg. Our results correlated with our data on the effects of extracellular l-Arg on uptake of l-Arg transport in chick IEC (Figs. 2, 3) suggested that the transport of extracellular l-Arg and NO production are interrelated. Moreover, the production of l-[3H]-citrulline increased with the increasing extracellular concentrations of l-Arg, which might be related to the upregulation of iNOS (Fig. 4b).
iNOS, a calcium-independent enzyme that is upregulated in response to inflammation and other stimuli, remains activated for longer periods of time when compared with the other isoforms and generates a sustained increase in NO [36]. Despite a number of articles describing the expression of iNOS in macrophages and neutrophils, but with potent signals for induction, such as cytokines or bacterial lipopolysaccharides, it may also be observed in epithelial cells and neurons [17]. Current results showed that the mRNA abundance of iNOS increased with the increasing extracellular concentrations of l-Arg from 10 to 400 μM. l-Arg is the substrate of the enzyme NOS for the production of NO, which is thought to modulate intestinal water and electrolyte transport and mucosal permeability [37, 38] and has a role in maintaining intestinal microvascular integrity by modulating intestinal blood flow and inhibiting platelet aggregation and leukocyte adhesion for cell survival [39, 40]. However, other studies in mammalian IEC found that l-Arg-induced stimulation of cell survival was not mediated by NO [20, 38, 40, 41]; the variances in these findings might be attributed to the different animal species, and l-Arg might be nutritionally essential for other important functions related to NO in chick intestine rather than its role in protein synthesis.
The expression of amino acid transporters in chick small intestines during the stages of embryo completion (embryonic day 8 to 18) has been documented [25]. The fact that the expression of CAT-4 is not sufficient to induce amino acid transport activity in cells had been reported in many previous studies, and the mRNA abundance of CAT-4 was the lowest among the CAT transporters in chick embryos aged 9–19 [15, 25]. The mRNA expression of CAT-4 in IEC isolated from 14-day-old embryo was not affected by the extracellular concentrations of l-Arg from 10 to 400 μM, so we can suggest that CAT-4 was either not an amino acid transporter for arginine or that it may not be crucial for the embryos intestine. Of the known transport systems for cationic amino acids, system y+ has been represented by the CAT-1 transporter, which is likely to be the most relevant with regard to quantitative transport of l-Arg as it exhibits a high transport capacity [42], for example, it is responsible for 70–95 % of l-Arg uptake in endothelial cells [43, 44]. In this study, we found both mRNA and protein abundance of CAT-1 in 100, 200, or 400 μM l-Arg groups were significant higher than that in 10 μM l-Arg group, and this was accompanied by an increase in cell viability. This result suggested that extracellular l-Arg was involved in the regulation of chick intestinal CAT-1 transporter, which mediated l-Arg-uptake for cell growth, because CAT-1 allows cells to resume growth as soon as amino acids become available [45].
In conclusion, addition of 100, 200, or 400 μM l-Arg to the culture medium stimulated the growth of chick IEC. The effect of l-Arg on CAT-1 and iNOS levels would potentially increase the availability of extracellular l-Arg and the conversion of l-Arg to NO. Taken together, we suggest that the CAT-1 isoform plays a role in l-Arg uptake, and l-Arg-mediated elevation of NO via iNOS promotes the growth of chick IEC.
References
Alberts B, Bray D, Lewis J et al (2002) Molecular biology of the cell, 4th rev. edn. Garland Publishing Inc., New York
Rhoads JM, Wu GY (2009) Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 37:111–122
Rhoads JM, Chen W, Gookin J et al (2004) Arginine stimulates intestinal cell migration through a focal adhesion kinase dependent mechanism. Gut 53:514–522
Rhoads JM, Liu YY, Niu XM et al (2008) Arginine stimulates cdx2-transformed intestinal epithelial cell migration via a mechanism requiring both nitric oxide and phosphorylation of p70 S6 kinase. J Nutr 138:1652–1657
Tan B, Yin YL, Kong XF et al (2010) l-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids 38:1227–1235
D’Amato JL, Humphrey BD (2010) Dietary arginine levels alter markers of arginine utilization in peripheral blood mononuclear cells and thymocytes in young broiler chicks. Poult Sci 89:938–947
Pance A (2006) Nitric oxide and hormones in breast cancer: allies or enemies? Future Oncol 2:275–288
Wu GY, Morris SM (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Mann GE, Yudilevich DL, Sobrevia L (2003) Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev 83:183–252
Closs EI (2002) Expression, regulation and function of carrier proteins for cationic amino acids. Curr Opin Nephrol Hypertens 11:99–107
Verrey F, Closs EI, Wagner CA et al (2004) CATs and HATs: the SLC7 family of amino acid transporters. Pflug Arch Eur J Phy 447:532–542
Baydoun AR, Wileman SM, Wheeler-Jones CP et al (1999) Transmembrane signalling mechanisms regulating expression of cationic amino acid transporters and inducible nitric oxide synthase in rat vascular smooth muscle cells. Biochem J 344:265–272
Simmons WW, Closs EI, Cunningham JM et al (1996) Cytokines and insulin induce cationic amino acid transporter (CAT) expression in cardiac myocytes. Regulation of l-arginine transport and no production by CAT-1, CAT-2A, and CAT-2B. J Biol Chem 271:11694–11702
Vekony N, Wolf S, Boissel JP et al (2001) Human cationic amino acid transporter hCAT-3 is preferentially expressed in peripheral tissues. Biochemistry 40:12387–12394
Wolf S, Janzen A, Vekony N et al (2002) Expression of solute carrier 7A4 (SLC7A4) in the plasma membrane is not sufficient to mediate amino acid transport activity. Biochem J 364:767–775
Cho CH (2001) Current roles of nitric oxide in gastrointestinal disorders. J Physiol Paris 95:253–256
Wallace JL, Miller MJ (2000) Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology 119:512–520
Nathan C, Xie QW (1994) Nitric oxide synthases: roles, tolls, and controls. Cell 78:915–918
Yuan C, He Q, Li JM, Azzam MMM et al (2014) Evaluation of embryonic age and the effects of different proteases on the isolation and primary culture of chicken intestinal epithelial cells in vitro. Anim Sci J (in press)
Kong XF, Tan B, Yin YL et al (2012) l-arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J Nutr Biochem 23:1178–1183
Nelin LD, Nash HE, Chicoine LG (2001) Cytokine treatment increases arginine metabolism and uptake in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 281:1232–1239
Zharikov SI, Krotova KY, Belayev L, Block ER (2004) Pertussis toxin activates l-arginine uptake in pulmonary endothelial cells through downregulation of PKC-alpha activity. Am J Physiol Lung Cell Mol Physiol 286:974–983
Schapira RM, Wiessner JH, Morrisey JF et al (1998) l-arginine uptake and metabolism by lung macrophages and neutrophils following intratracheal instillation of silica in vivo. Am J Respir Cell Mol Biol 19:308–315
Humphrey BD, Stephensen BC, Calvert CC, Klasing KC (2006) Lysine deficiency and feed restriction independently alter cationic amino acid transporter expression in chickens (Gallus gallus domesticus). Comp Biochem Physiol A Mol Integr Physiol 143:218–227
Zeng PL, Li XG, Wang XQ et al (2011) The relationship between gene expression of cationic and neutral amino acid transporters in the small intestine of chick embryos and chick breed, development, sex, and egg amino acid concentration. Poult Sci 90:2548–2556
Li YP, Ingmer H, Madsen M, Bang DD (2008) Cytokine responses in primary chicken embryo intestinal cells infected with Campylobacter jejuni strains of human and chicken origin and the expression of bacterial virulence-associated genes. BMC Microbiol 8:107
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using Real-Time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408
Meininger CJ, Wu GY (2002) Regulation of endothelial cell proliferation by nitric oxide. Method Enzymol 352:280–295
Wu GY (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Caso G, Mcnurlan MA, Mcmillan ND et al (2004) Tumour cell growth in culture: dependence on arginine. Clin Sci 107:371–379
Blachier F, Davila AM, Benamouzig R, Tome D (2011) Channelling of arginine in NO and polyamine pathways in colonocytes and consequences. Front Biosci (Landmark Ed) 16:1331–1343
Abdelmagid SA, Too CK (2008) Prolactin and estrogen up-regulate carboxypeptidased to promote nitric oxide production and survival of mcf-7 breast cancer cells. Endocrinology 149:4821–4828
Allen A, Flemstrom G, Graner A, Kivilaakso E (1993) Gastroduodenal mucosal protection. Physiol Rev 73:823–857
Brown JF, Keates AC, Hanson PJ, Whittle BJ (1993) Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am J Physiol Gastrointest Liver Physiol 265:418–422
Lanas A (2008) Role of nitric oxide in the gastrointestinal tract. Arthritis Res Ther. doi:10.1186/ar2465
Nathan C (1997) Inducible nitric oxide synthase: what difference does it make? J Clin Invest 100:2417–2423
Kubes P (1992) Nitric oxide modulates epithelial permeability in the feline small intestine. Am J Physiol Gastrointest Liver Physiol 262:1138–1142
Barry MK, Aloisi JD, Pickering SP, Yeo CJ (1994) Nitric oxide modulates water and electrolyte transport in the ileum. Ann Surg 219:382–388
Hutcheson IR, Whittle BJ, Boughton-Smith NK (1990) Role of nitric oxide in maintaining vascular integrity in endotoxin-induced acute intestinal damage in the rat. Br J Pharmacol 101:815–820
Alican I, Kubes P (1996) A critical role for nitric oxide in intestinal barrier function and dysfunction. Am J Physiol Gastrointest Liver Physiol 270:225–237
Bauchart-Thevret C, Cui LW, Wu GY, Burrin DG (2010) Arginine-induced stimulation of protein synthesis and survival in IPEC-J2 cells is mediated by mTOR but not nitric oxide. Am J Physiol Endocrinol Metab 299:899–909
Strobel J, Mieth M, Endress B et al (2012) Interaction of the cardiovascular risk marker asymmetric dimethylarginine (ADMA) with the human cationic amino acid transporter 1 (CAT1). J Mol Cell Cardiol 53:392–400
Greene B, Pacitti AJ, Souba WW (1993) Characterization of l-arginine transport by pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 264:351–356
Zharikov SI, Block ER (1998) Characterization of l-arginine uptake by plasma membrane vesicles isolated from cultured pulmonary artery endothelial cells. BBA Biomembr 1369:173–183
Hatzoglou M, Fernandez J, Yaman I, Closs E (2004) Regulation of cationic amino acid transport: the story of the CAT-1 transporter. Annu Rev Nutr 24:377–399
Acknowledgments
This research was supported by the earmarked fund for Modern Argo-Industry Technology Research System of China (No. CARS-41-K17) and the Program for Zhejiang Leading Team of S&T Innovation (No. 2011R50025-04).
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yuan, C., Zhang, X., He, Q. et al. l-arginine stimulates CAT-1-mediated arginine uptake and regulation of inducible nitric oxide synthase for the growth of chick intestinal epithelial cells. Mol Cell Biochem 399, 229–236 (2015). https://doi.org/10.1007/s11010-014-2249-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2249-2