Abstract
Dietary arginine (Arg) could improve the intestinal structure and absorption of grass carp (Ctenopharyngodon idellus); however, the mechanism of Arg on intestinal morphology improvement was unclear. The present study aimed to explain the possible mechanism of the positive effect of Arg on intestinal epithelial cells of grass carp. An in vitro study was conducted through a primary culture model to assess the growth, cell viability, mRNA expressions of TOR signal pathway, and tight junction proteins of enterocytes after culture in the medium with 6 levels of Arg (0, 0.1, 0.2, 0.5, 1.0, and 2.0 mmol/L). The results showed that 0.5 mmol/L Arg improved the cell number and decreased the lactate dehydrogenase and creatine kinase activities in culture medium (P < 0.05). The alkaline phosphatase activity in cell lysis buffer was depressed by 1 and 2 mmol/L Arg (P < 0.05). The nitric oxide (NO) content showed an increasing trend with the Arg content (P < 0.05), whereas the NO synthase activity showed an opposite trend to NO. TOR expression was higher in 0.2 and 0.5 mmol/L groups, whereas S6K1 expression in 1.0 mmol/L and 2.0 mmol/L groups were lower (P < 0.05). The mRNA expressions of occludin, claudin 3, and claudin c in 0.5 mmol/L group were the highest, while ZO-1 and claudin b expressions were higher in 0.2 and 0.5 mmol/L groups (P < 0.05). This study indicated that Arg enhanced the growth and integrity of intestinal epithelial cells of grass carp through upregulation of mRNA expression of TOR signal pathway and tight junction proteins at an optimal Arg content of 0.2–0.5 mmol/L.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal epithelium, composed of a single layer of cells, shares two important functions: absorbing helpful substances and acting as a barrier against harmful substances (Günzel and Yu 2013), due to the presence of a brush border on the lumenal cell surface and tight junctions between the epithelial cells thus forming a relatively impermeable membrane (Rescigno et al. 2001). Tight junction, including occludin, claudin family, and zonula occludens (ZOs), is a highly dynamic intercellular junction with a complex multiprotein structure. A large family of transmembrane proteins called claudins was reported (Van Itallie and Anderson 2004). Structurally, tight junction is composed of transmembrane proteins (occludin and claudins) linked to the actin cytoskeleton via cytoplasmic ZO proteins (Anderson and Van Itallie 1995; Stevenson et al. 1986). The integrity of the intestinal epithelial barrier structure is inseparable from the health and nutrient absorption of the intestine (Xia et al. 2016).
Arginine (Arg), which is an essential amino acid for finfish species (Wilson 2002), shares multiple biological properties. More attention has been paid to the protective effect of Arg on intestinal health. Arginine has been shown to improve intestinal weight and mucosal cell proliferation, recover intestinal absorptive function (Sukhotnik et al. 2005), and reduce the apoptosis of intestinal epithelial cells (Wu et al. 2007). Arginine activates the target of mammalian rapamycin (mTOR) signaling pathway in intestinal cells when entering the cells (Corl et al. 2008; Rhoads et al. 2008). Besides, Arg serves as the substrate for synthesis of nitric oxide (NO) through the catalysis of NOS (Zhan et al. 2008), which can stimulate intestinal cell migration, maintain the intestinal mucosal structure integrity, and prevent the intestine from injuries (Koppelmann et al. 2012; Stettner et al. 2018). Arginine also can increase the gene expressions of the claudin-1 and occludin in porcine small intestinal epithelial cell line (Xia et al. 2016) and protect permeability and tight junction protein expressions (ZO-1 and occludin) in MTX-treated intestinal epithelial cells (Beutheu et al. 2013).
Leakage of the intracellular lactate dehydrogenase (LDH) and creatine kinase (CK) into the culture medium indicates cell damage (Fredsted et al. 2005). Alkaline phosphatase (AKP) is an indicator of enterocyte differentiation, in addition, cytosolic activities of Na+, K+-ATPase, and AKP reflect the absorptive function of enterocytes (Jiang et al. 2013).
Grass carp (Ctenopharyngodon idellus) is a widely cultured freshwater species with delicious taste and high production. Intestine is an important organ for digestion, absorption, transport of nutrients, and ion regulation (Grosell et al. 2011; Minghetti et al. 2017); the health of grass crap intestine is getting more attention. Promoting effects of dietary Arg on mRNA expressions of y+L amino acid transporters (y+LAT1 and y+LAT2) and peptide transporter-1 (PepT1), as well as villus morphology in foregut of grass carp have been confirmed (Chen 2017). However, information regarding the mechanism of Arg on intestinal morphology of grass carp was unknown. The objective of the present study was to investigate the effects of different Arg contents in culture media on cell growth, viability, mRNA expressions of TOR signal pathway, and tight junction proteins of intestinal epithelial cells of grass carp by in vitro primary culture, so as to demonstrate the protective mechanism of Arg on intestinal health.
Materials and methods
Culture reagents
Arginine-free high-glucose formulation Dulbecco’s Modified Eagle’s Medium (DMEM) medium (1 × concentrate) was purchased from Gibco Life Technologies (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from (Sciencell Research Laboratories, Carlsbad, CA). Additives to some of the media included glutamine (Aladdin), lysine (Aladdin), arginine hydrochloride (Aladdin), epidermal growth factor (EGF; Sigma), and fibroblast growth factor (FGF; Sigma); penicillin streptomycin solution (100×; Hyclone); gentamycin sulfate (50 mg/mL; Sigma); solubilized amphotericin B (0.25 mg/mL; Biosharp); collagenase I and collagenase IV (Sigma); and Poly-lysine (Sigma). All reagents were cell culture grade.
Animals
Grass carp (about 1 kg body weight) for isolation of intestinal epithelial cells were cultured in an indoor aquarium for 2 weeks with continuous aeration before use. Healthy fish were starved for 24 h before they were sacrificed for enterocytes isolation. Animal treatments in the present study were conducted under principles of good laboratory animal care and were approved by the Ethical Committee for Laboratory Animals Care and Use of Huazhong Agricultural University.
Cell isolation and cell culture
Isolation of the intestinal epithelial cells for primary culture was conducted according to the methods described by Li et al. (2016) with slight modifications. In brief, fish were anesthetized with tricaine methanesulfonate (MS-222) in dechlorinated water, cleared of blood from the caudal vein with an injector, sterilized by 75% ethanol, and then dissected under sterile conditions. The foregut and midgut were rapidly removed from the carcass, opened and rinsed with ice-cold phosphate buffer solution (PBS) to wash the intestinal lumen three times, followed by soaking in DMEM solution containing 3% FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin, 12.5 μg/mL amphotericin B, and 500 μg/mL gentamycin sulfate for 2 h. After that, the intestine was minced into <1 mm3 pieces. Then, the enterocytes were isolated by adding digestion solution (PBS with 0.1 mg/mL of collagenase I and 0.1 mg/mL of collagenase IV respectively, filtered with 0.22 uM and stored at − 20 °C before use) and shaking at 28 °C for 30 min. After then, digestion was terminated by adding FBS at a volume ratio of 1:19 (FBS: digestion solution). Cells were cleared, collected, and resuspended in a DMEM solution containing 3% FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin, 12.5 μg/mL amphotericin B, and 500 μg/mL gentamycin sulfate.
Cells were plated at a density of 1.3 × 105 viable cells/cm2 in 24-well polylysine (0.1 mg/mL)-coated microplates for viability and enzyme activity assays, or 1.0 × 105 viable cells/cm2 in 6-well polylysine-coated microplates for gene expression. The cells were suspended in modified Arg-free DMEM medium containing 15% FBS, 3% sorbitol, 2 μg/mL EGF, 25 ng/mL FGF, 100 IU/mL penicillin, 100 μg/mL streptomycin, 12.5 μg/ml amphotericin B, and 500 μg/ml gentamycin sulfate. On the following days, the cells were incubated in different media of Arg-free DMEM supplemented with different concentrations of Arg (0, 0.1, 0.2, 0.5, 1, and 2 mmol/L) to attach and grow at 28 ± 0.5 °C for various time in a humidified atmosphere incubator (Jinghong Laboratory Instrument Co., Ltd., Shanghai, China). The optimal culture time for fish primary enterocytes was firstly established based on cell growth with trypan blue staining and cell viability assays results by sampling at 24 h, 48 h, 72 h, and 96 h after Arg treatment. After then, cells were cultured in various concentrations of Arg for 72 h (optimal culture time) for further sampling and assays. Four wells were used for each treatment. After the indicated time of incubation, the culture media and the cells were collected separately and stored at − 80 °C immediately for various assays.
Assays of cell growth and viability
Samples of each group at 24 h, 48 h, 72 h, and 96 h after Arg treatment were subject to cell growth and viability assays. The growth of cells was determined by counting cells with a hemocytometer after 0.4% trypan blue staining. Analysis of enterocyte viability was performed by an MTT assay as described by Fukamachi (1992).
Enzyme activity assays
After 72 h primary culture, the activities of lactic dehydrogenases (LDH) and creatine kinase (CK) in the culture media were determined to assess membrane integrity using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. The collected cells were treated by ultrasonic wave to release the proteins for activity assays of AKP, Na+, K+-ATPase, nitric oxide synthase (NOS), and NO using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China).
mRNA expression detection of TOR signal pathway and tight junction protein
Relative quantification of the target gene transcripts was made using β-actin and GAPDH as reference genes and 0 mmol/L Arg as the control according to the optimized comparative Ct (2−△△ct) value method described by Schmittgen and Livak (2008). For each target gene, specific primer pairs (Table 1) were designed through Primer Premier 5.0 software using known sequences in NCBI database. Total RNA was isolated from the cell with RNAiso Plus Kit (Takara, Dalian, China) according to the manufacturer’s instructions. RNA quality and quantity were assessed by electrophoresis on 1% agarose gel and by NanoDrop® ND-1000 UV-vis spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at 260 nm and 280 nm (260/280). One microgram of total RNA was used for reverse transcription with PrimeScript™ RT reagent Kit (Takara, Dalian, China) according to the manufacturer’s recommendations. Quantitative real time PCR analyses for mRNA expressions were performed using a quantitative thermal cycler (Light Cycler 480II, Roche) with the UltraSYBR Master Mix (CWBIO, China) in a total volume of 20 μL reaction mixture containing reverse transcriptase. The PCR parameters were 40 cycles at 95 °C for 5 s, annealing temperature (according to specific primer pairs) for 10 s and 72 °C for 15 s, with an additional initial 30 s pre-denaturation step at 95 °C. Melting curves were systematically monitored (temperature gradient at 0.5 °C/s from 55 to 94 °C) at the end of the last amplification cycle to confirm that only one fragment was amplified.
Statistics
Values were expressed as mean ± SE of four replicates. Factorial ANOVA was performed to assess the effects of culture duration and Arg content on cell growth and viability. The effects of Arg treatment were assessed by one-way ANOVA and differences between group means were compared by post-hoc Duncan’s tests with the SPSS 19 (IBM Inc., Chicago, IL, USA) computer program for Windows. Differences between treatments were considered significantly when P < 0.05 occurred.
Results
Growth and cell viability of intestinal epithelia cells
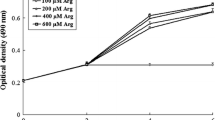
The effect of Arg on growth and cell viability of enterocytes of grass carp was shown in Fig. 1 and Table 2. Factorial ANOVA results showed that culture duration and Arg content in the media both significantly affected the cell growth and cell viability, and significant interaction between culture duration and Arg content was observed for cell growth and cell viability (P < 0.05). The live cell number (Fig. 1) showed a first increasing then decreasing trend as the culture time prolonged and was highest at 72 h after treatments (P < 0.05). Multiple range tests showed that the optimal Arg content for cell growth was 0.5 mmol/L. Table 2 also showed that cell viability in all Arg treated groups showed a first increasing trend from 0 to 72 h and then kept stable at 96 h (P < 0.05), while the effect of Arg content on cell viability varied at the different sampling time.
Growth of intestinal enterocytes of grass carp responding to culture days and arginine contents. Values are presented as Mean ± SE (n = 4). Two-way factorial ANOVA indicated that an interaction between time and Arg contents on the cell growth (cell number) (P < 0.05). Cell growth between Arg treatments at 72 h was compared by one-way ANOVA; the values with different letters mean significant difference (P < 0.05)
Effect of Arg on enzyme activities in culture medium and lysate solution of intestine epithelial cell of grass crass
The effects of Arg on LDH, CK activity in the culture media after 72 h primary culture of grass crass enterocytes are presented in Table 3. LDH activity decreased first then increased as the Arg content in culture medium increased, which showed higher values in Arg-free and 2 mmol/L groups than other groups, and was significantly lower in 0.5 mmol/L group (P < 0.05). The same trend was shown in CK activity, except that CK activity was significantly lower in 0.2 mmol/L and 0.5 mmol/L groups (P < 0.05).
The activities of AKP, Na+, K+-ATPase, NOS, and NO content in cell lysis solution were shown in Table 4. The AKP activity was depressed by higher Arg content (1 mmol/L and 2 mmol/L) (P < 0.05). NO content showed an increasing trend as the culture medium Arg content increased, which was highest in 2 mmol/L group (P < 0.05). In contrast, NOS activity showed an opposite trend to NO content, which was the lowest in 2 mmol/L group (P < 0.05). Na+, K+-ATPase was not affected by Arg content (P > 0.05).
Effect of Arg on mRNA expressions of TOR signal pathway and tight junction proteins
As shown in Fig. 2, the gene expression levels of tor and s6k1 in enterocytes cultured for 72 h in media with different Arg content both showed first increasing and then decreasing trends as the Arg content increased (P < 0.05). The TOR expression was highest in 0.5 mmol/L and lowest in Arg-free and 2 mmol/L groups (P < 0.05). Accordingly, the expression levels of s6k1 in 1.0 mmol/L and 2.0 mmol/L groups were significantly lower than in other groups (P < 0.05). However, the expression of 4e-bp1 was not affected by Arg content in culture media (P > 0.05),
The gene expression levels of tight junction proteins in enterocytes all showed first increasing and then decreasing trends as Arg content in culture medium increased (Fig. 3). zo-1 expression in 0.2 mmol/L and 0.5 mmol/L groups were significantly higher than those in other groups (P < 0.05), and occludin expression level in 0.5 mmol/L group was significantly higher than those in 1.0 mmol/L and 2.0 mmol/L groups (P < 0.05). The expression levels of claudin b in 0.1 mmol/L, 0.2 mmol/L, and 0.5 mmol/L groups were significantly higher than that in 2.0 mmol/L group (P < 0.05). Claudin 3 level in 0.5 mmol/L group was significantly higher than those in Arg-free, 0.1 mmol/L, and 2.0 mmol/L groups. The highest expression values of claudin c, claudin 12, and claudin 15a were all observed in 0.5 mmol/L group (P < 0.05).
Discussion
Intestinal epithelial cells play an important barrier function for intestine protection, and are easily affected by dietary nutritional status and environmental factors (Gewirtz et al. 2002). Our previous study has shown that dietary Arg improved intestinal morphology and mRNA expression of amino acid transporters of juvenile grass carp (Chen 2017). The present study tried to elucidate the mechanism of Arg on intestinal health of grass carp by an in vitro primary culture model. The suitable Arg concentration and culture duration for cell culture of grass carp enterocytes were firstly investigated in this study by the determination of cell growth and viability. Results indicated that Arg content and culture duration both significantly affected cell growth, which is similar to the results in IPEC-1 cells reported by Tan et al. (2010). The cell growth and cell viability in this study increased first and then decreased as the primary culture time extended, and reached the peak at 72 h. So cell culture for 72 h in media with different Arg concentrations was selected for further studies. In addition, the variation of MTT value between different Arg treatments that was not consistent at different time might be due to the strong interaction between culture duration and Arg content, and the liability of the primary cells.
Arginine has been reported to stimulate cell proliferation and protein synthesis (Tan et al. 2010). The current study supported this as cell growth assessed by trypan blue staining increased with Arg content (from 0 to 0.5 mmol/L), whereas excess Arg in the media tended to depress the growth of enterocyte. The results suggest that an adequate concentration of Arg is critical for optimal intestinal protein synthesis, cell migration, and intestinal integrity of Cdx2-IEC (intestinal epithelial cell) (Rhoads et al. 2008). The proliferation of enterocytes may directly affect the formation of intestinal folds (Jiang et al. 2015). Thus, the present result may partly explain our observation that dietary Arg improved intestinal morphology of grass carp (Chen 2017).
The positive effects of Arg on cell growth and proliferation are correlated to some enzyme activities. LDH is highly sensitive to assess the cell injury in various cell types, including intestinal epithelial cell lines (Aherne et al. 2007; Maher et al. 2010). CK activity is commonly used to reflect the integrity of cell structure when released from cells (Hurst et al. 2010). In this study, at the 72-h time point, LDH activity was significantly lower in 0.1, 0.2, 0.5, and 1 mmol/L groups than that in 0 and 2 mmol/L groups, while CK activity in culture medium was significantly lower in 0.2 and 0.5 mmol/L groups than that in other groups, which indicates that optimal Arg contents can protect the cell integrity and reduce leakage of the membrane-bound enzymes LDH and CK into culture supernatants. This is consistent with the finding that supplementation of 100 uM and 350 uM Arg in the culture medium showed protective effect on the LPS-treated porcine intestinal epithelial cells (Tan et al. 2010). Intestinal AKP, a marker enzyme of the brush border membrane (Villanueva et al. 1997), reflects the epithelium differentiation (He et al. 1993; Jiang et al. 2013). The AKP activity in the cell lysis solution dropped in 1 and 2 mmol/L groups compared with other groups in this study, which suggests that the high levels of Arg over 1 mmol/L may be detrimental to the differentiation of grass carp enterocytes. This could also explain that the cell growth counting after 0.4% trypan blue staining in the higher Arg levels group seemed to be depressed compared to the 0.5 mmol/L group. Similar result was reported that a high Arg concentration (3.6 mmol N L−1) inhibited the growth of M. aeruginosa (Dai et al. 2019).
Arginine may exert its protective action on cells through NO- and polyamine-mediated mechanisms to repair the damaged cells and tissues (Tan et al. 2010). NO is endogenously synthesized from Arg as substrate in the vascular endothelium by NOS (Ramprasath et al. 2012; Stettner et al. 2018). Arginine has been reported to regulate the cardiovascular function through the inhibition of cell apoptosis by NO (Alef et al. 2011). In this study, the NO content in the cell lysate solution of intestinal epithelial cells showed an increasing trend with the increase of Arg content, while the NOS activity showed an opposite trend, which indicates that exogenous Arg could promote the proliferation of enterocytes of grass carp through NO-mediated mechanism to reduce the cell damage. However, when comparing the inhibited cell growth and the increasing NO content in 1 and 2 mmol/L groups, the results indicate that high levels of Arg might exhibit detrimental effect on cell proliferation through NO-independent effect (Tan et al. 2010). The negative correlation between Arg content and NOS activity in this study is consistent with the previous result that Arg negatively regulates NOS activity (Munder et al. 1999), and the reduced NOS activity might be caused by the negative feedback of NO.
Cell growth depends on the balance between protein synthesis and degradation (Wu 2009). Our results indicate that the mechanisms responsible for the stimulatory effect of Arg on protein accretion in enterocytes of grass carp involve the activation of cell signaling pathways that regulate the machinery of intracellular protein turnover. Similarly, optimal dietary Arg supplementation upregulates the fish growth by activating the TOR signaling pathway in the muscle and hepatopancreas (Chen et al. 2012; Chen 2017; Tu et al. 2015). Growing evidences show that Arg can activate mTOR and two key downstream targets, S6K1 and 4E-BP1, in intestinal epithelial cells (Hidetoshiban et al. 2004; Rhoads et al. 2006). Consistent with these reports, our results indicate that Arg supplementation to the culture medium can promote the enterocytes proliferation by activating the TOR signaling pathway as TOR and S6K1 expressions tended to increase at the concentration of 0.5 mmol/L. However, higher Arg level in culture medium depressed the TOR pathway expression, which is consistent with the depressed cell growth and further supports that Arg-regulating cell growth involves the TOR signaling pathway to regulate the protein synthesis.
Intestinal mucosa is covered by a single layer of epithelial cells that are inaccessible to pathogens due to the presence of a brush border on the lumenal cell surface and of tight junctions between cells to form an impermeable membrane (Rescigno et al. 2001). Tight junction seals cells to create the primary barrier to the diffusion of solutes through the paracellular pathway (Tsukita and Tstlkita 1993), which is important in the maintenance of epithelial integrity. Claudins are required to restrict the paracellular diffusion of small water-soluble molecules. To date, the claudin family has been identified to contain 24 members, and most epithelia simultaneously express several different claudin isoforms (Günzel and Yu 2013). Claudin 3, claudin 12, claudin 15a, claudin b, and claudin c expression in intestine of grass carp had been previously detected (Luo et al. 2014). In this study, expressions of ZO-1, occludin, claudin 3, claudin 12, claudin 15a, claudin b, and claudin c were all significantly upregulated by optimal Arg in culture medium and peaked at 0.5 mmol/L group, which indicated that optimal Arg content could enhance the barrier function of intestine, because the downregulation of claudin expressions in mouse mammary cell line has been reported to cause the decrease in barrier function (Reiter et al. 2006). Consistent results have been reported that Arg can protect integrity of gill epithelial cell barrier structure by regulating tight junction protein gene expression in grass carp (Wang et al. 2015), and can prevent the reduction of ZO-1 and occludin gene expression in methotrexate-treated Caco-2 cells (Beutheu et al. 2013). All these results suggest that Arg can upregulate gene expressions of occludin and claudins to enhance the “kisses” structure, which closely interrelate with ZO-1 to maintain the integrity and function of the intestine epithelial cells. This may partially explain that dietary Arg improved intestinal morphology of grass carp in our previous study (Chen 2017).
In conclusion, the current study suggests that Arg can affect the cell growth, proliferation, and integrity of intestine epithelial cell of grass carp through regulation on TOR signal pathways and tight junction proteins expression based on the intestine epithelial cell primary culture. Inclusion of 0.2–0.5 mmol/L Arg in the culture medium enhanced the expressions of TOR and tight junction protein mRNA levels so as to increase the cell growth and integrity of enterocytes.
References
Aherne SA, Kerry JP, O'brien NM (2007) Effects of plant extracts on antioxidant status and oxidant-induced stress in Caco-2 cells. Br J Nutr 97:321–328
Alef MJ, Vallabhaneni R, Carchman E, Morris SM Jr, Shiva S, Wang Y, Kelley EE, Tarpey MM, Gladwin MT, Tzeng E, Zuckerbraun BS (2011) Nitrite-generated NO circumvents dysregulated arginine/NOS signaling to protect against intimal hyperplasia in Sprague-Dawley rats. J Clin Invest 121:1646–1656
Anderson JM, Van Itallie CM (1995) Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol-Gastr L 269:G467–G475
Beutheu S, Ghouzali I, Galas L, Déchelotte P, Coëffier M (2013) Glutamine and arginine improve permeability and tight junction protein expression in methotrexate-treated Caco-2 cells. Clin Nutr 32:863–869
Chen J (2017) Effects of arginine on growth performance, intestinal morphology regulation and mechanism research of juvenile grass carp (Ctenopharyngodon idellu). Dissertation. Huazhong Agricultural University of China
Chen G, Feng L, Kuang S, Liu Y, Jiang J, Hu K, Jiang W, Li S, Tang L, Zhou X (2012) Effect of dietary arginine on growth, intestinal enzyme activities and gene expression in muscle, hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Br J Nutr 108:195–207
Corl BA, Odle J, Niu XM, Moeser AJ, Gatlin LA, Phillips OT, Blikslager AT, Rhoads JM (2008) Arginine activates intestinal p70S6k and protein synthesis in piglet rotavirus enteritis. J Nutr 138:24–29
Dai R, Zhou Y, Chen Y, Zhang X, Yan Y, An D (2019) Effects of arginine on the growth and microcystin-LR production of Microcystis aeruginosa in culture. Sci Total Environ 651:706–712
Fredsted A, Mikkelsen UR, Gissel H, Clausen T (2005) Anoxia induces Ca2+ influx and loss of cell membrane integrity in rat extensor digitorum longus muscle. Exp Physiol 90:703–714
Fukamachi H (1992) Proliferation and differentiation of fetal rat intestinal epithelial cells in primary serum-free culture. J Cell Sci 103:511–519
Gewirtz AT, Liu Y, Sitaraman SV, Madara JL (2002) Intestinal epithelial pathobiology: past, present and future. Best Pract Res Clin Gastroentero 16:851–867
Grosell M, Farrell AP, Brauner CJ (eds) (2011) The multifunctional gut of fish. Elsevier Inc, Amsterdam
Günzel D, Yu ASL (2013) Claudins and the modulation of tight junction permeability. Physiol Rev 93:525–569
He Y, Chu SH, Walker WA (1993) Nucleotide supplements alter proliferation and differentiation of cultured human (Caco-2) and rat (IEC-6) intestinal epithelial cells. J Nutr 123:1017–1027
Hidetoshiban KS, Yamatsuji T, Gunduz M, Tanaka N, Naomoto Y (2004) Arginine and leucine regulate p70S6 kinase and 4E-BP1 in intestinal epithelial cells. Int J Mol Med 13:537–543
Hurst RD, Wells RW, Hurst SM, McGhie TK, Cooney JM, Jensen DJ (2010) Blueberry fruit polyphenolics suppress oxidative stress-induced skeletal muscle cell damage in vitro. Mol Nutr Food Res 54:353–363
Jiang W, Kuang S, Liu Y, Jiang J, Hu K, Li S, Tang L, Feng L, Zhou X (2013) Effects of myo-inositol on proliferation, differentiation, oxidative status and antioxidant capacity of carp enterocytes in primary culture. Aquac Nutr 19:45–53
Jiang J, Shi D, Zhou XQ, Yin L, Feng L, Liu Y, Jiang WD, Zhao Y (2015) Effects of glutamate on growth, antioxidant capacity, and antioxidant-related signaling molecule expression in primary cultures of fish enterocytes. Fish Physiol Biochem 41:1143–1153
Koppelmann T, Pollak Y, Mogilner J, Bejar J, Coran AG, Sukhotnik I (2012) Dietary L-arginine supplementation reduces methotrexate-induced intestinal mucosal injury in rat. BMC Gastroenterol 12:1–9
Li XY, Liu Y, Jiang WD, Jiang J, Wu P, Zhao J, Kuang SY, Tang L, Tang WN, Zhang YA, Zhou XQ, Feng L (2016) Co- and post-treatment with lysine protects primary fish enterocytes against Cu-induced oxidative damage. PLoS One 11:e0147408
Luo JB, Feng L, Jiang WD, Liu Y, Wu P, Jiang J, Kuang SY, Tang L, Zhang YA, Zhou XQ (2014) The impaired intestinal mucosal immune system by valine deficiency for young grass carp (Ctenopharyngodon idella) is associated with decreasing immune status and regulating tight junction proteins transcript abundance in the intestine. Fish Shellfish Immunol 40:197–207
Maher S, Devocelle M, Ryan S, McClean S, Brayden DJ (2010) Impact of amino acid replacements on in vitro permeation enhancement and cytotoxicity of the intestinal absorption promoters, melittin. Int J Pharm 387:154–160
Minghetti M, Drieschner C, Bramaz N, Schug H, Schirmer K (2017) A fish intestinal epithelial barrier model established from the rainbow trout (Oncorhynchus mykiss) cell line, RTgutGC. Cell Biol Toxicol 33(6):539–555
Munder M, Eichmann K, Morán JM, Centeno F, Soler G, Modolell M (1999) Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol 16:3771–3777
Ramprasath T, Kumar PH, Puhari SSM, Murugan PS, Vasudevan V, Selvam GS (2012) l-Arginine ameliorates cardiac left ventricular oxidative stress by upregulating eNOS and Nrf2 target genes in alloxan-induced hyperglycemic rats. Biochem Biophys Res Commun 428:389–394
Reiter B, Kraft R, Günzel D, Zeissig S, Schulzke JD, Fromm M, Harteneck C (2006) TRPV4-mediated regulation of epithelial permeability. FASEB J 20:1802–1812
Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P (2001) Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2:361–367
Rhoads JM, Niu X, Odle J, Graves LM (2006) Role of mTOR signaling in intestinal cell migration. Am J Physiol-Gastr L 291:G510–G517
Rhoads JM, Liu Y, Niu X, Surendran S, Wu G (2008) Arginine stimulates cdx2-transformed intestinal epithelial cell migration via a mechanism requiring both nitric oxide and phosphorylation of p70S6 kinase. J Nutr 138:1652–1657
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
Stettner N, Rosen C, Bernshtein B, Gur-Cohen S, Frug J, Silberman A, Sarver A, Carmel-Neiderman NN, Eilam R, Biton I, Pevsner-Fischer M, Zmora N, Brandis A, Halpern KB, Mazkereth R, di Bernardo D, Brunetti-Pierri N, Premkumar MH, Dank G, Nagamani SCS, Jung S, Harmelin A, Erez A (2018) Induction of nitric-oxide metabolism in enterocytes alleviates colitis and inflammation-associated colon cancer. Cell Rep 23:1962–1976
Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA (1986) Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 103:755–766
Sukhotnik I, Helou H, Mogilner J, Lurie M, Bernsteyn A, Coran AG, Shiloni E (2005) Oral arginine improves intestinal recovery following ischemia-reperfusion injury in rat. Pediatr Surg Int 21:191–196
Tan B, Yin Y, Kong X, Li P, Li X, Gao H, Li X, Huang R, Wu G (2010) l-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids 38:1227–1235
Tsukita S, Tstlkita S (1993) Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123:1777–1788
Tu Y, Xie S, Han D, Yang Y, Jin J, Zhu X (2015) Dietary arginine requirement for gibel carp (Carassis auratus gibelio var. CAS III) reduces with fish size from 50g to 150g associated with modulation of genes involved in TOR signaling pathway. Aquaculture 449:37–47
Van Itallie CM, Anderson JM (2004) The role of claudins in determining paracellular charge selectivity. Proc Am Thorac Soc 1:38–41
Villanueva J, Vanacore R, Goicoechea O, Amthauer R (1997) Intestinal alkaline phosphatase of the fish Cyprinus carpio: regional distribution and membrane association. J Exp Zool 279:347–355
Wang B, Feng L, Jiang WD, Wu P, Kuang SY, Jiang J, Tang L, Tang WN, Zhang YA, Liu Y, Zhou XQ (2015) Copper-induced tight junction mRNA expression changes, apoptosis and antioxidant responses via NF-κB, TOR and Nrf2 signaling molecules in the gills of fish: preventive role of arginine. Aquat Toxicol 158:125–137
Wilson RP (2002) Protein and amino acids. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Elsevier Science, San Diego, USA, pp 144–179
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu CT, Ren YF, Liu JF, Luo YQ, Liao KX (2007) l-Arginine reduces intestinal epithelial cell apoptosis in rats with severe abdominal infection. J S Med Univ 27:1728–1730 (In Chinese with English abstract)
Xia M, Ye L, Hou Q, Yu Q (2016) Effects of arginine on intestinal epithelial cell integrity and nutrient uptake. Br J Nutr 116:1675–1681
Zhan Z, Ou D, Piao X, Kim SW, Liu Y, Wang J (2008) Dietary arginine supplementation affects microvascular development in the small intestine of early-weaned pigs. J Nutr 138:1304–1309
Acknowledgements
This study was supported by Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry of China, the Fundamental Research Funds for the Central Universities (2662016PY105), and National Undergraduate Innovation and Entrepreneurship Training Plan of Huazhong Agricultural University (201510504044).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were conducted under principles of good laboratory animal care and were approved by the Ethical Committee for Laboratory Animals Care and Use of Huazhong Agricultural University.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, J., Zhang, D., Tan, Q. et al. Arginine affects growth and integrity of grass carp enterocytes by regulating TOR signaling pathway and tight junction proteins. Fish Physiol Biochem 45, 539–549 (2019). https://doi.org/10.1007/s10695-019-00613-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-00613-w