Abstract—
Tensile stresses present in the pipe steels exposed to corrosive environment can result in the corrosion cracking of the pipe material. The standard procedure used for assessing the susceptibility of steels to stress corrosion cracking (for about 720 h) often does not fully provide insight into the characteristics of the material. The goal of the study is to develop a more rapid test procedure, which can provide reliable and complete information about the material placed in a corrosive environment under stress. Accelerated test for stress corrosion cracking of pipe steels with a relative strain rate of ~10–6 s–1 is proposed. The results of testing two materials at different deformation rates placed in different corrosive environments are presented. The tensile diagrams of the specimens tested in air and corrosive environments containing hydrogen sulfide and carbon dioxide, as well as the measurement of the relative elongation and relative contraction of fractured specimens, were used to determine a degree of the susceptibility of the pipe steels, which differ in the strength characteristics to stress corrosion cracking. It is shown that the degree of susceptibility of steel to stress corrosion cracking depends on the characteristics of the corrosive environment and the strength of the pipe steel. Tests under low rate of loading compared to tests with static load of the specimens revealed reduced duration of analysis from 720–1000 to 25–100 h.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Stress corrosion cracking (SCC) is observed for most structural metal materials in various corrosive environments [1–4]. In this regard, great attention is paid to the study of the reasons leading to SCC of structural steels and alloys, and the development of test methods that allow determining the boundary conditions under which the susceptibility to SCC does not appear.
In the oil and gas industry, where steels are used that differ in chemical composition, structure and strength level, the main component of the external environment that can cause SCC of pipe steels is an aqueous solution, containing dissolved aggressive substances, that accompanies the main transported product (oil and gas). These substances include dissolved salts, most of which are chlorides, as well as dissolved gases such as hydrogen sulfide and carbon dioxide.
The most dangerous is hydrogen sulfide. In an aqueous solution on the surface of iron, hydrogen sulfide behaves like acid and interacts with iron as follows [5–7]:
This process can be interpreted as an electrochemical one consisting of an anodic reaction
and cathodic reaction
The resulting FeS has poor solubility and can be represented as products of ion concentrations:
Therefore, it is precipitated on the iron surface and prevents further corrosion of the steel. However, if the medium is acidic, then FeS will pass into solution:
In solution, H2S dissociates with the formation of H+ and SH– ions
Hydrogen ions are discharged by the reaction (3) and atomic hydrogen is formed on the surface of the metal. SH– sulfide ions prevent the hydrogen atoms from joining into molecules. This leads to a significant part of the atomic hydrogen penetrates into the metal. The susceptibility of pipe steels to hydrogen sulfide stress cracking (SSC) is possible if the partial pressure of hydrogen sulfide is more than 0.35 kPa.
At the end of the 20th century, it was found that low-alloy steels undergo SCC in aqueous solutions containing dissolved carbon dioxide [8–11]. The sensitivity of the investigated steels to SCC in the H2O–CO2 system increases with the addition of up to 5% sodium chloride to the solution and with an increase in the yield stress of the steel. However, SCC requires higher levels of applied stress and plastic deformation than SSC in the presence of H2S. Moreover, cracking in all cases is intergranular.
The existing methods for testing steels and alloys for stress corrosion cracking (by the type of specimens, the composition of the corrosive environment, by the test conditions—temperature, pressure, external polarization) can be divided into three groups depending on the method of creating a stressed state in specimens. These are tests under constant strain, constant load, or constant strain rate.
The first two types of tests are widely used for material performance testing in terms of resistance SCC and SSC under the conditions of operating loads of a certain level and a specific corrosive environment. Constant load testing is also used to determine tensile stress thresholds [12] or stress intensity factor KISCC for fatigue crack specimens [13], below which the material does not show the susceptibility to corrosion cracking. The main disadvantage of these methods is the test duration, varying from 720 to 1000 hours, in some cases several thousand hours.
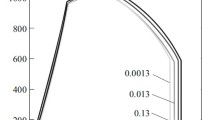
The slow strain rate test (SSRT) method has been used to determine the susceptibility to SCC of steels and alloys since the second half of the 20th century. The development of corrosion cracks under static loading of specimens and loading at a slow speed is established to proceed according to a single mechanism. In addition, data obtained using different loading methods allows to compare different materials according to the degree of SCC susceptibility [14, 15]. When performing stress corrosion cracking tests by the SSRT method, there are two options for the effect of the strain rate on the susceptibility of metal materials to stress corrosion cracking [16], as shown in Fig. 1 [17].
For aqueous solutions of electrolytes, the initiation and growth of cracks during stress corrosion cracking begins with the anodic dissolution of the most active areas on the metal surface (non-metallic inclusions, mechanical damage, etc.). If a protective oxide film is formed on metal in a corrosive environment, then local corrosion hot spots may occur at a certain deformation rate equal to or slightly higher than the recovery rate of this film, which is destroyed under the action of tensile stresses (deformation). Due to the growing stresses, some local hot spots are transformed into a corrosion crack, the growth rate of which will depend on the rate of alternating stages of anodic dissolution and stress growth (plastic deformation at the tip of a developing crack). Corrosion cracking will be difficult or completely impossible if the test strain rate is lower than the recovery rate of a locally breaking oxide film. At a high deformation rate, the film on the surface begins to break down, but the corrosion processes will not have time to lead to the appearance of a crack, and simply mechanical destruction will be observed when the ultimate strength of the material is exceeded (Fig. 1, curve 1). Such an effect of the strain rate on the susceptibility to stress corrosion cracking is characteristic of steels and alloys with the ability to passivate, for example, aluminum and titanium alloys, stainless steels. A similar character was found in low-alloy steels when tested in a carbonate solution and a weakly alkaline medium with polarization in a narrow potential range, where an unstable passive film is formed [18].
In acidic saline water solutions, low-alloy steels do not form a protective oxide film in the presence of H2S or CO2. The development of cracks from local surface defects occurs due to the diffusion of atomic hydrogen and embrittlement of the metal, i.e. reduction of plastic deformation at the crack tip, resulting in crack breakthrough by the size of the embrittled layer. Since all low alloy steels have a bcc crystal lattice, the hydrogen diffusion rate will be approximately the same regardless of the strength level of the steel. Hence, the rate of deformation, i.e. the time required to reach the maximum hydrogen concentration is also approximately the same. In this case, the rate of deformation should be such that, during the tests, the achievement of the maximum oxygen concentration inside the metal and the magnitude of stresses (strains) sufficient for crack growth was ensured. Such a development of events will lead to the susceptibility of steel to stress corrosion cracking to a gradual increase up to the maximum value, after which it will no longer change (Fig. 1, curve 2).
The purpose of this research is to develop a methodology for accelerated testing of pipe steels for sulfide and stress corrosion cracking, the duration of which does not exceed 100 hours. To rank steels that slightly differ in chemical composition, according to their tendency to such types of corrosion damage, it was necessary to select appropriate sensitive evaluation criteria, as well as threshold values of these criteria, allowing the steel to be considered suitable for using in environments containing H2S or CO2.
RESEARCH METHODS
The studies were carried out on two pipe steels of different strength—34KhMA and SAWL485FD with yield strengths of 980 and 540 MPa. Both steels are low-alloy; their chemical composition is given in Table 1.
The corrosive environment was a 5% aqueous solution of sodium chloride acidified to pH 2.8 with an acetic acid solution, which corresponds to solution A recommended by the NACE TM 0177 standard for testing sulphide and corrosion cracking of pipe steels. In the course of the experiment on the SSC, the solution was additionally saturated with hydrogen sulfide, and during the tests on the SCC, with carbon dioxide.
When the method was developed, the slow strain rate testing method (SSRT) was taken as a basis, which is used to determine the susceptibility of steels and alloys to SCC. The development of corrosion cracks under static loading of specimens and loading at a slow rate is established to occur according to a single mechanism. Moreover, the data obtained for different methods of load application make it possible to compare different materials with each other in terms of their susceptibility to SCC [14, 15]. Smooth cylindrical specimens with a diameter of the working part of 6.35 mm according to the NACE TM 0177 standard, intended for tests under static load, were selected for testing. The studies were carried out on a modernized tensile testing machine UME-10T, which allowed the specimen to be stretched at a rate of 1.3 to 1.3 × 10–3 mm/min.
The selection of the required strain rate during stress corrosion cracking tests is of great practical importance. The deformation rate should be such that the corrosion processes that contribute to the initiation and propagation of cracks have time to take place. This means that the rate of deformation should be close to the rate of short-term creep of steel caused by the action of applied tensile stresses concentrated in the zone of the developing corrosion defect, which acts as a stress concentrator on the specimen’s surface. If the strain rate is too low, then this significantly increases the duration of the test.
Figure 2 presents the experimental data obtained in the study of the effect of the strain rate on the susceptibility to stress corrosion cracking of two investigated low-alloy steels of different strength in an aqueous solution of chlorides at pH 3 in the presence of hydrogen sulfide at room temperature. At the same time, the stresses leading to the destruction of the specimens are taken for the stronger steel 34KhMA as the main criterion for susceptibility to SSC. For SAWL485FD steel, the breaking stresses always exceeded their yield strength and slightly differed from each other at all deformation rates. Therefore, the susceptibility to SSC was determined from the relative deformation at which the specimen was destroyed.
By contrast with tests in air, tests in a solution saturated with hydrogen sulfide showed a significant effect of the deformation rate on the mechanical properties of steels 34KhMA and SAWL485FD. A decrease in the strain rate from ~10–4 to ~10–6 s– 1 leads to a noticeable decrease in these characteristics when tested in a hydrogen sulfide environment. A further decrease in the strain rate to ~10–7 s–1 practically does not affect the values of the obtained fracture characteristics. The authors observed a similar dependence when testing shipbuilding steels in seawater [14]. At high strain rates, the diffusion mobility of atomic hydrogen does not have time to create at the tip of the developing crack the hydrogen concentration necessary for further advancement. Therefore, a higher level of applied stresses is required, leading to the destruction of the specimen. For this reason, a decrease in the strain rate down to 10–6 s–1 leads to a decrease in the stresses (or deformation) required for stress corrosion cracking of the steels under study. Such deformation rates are sufficient to exclude the inhibitory effect of the formed corrosion products on local foci of surface defects, which serve as the place of crack initiation and growth. Therefore, the crack initiation period in this range of strain rates is an insignificant part of the test duration.
A further decrease in the strain rate to 10–7 s–1 had practically no effect on the value of threshold stresses or strains. A certain level of applied stresses is required for crack growth according to the current understanding of the mechanism of steels cracking caused by the action of hydrogen penetrating into the metal [1, 2, 18]. And since, in this case, stresses (deformations) coincided at which the specimen fractured at deformation rates of 10–6 and 10–7 s– 1, then the obtained stresses can be considered as threshold. Thus, all further tests should be carried out at a rate deformation 10–6 s–1 within deformation rates under investigation.
Based on the above data, all further studies for the susceptibility of pipe steels to SSC and SCC were carried out at a strain rate of 8.5 × 10–7 s–1 (0.0013 mm/min) in environments containing H2S or CO2. Figures 3 and 4 show the diagrams of the destruction of these steels in the indicated solutions and for comparison in air. Table 2 indicates the main characteristics of strength and ductility obtained from the corresponding diagrams. In addition, the results of measurements of the relative elongation and relative contraction of the specimens are presented.
DISCUSSION AND RESULTS
The obtained data shows that both steels 34KhMA and DNV SAWL 485FD tend to SSC and SCC, although there are certain differences in their behavior. In an environment with CO2, the specimen destruction of both steels occurs due to SCC only at stresses exceeding their yield strength, i.e. in the field of plastic deformation. In the presence of H2S, the less strong SAWL 485FD steel breaks down according to the SSC scheme also at stresses above the yield point, while 34KhMA steel—already in the elastic stress region, i.e. below the yield point. In an environment saturated with carbon dioxide, the destruction of specimens is accompanied by noticeable plastic deformation with the formation of a neck at the fracture site. Therefore, to assess the degree of the susceptibility of steels to SCC in a CO2 solution, one can use the ratios of the values of the relative elongation and relative contraction of the specimens obtained when tested in a corrosive environment and in air. Comparison of steels with respect to breaking stresses is less informative, since fracture always occurs at stresses above the yield point and the obtained values differ little from each other.
In an environment saturated with hydrogen sulfide, the destruction of samples is fragile. Even on specimens of the weaker 485FD steel, it was difficult to measure tension and elongation. Therefore, to determine the susceptibility to SSC of steel in a H2S solution, it is more convenient to use the ratio of the breaking stress in the environment to the breaking stress in air or a similar ratio of total deformations (elastic + plastic). For comparison, similar specimens of the investigated steels were tested according to the standard method under static loads. In an acidic solution saturated with H2S, specimens of the stronger 34KhMA steel failed at an applied stress of 725 MPa (70% of the yield point) in 18–25 hours. Only when the stresses were reduced to 500 MPa, they did not fail during the selected test time of 720 hours. The 485FD steel showed no susceptibility to SSC or SCC at 70% yield strength. Similar data were obtained when tested in a solution saturated with CO2. Therefore, cracking of the specimens occurred only at stresses above the yield point in the plastic deformation region when these steels were tested at a slow strain rate under conditions of static loading, they did not show susceptibility to SSC and SCC.
CONCLUSIONS
Thus, the proposed method of accelerated testing of pipe steels on SSC and SCC determines quite quickly (in no more than 100 hours) their susceptibility to these types of corrosion damage.
REFERENCES
Azhogin, F.F., Korozionnoe rastreskivanie i zashchita vysokoprochnykh stalei (Corrosion Cracking and Protection of High-Strength Steels), Moscow: Metallurgiya, 1974.
Karpenko, G.V. and Vasilenko, I.I., Korrozionnoe rastreskianie stalei (Corrosion Cracking of Steels), Kiev: Tekhnika, 1971.
Sinyavskii, V.S. and Lukina, S.I., Corrosion cracking of titanium alloys in neutral aqueous solutions, Tekhnol. Legk. Splavov, 1980, no. 8, pp. 72–83.
Bobylev, A.V., Korrozionnoe rastreskivanie latuni (Corrosion Cracking of Brass), Moscow: Metallurgizdat, 1956.
Beloglazov, S.M., Navodorozhivanie stali pri elektrokhimicheskikh protsessakh (Steel Hydrogenation during Electrochemical Processes), Leningrad: Leningr. Gos. Univ., 1975.
Shreider, L.V., Shparber, I.S., and Archakov, Yu.I., Vliyanie vodoroda na neftyanoe i khimicheskoe oborudovanie (The Effect of Hydrogen on Petroleum and Chemical Equipment), Moscow: Mashinostroenie, 1976.
Perez, T.I., Corrosion in the oil and gas industry: an increasing challenge for materials, JOM, 2013, vol. 65, no. 8, pp. 1033–1042. https://doi.org/10.1007/s11837-013-0675-3
Burke, P.A., Recent progress in the understanding of CO2 corrosion, in Advances in CO 2 Corrosion, Houston, TX: Natl. Assoc. Corros. Eng., 1984, vol. 1, pp. 3–9.
Schmitt, G., Fundamental aspects of CO2 corrosion, in Advances in CO 2 Corrosion, Houston, TX: Natl. Assoc. Corros. Eng., 1984, vol. 1, pp. 10–19.
Schmitt, G. and Horstemeier, M., Fundamental aspects of CO2 metal loss corrosion, Part II: Influence of different parameters on CO2 corrosion mechanisms, Proc. NACE-Corrosion 2006 Meeting, March 11–16, 2006, San Diego, Houston, TX: Natl. Assoc. Corros. Eng., 2006, no. NACE-06112.
Schmitt, G. and Schierkmann, G., Corrosion cracking of steel in the system CO2/H2O, Proc. 8th Int. Congr. on Metallic Corrosion, Frankfurt on Main: Deutsh. Ges. Chem. App., 1981, vol. 1, pp. 426.
TM0177-2016-SG: Laboratory Testing of Metals for Resistance to Sulfide Stress Cracking and Stress Corrosion Cracking in H 2 S Environments, Houston, TX: NACE Int., 2016, p. 61.
GOST (State Standard) 9.903-81: Unified System of Corrosion and Ageing Protection. High-Strength Steels and Alloys. Accelerated Test Methods for Corrosion Cracking, Moscow: Izd. Standartov, 1982.
Kharkov, A.A., Nemchikova, L.G., Mikhnevich, A.P., and Bilina, S.Yu., Evaluation of the tendency of steel to corrosion cracking when tested with a slow strain rate, Tekhnol. Sudostr., 1990, no. 3, pp. 10–13.
Oryshchenko, A.S., Mushnikova, S.Y., Kharkov, A.A., and Kalinin, G.Y., Study of stress corrosion cracking of austenitic steels in seawater, Proc. European Corrosion Congr., EUROCORR 2010, Moscow, 2010.
Parkins, R.N., The controlling parameters in stress corrosion cracking, Proc. Fifth Symp. on Line Pipe Research, Chantilly, VA: Pipeline Res. Counc. Int., 1974, no. L30174.
ASTM G129: Standard Practice for Slow Strain Rate Testing to Evaluate the Susceptibility of Metallic Materials to Environmentally Assisted Cracking, West Conshohocken, PA: ASTM Int., 2013.
Corrosion, Shreir, L.L., Ed., London: Newnes-Butterworths, 1976.
Funding
The work is partially funded by the Ministry of Science and Higher Education of the Russian Federation as part of World-class Research Center program: Advanced Digital Technologies, grant no. 075-15-2020-934 dated to November 17, 2020.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alkhimenko, A.A., Kharkov, A.A., Shemyakinskiy, B.A. et al. Development of the Methodology of Accelerated Testing of Oil-Gas Pipe Steels for Stress Corrosion Cracking. Inorg Mater 57, 1541–1546 (2021). https://doi.org/10.1134/S0020168521150024
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168521150024