Abstract
Antimicrobial peptides (AMPs) are small cationic or amphipathic molecules that are produced by both prokaryotic and eukaryotic species. The latest findings in the field of dermatology point to the potential significance of AMPs in the battle against skin microbial infections. AMPs additionally function as multifunctional immune effectors, promoting angiogenesis, wound healing, and the production of cytokines and chemokines. In human skin, AMPs such as β-defensin, S100, and cathelicidin are primarily secreted by keratinocytes, neutrophils, sebocytes, or sweat glands. These substances are either produced continuously or expressed in reaction to certain inflammatory stimuli, thus playing a role in the development of various skin diseases in humans. Furthermore, in contrast to other human skin conditions, the level of AMP synthesis decreases as the disease progresses. In this review, we provide data supporting the role of AMPs as natural mediators of dermatological problems, as well as their potential for being used as therapeutic agents in the treatment of skin diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To resist microbial invasion, the human epidermis has developed a protective shield consisting of a strong mechanical barrier made of the cornified envelope, keratin, and lipid layers. However, resident epidermal cells not only form a passive mechanical barrier, but they also actively secrete chemicals to combat external infections, such as antimicrobial peptides. There is evidence that AMPs are present in vertebrates, and they have been discovered to be active in organisms that have complex adaptive immune systems as well as those that do not have an acquired immune system (Gallo et al. 1994a, b). Numerous peptides exhibit broad-spectrum antimicrobial activity against bacteria, fungi, and viruses, despite the variable sequences of AMPs. Considerable research has been devoted to these evolutionarily conserved molecules, which have been shown to possess various functionalities not only against microorganisms but also against the host cells. Our understanding of the innate immune systems of numerous species, including humans, is expanding at a rapid rate as a result of ongoing research into novel facets of the behaviour of these ubiquitous and unique small peptides (Chen and Jiang 2023; Zhang and Yang 2022). The skin of humans is an important reservoir of AMPs (Niyonsaba et al. 2017). Defensins, cathelicidins, dermcidin, and other short proteins initially identified for alternative biological functions, such as chemokines and neuropeptides, are among the AMPs generated in the human epidermis (Christophers and Schröder 2022). Additional larger proteins that possess direct antibacterial properties may also be detected in the skin, including lysozyme, elastase, complement, S100 proteins, and several others (Herman and Herman 2019).
Nevertheless, AMPs are not just antibiotics that occur naturally. Recent research has established a connection between an imbalance in AMPs and several common skin conditions that affect humans, including rosacea, psoriasis, and atopic dermatitis (AD) (Herman and Herman 2019; Yamasaki and Gallo 2008). It is not possible to exclusively attribute these skin diseases to microorganisms. In contrast to their antimicrobial designation, AMPs modulate host inflammatory responses through diverse mechanisms, such as functioning as chemotactic agents, angiogenic factors, and regulators of cell proliferation, in addition to directly destroying or impeding the growth of microorganisms (Yamasaki and Gallo 2008). This review article provides a concise overview of AMPs, their role in skin, and the many AMPs produced by both skin and commensal bacteria. Afterwards, we established the relationship between AMPs and the skin barrier, and also evaluated the immunomodulatory effects of these AMPs. Subsequently, we thoroughly examined the literature about to skin disease and wound healing, which is a crucial aspect of this paper. Subsequently, the therapeutic action of AMPs is discussed. Furthermore, we analyzed the difficulties encountered in the field and provided potential avenues for further study. The primary objective of this article is to offer a comprehensive analysis of the latest research on skin-related disorders.
Antimicrobial Peptides (AMPs)
AMPs, or antimicrobial peptides, are positively charged and have both hydrophobic and hydrophilic properties. They are part of the body’s natural defence system and are composed of a very small number of amino acid residues, often ranging from 10 to 50 (Pirtskhalava et al. 2016). Natural selection has maintained AMP genes unmodified, and they are produced by nearly all known forms of life, including single-celled bacteria, plants, invertebrates, and vertebrates. In bacteria, AMPs kill out other bacteria that threaten their biological niche. However, in more sophisticated animals, AMPs play an important role in natural immunity and help protect the host from harmful viruses (Hassan et al. 2012). Fungi, viruses, and unicellular protozoa are among the various organisms that are impacted by AMPs (Marr et al. 2006). As a primary defensive mechanism against microbial infections, many plants also include AMP-generating genes, which cause the formation of AMPs rich in cysteine and disulfide bonds (Tam et al. 2015). Vertebrates have been shown to contain a diverse range of AMPs. For example, AMPs are found in the neutrophil granules of mammals and are released by epithelial cells. Over 500 AMPs have been discovered in amphibian skin glands thus far (Tam et al. 2015). In vertebrates, two of the most important AMPs are cathelicidin and defensins (Jin and Weinberg 2019).
Mode of Action of Antimicrobial Peptides
The cationic structure of AMPs allows them to effectively interact with bacterial and fungal membranes, which are negatively charged and rich in lipopolysaccharides and lipoteichoic acid. Most AMPs act by damaging membrane integrity (Hancock and Sahl 2006; Mustafa et al. 2018). In addition, the hydrophobic portions of the peptide interact with the lipid double layer, as predicted by the principal peptide-membrane interaction models, which initially resulted in the formation of an amphiphilic structure (13). This structure is generated through the interaction between the peptide hydrophilic domain and the phospholipid charged groups. The accompanying models delineate the various methods by which this may occur (Huan et al. 2020). Parallel to the phospholipids, peptide aggregates traverse the membrane in the “barrel-stave” model, producing channels that result in cytoplasmic loss until the membrane collapses. Within the “carpet-like” structure, the AMPs amass on the membrane’s surface, where they engage in interactions via their lipid domains, thereby forming a structure resembling a carpet. When a specific concentration of peptides is attained, they initiate a purifying process that ultimately results in the membrane’s rupture (Seyfi et al. 2020; Zhang et al. 2021a, b). The “toroidal pore or wormhole” model, which is the third model, consists of AMPs accumulating in the membrane perpendicularly inserted and bending to form a nanometric-sized circular pore (Fig. 1). Additionally, a framework has been proposed that elucidates the process by which aggregates of peptides and phospholipids form, facilitating the passage of AMPs across the membrane (Seyfi et al. 2020; Zhang et al. 2021a, b). In conclusion, AMPs have the potential to alter the bacterial membrane’s stability by altering its thickness or triggering the aggregation and collapse of phospholipid heads. Additionally, they have the power to enhance the permeability of the membrane (Seyfi et al. 2020; Zhang et al. 2021a, b). It has also been suggested that there are non-membrane lytic pathways in which AMP crosses the membrane and affects a specific target located inside the cell (Le et al. 2017). Peptide aggregation is a process that is frequently overlooked, yet it has the potential to considerably influence AMP selectivity (Vaezi et al. 2020). The hydrophobicity of peptides and their attraction to neutral membranes, like those of healthy eukaryotic cells, can be significantly reduced by aggregation (Vaezi et al. 2020). Recent studies have examined the action mechanisms of four AMPs that show great promise in combating the most dangerous multidrug-resistant nosocomial bacterial infections: Pardaxin, MSI-78, DMPC, and Cecropin B (Lin et al. 2022; Bellotti and Remelli 2022). Based on the results, the first two peptides are the most effective against the investigated bacteria through membrane damage and destruction of their membranes. Paradoxin’s capacity to enter the bacterial cytoplasmic membrane alone is what allows it to perform its function even at very low doses (Bellotti and Remelli 2022).
Mechanism of action of antimicrobial peptides against microbes
Peptides with antimicrobial properties destroy microorganisms via direct and indirect mechanisms. The direct approach involves targeting the membrane, whereby antimicrobial peptides eliminate microorganisms through three distinct mechanisms of membrane destruction. Conversely, intracellular microbe components including DNA, RNA, and proteins may be targeted. However, the indirect approach involves stimulating the immune system, which eliminates microorganisms via the release of inflammatory factors such as cytokines and chemokines and the activation of immune cells
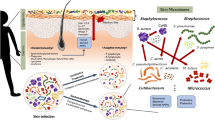
The Activity of AMPs in the Skin
As a result of its inherent resilience to infections, human skin is constantly exposed to microbes but rarely becomes infected. The presence of constitutively and inducibly produced AMPs may be one of the main reasons for this (Bellotti and Remelli 2022). Indeed, keratinocytes serve as the primary producers of AMPs on the human skin. A diverse array of cutaneous AMPs comprises defensins, cathelicidins, dermcidin (DCD), and additional small peptides, such as neuropeptides and chemokines (Yamasaki and Gallo 2008; Schittek et al. 2008; Zhang and Gallo 2016). Over the past few years, there has been considerable interest in elucidating the mechanisms by which constitutively expressed and inducible AMPs contribute to infectious and inflammatory diseases. The ultimate goal is to harness the therapeutic potential of these peptides, specifically for the treatment of skin diseases. Following a concise overview of the most significant human AMPs in the ensuing section, their function in infectious and inflammatory skin diseases is clarified (Table 1).
The Type of AMPs
Cathelicidin Antimicrobial Peptides (CAMPs)
The first AMP found in mammalian skin was cathelicidin (Gallo et al. 1994a, b). The precursor protein hCAP18 is encoded by a single cathelicidin antimicrobial peptide gene (CAMP) in humans (Larrick et al. 1996). Proteases cleave hCAP18 in multiple manners to produce active AMPs, including the 37-amino acid peptide LL-37 (Gudmundsson et al. 1996). To defend the skin barrier, keratinocytes produce different AMPs at low levels in normal skin (Murakami et al. 2002), whereas resident mast cells express cathelicidin precursor protein and mature peptide in the highest abundance (Nardo et al. 2003). One possible evolutionary pathway for HDPs in multicellular organisms is their defense against pathogens, especially bacteria (Boman 2003). According to this principle, HDP sequences were likely optimized for antibacterial potency through evolution as a result of host-microbial interactions and direct antimicrobial activity. Having said that, in host-like settings, the antibacterial effectiveness of the majority of HDPs is still fairly weak. The capacity of LL-37 to inhibit pro-inflammatory signaling is one of its remarkable properties. Undoubtedly, this is accomplished through a web of interconnected direct and indirect processes (Koo and Seo 2019). In terms of direct mechanisms, lipopolysaccharide (LPS) and lipoteichoic acid (LTA) are examples of bacterial toll-like receptor (TLR) ligands that cathelicidins bind to and neutralize (Kahlenberg and Kaplan 2013). If TLRs were engaged, these ligands would activate TLRs and cause inflammatory processes linked to the cascade activation of immune cells (Horibe et al. 2013). The host-cell-directed action of cathelicidins, which is not dependent on bacterial ligand binding, is supported by many lines of evidence. For instance, irrespective of LPS, LTA, and/or flagellin stimulation, LL-37 controls over a dozen signaling pathways, including the p38, Erk1/2, JNK MAP-kinases, NFκB, PI3K/Akt, Src family kinase, TRIF-IRF, TREM, Wnt/β-Catenin, JAK-STAT, and autophagy signaling pathways (Scott et al. 2002). Samples from patients with non-small cell lung cancer also showed activation of Wnt/β-Catenin and PI3K/Akt signaling cascades triggered by LL-37 (Ji et al. 2019) with a significant connection found between LL-37 content in tissues and related gene expression. Similarly, LL-37 induced proliferation in the A549 pneumocyte cell line in vitro in a way that was TLR-independent but reliant on β-catenin (Ji et al. 2019). In general, the findings of these studies indicate that LL-37 interacts with numerous intracellular and surface targets (Hilchie et al. 2013) that play a role in the biological processes of this HDP in vivo. These interactions are mediated by a wide range of signaling events and result in diverse outcomes (Alford et al. 2020). Cathelicidins can influence the expression of TLRs on a variety of cell types, including monocytes, neutrophils, renal cells, epithelia lining the colon, and other mucosal surfaces, in addition to inhibiting proinflammatory signaling via TLRs (Agier et al. 2018). The effect of LL-37 on TLR expression varies with time and with tissues, which is rather intriguing. As an example, when stimulated in vitro with LL-37, the levels of many toll-like receptors (TLRs) in the mast cells of Wistar rats rose time-dependently, reaching a maximum 3 h after stimulation (Agier et al. 2018). Moreover, it was observed that LL-37 inhibited the expression of TLR and co-receptors that were activated by LPS in human gingival fibroblasts. However, this effect was not observed in untreated cells (Inomata et al. 2020). However, the significance of this finding is uncertain given that LL-37 significantly decreases the expression of proinflammatory cytokines mediated by LPS/LTA (Kandler et al. 2006). In general, rather than exerting direct microbicidal effects, the characteristics of LL-37 that promote immune system modulation appear to aid in the resolution of infections and regulate detrimental inflammation. Cathelicidins exhibit immunomodulatory properties through various mechanisms, such as direct and indirect chemotaxis-mediated enhancement and reduction of inflammation, induction of pro-inflammatory and anti-inflammatory cytokine secretion, inhibition of TLR activation and subsequent signaling pathways, and facilitation of adaptive immunity activation.
Defensins
Defensins, which are classified as cationic microbial peptides, comprise three pairs of intramolecular disulfide bonds formed by six conserved cysteine residues (Lai and Gallo 2009). Humans possess several defensin genes that constitute various gene clusters, in contrast to the single human cathelicidin gene CAMP. For instance, six human α-defensins have been found (human neutrophil peptide [HNP]1–6). Neutrophils and Paneth cells are the primary sources of α-defensins. HNP1, HNP2, and HNP3 have been detected in the skin by the analysis of extracts taken from psoriatic scales (Harder and Schröder 2005). A total of 90 β-defensin genes have been discovered in both mice and humans. There are four human β-defensins (hBD-1–4) that exhibit a wide range of antibacterial activity and immune-modulating effects. These defensins are expressed in epithelial cells and peripheral blood cells. HBD-1 is consistently expressed in epithelial cells, whereas hBD-2–4 is only activated when exposed to pro-inflammatory cytokines and microbial metabolites (Lai and Gallo 2009). Comparable to cathelicidin, β-defensins are produced in the form of propeptides, with the precise details of the processing mechanism yet to be elucidated (Schutte and McCray 2002). High levels of TNF- and IFN-, which are present in the skin lesions of psoriasis, induce hBD (Harder et al. 1997). Notably, the collaborative action of TNF-α and IL-17 A effectively enhances the secretion of hBD-2 (Chiricozzi et al. 2011) through the stimulation of transcription factors such as OCT-1, NF-κB, and AP-1 (Johansen et al. 2016). Mounting evidence suggests that the pivotal role of defensins in governing the antibacterial immune response extends beyond their direct bactericidal activity. Notably, defensins play a crucial role in regulating host immune homeostasis by modulating both innate and adaptive immune responses and functioning as immune regulatory factors (Hilchie et al. 2013). Defensins exhibit the ability to modulate acquired immune responses. Moreover, in a state of nutritional deficiency, the persistent activation of α-defensins plays a role in conferring resistance to enteric pathogen invasion, operating through the mTOR-Hes1-Atoh1-MMP7-α-defensins axis (Liang et al. 2019). Moreover, defensins showcase their prowess in orchestrating the symphony of inflammatory factors. In a captivating study, Koeninger et al. illuminated the role of hBD2, unveiling its ability to elevate disease activity indices and thwart colitis-associated weight loss across a trio of mouse models—namely, dextran sodium sulfate (DSS), 2,4,6-Trinitrobenzenesulfonic acid (TNBS), and T-cell transfer into immunodeficient recipient mice (Koeninger et al. 2020). Moreover, their research revealed that human beta-defensin 2 (hBD2) interacts with chemokine receptor 2 (CCR2) on dendritic cells (DCs), effectively suppressing nuclear factor-kappa B (NF-κB) activity and fostering cAMP response element-binding protein (CREB) phosphorylation. This dual action serves to diminish the expression of inflammatory mediators. In our prior investigations, we demonstrated that porcine beta-defensin 2 (pBD2) competitively hinders the activation of NF-κB signaling through Toll-like receptor 4 (TLR4) in response to lipopolysaccharide (LPS) and dextran sulfate sodium (DSS), thereby attenuating the release of inflammatory cytokines (Han et al. 2015). Likewise, observations by Zhang et al. and Lian et al. underscore that porcine beta-defensin 2 (pBD2) diminishes Escherichia coli adherence to cellular structures and mitigates inflammation through modulation of the TAK1-NF-κB pathway (Zhang et al. 2022). Two powerful pro-inflammatory cytokines, TNF-α and IL-6, were discovered by Semple and colleagues to be strongly inhibited by hBD3 (Semple et al. 2010a, b). Neutrophils, being the foremost and most plentiful cells to arrive at the site of inflammation-induced injury, discharge a significant volume of defensin HNP1 (Brook et al. 2016). Additionally, HNP1-3 secreted by neutrophils can enhance bacterial phagocytosis by stimulating macrophages to produce TNF and IFN at an accelerated rate (Soehnlein et al. 2008). The induction of human beta-defensin 2 (hBD2) occurs through Nod1-dependent activation of NF-κB following infections with Helicobacter pylori or Pseudomonas aeruginosa. Likewise, infection with H. pylori leads to an upregulation in the production of hBD3, facilitated by the epidermal growth factor receptor (EGFR)-dependent activation of the MAP kinase and Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways (Grubman et al. 2010) (Huang 2014) (Bauer et al. 2012). Additionally, a captivating study unveiled the presence of a signaling pathway crucial for skin resilience against pathogen infection. This pathway operates through the intricate interaction between the epithelium and neutrophils, mediated by defensins (Dong et al. 2022). Upon Staphylococcus aureus infection, keratinocytes initiate the production of defensins. Subsequently, these defensins activate Mrgpra2 receptors on neutrophils, leading to the generation of IL-1β and CXCL2. This orchestrated response serves to bolster the body’s resistance against the infection. Disruption of this sequential signaling cascade may compromise immune function and contribute to the formation of abscesses (Dong et al. 2022). Defensins have also been examined as possible therapeutic agents for anti-SARS-CoV-2 infection (Laneri et al. 2021). HD5 acts by blocking the binding of SARS-CoV-2 S1, which prevents pseudovirions from entering enterocytes. The protective mechanism is accomplished by HD5 competitively interacting with angiotensin-converting enzyme 2 (ACE2) (Wang et al. 2020). Defensins, beyond their antimicrobial properties, appear to play a dual role in both innate and adaptive immunity, as supported by the growing body of evidence demonstrating their multifaceted functions.
S100 Proteins
S100 proteins are an essential constituent of psoriasis and constitute a distinct category of antimicrobial peptides (AMPs). Proteins within this category have a low molecular weight (9–13 kDa) and are distinguished by a helix-loop-helix motif that binds calcium (Eckert et al. 2004). S100 proteins play a role in regulating various cellular processes, including protein phosphorylation, transcription factors, intracellular Ca2 + signaling, cytoskeletal membrane interaction, enzyme activities, cell cycle progression, differentiation, and inflammatory responses. Among the twenty-one known S100 proteins, S100A7 (psoriasin), S100A8 (calgranulin A), S100A9 (calgranulin B), S100A12 (calgranulin C), and S100A15 exhibit antimicrobial effects, with elevated expression levels observed in the lesional skin and serum of individuals with psoriasis (Büchau and Gallo 2007; Kurpet and Chwatko 2022). S100A7, in particular, has been intensively investigated and was originally identified in the epidermis of psoriasis patients (Madsen et al. 1991). Calcium, vitamin D, retinoic acid, bacterial metabolites, TNF-α, IL-17 A, and IL-22 are all known to induce S100A7. This protein is implicated in the pathogenesis of psoriasis through its chemotactic activity towards CD4 + T lymphocytes and neutrophils (Jinquan et al. 1996). Calcipotriol decreased the secretion of S100A7 and S100A15 when applied topically, according to Hegyi et al. This finding suggests that vitamin D derivatives may influence the epidermis in psoriatic lesions via one mechanism (Hegyi et al. 2012). Calprotectin is an additional antimicrobial agent belonging to the S100A family. It is composed of a heterocomplex formed by the calcium-binding proteins S100A8 and S100A9. Calprotectin demonstrates antibacterial and fungicidal activity against Candida albicans, E. coli, Klebsiella spp., S. aureus, and Staphylococcus epidermidis, among others (Madsen et al. 1991). The hypothesis has been raised that the complex may play a role in inflammatory skin diseases. In the epidermis, S100A8 and S100A9 are up-regulated in hyperproliferative keratinocytes like those in wound healing (Thorey et al. 2001) and in psoriatic lesions (Broome et al. 2003). S100 proteins, namely those found in keratinocytes, are recognized as the primary contributors to the development of psoriasis and AD, among other causative variables (Saito-Sasaki and Sawada 2023). Due to its significant involvement in enhancing the inflammatory process in psoriatic lesions, S100A7 emerges as a promising diagnostic and therapeutic target for psoriasis (Liang et al. 2023).
Dermcidin
Multiple research teams have verified that peptides originating from the AMP domain of the dermcidin precursor protein have a wide-ranging ability to combat many types of microorganisms while lacking any harmful effects on red blood cells (Li et al. 2009; Senyürek et al. 2009). The predominant antimicrobial DCD peptides found in sweat are the anionic DCD-1 L and DCD-1, as well as the cationic SSL-25 and SSL-23. These peptides exhibit a wide range of activities that overlap with each other, and this is not influenced by the overall charge of the peptides (Senyürek et al. 2009; Steffen et al. 2006). These peptides have antibacterial properties against harmful microbes such as Staphylococcus aureus, Escherichia coli, Enterococcus faecalis, and Candida albicans under laboratory circumstances that simulate human perspiration. Subsequent inquiries conducted unveiled a broader range of antimicrobial activity, encompassing Staphylococcus epidermidis, Pseudomonas aeruginosa, Pseudomonas putida, methicillin-resistant S. aureus, Listeria monocytogenes, and Salmonella typhimurium. Significantly, a number of workers successfully produced recombinant dermcidin in bacteria and isolated antimicrobial active DCD-1 or DCD-1 L by purification (Cipáková et al. 2006; Lai et al. 2005). These peptides, however, often exhibit reduced activity compared to manufactured peptides, perhaps because of the inclusion of extra amino acids in the peptide sequence resulting from tag cleavage. Studies on the antimicrobial action mechanism revealed that DCD peptides interact with bacterial membrane phospholipids and effectively eliminate gram-negative bacteria, regardless of their net charge. Notably, this process does not involve the formation of extensive holes in the bacterial membranes (Senyürek et al. 2009; Steffen et al. 2006). The continuous release of dermcidin, a substance formed from sweat, plays a role in the natural defensive mechanism of human skin by controlling the colonization of its surface. This is different from the response shown in the inducible peptides HBD-2 and − 3 or the human cathelicidin LL-37, which are only activated in the presence of damage or inflammation. Dermcidin may, therefore, aid in the prevention of both local and systemic pathogen invasions. One study demonstrated the therapeutic significance of dermcidin by revealing that individuals with atopic dermatitis have a decreased quantity of dermcidin-derived antimicrobial peptides (AMPs) in their sweat. This reduction is associated with a compromised innate defense mechanism of the human skin in a living organism (Rieg et al. 2005). The results indicate that the lower levels of dermcidin-derived peptides in individuals with atopic dermatitis are a contributing factor to their increased vulnerability to skin infections and changes in skin colonization. Niyonsaba et al. have revealed that dermcidin-derived peptides also have other activities in host defense (Niyonsaba et al. 2009). The researchers demonstrated that DCD-1 and DCD-1 L stimulate the activation of normal human keratinocytes by triggering the production of proinflammatory cytokines such as TNF-α, IL-8 (CXCL8), interferon-inducible protein 10 (CXCL10), and macrophage inflammatory protein-3α (CCL20). The activation of the NF-ĸB signaling pathway, as well as p38 and ERK, is regulated by the G-protein and MAPK pathways, leading to the generation of cytokines and the synthesis of chemokines. This study demonstrates that dermcidin has a role in controlling the innate immunity of the skin, not only by directly killing bacteria but also by regulating inflammation in the skin (Niyonsaba et al. 2009).
Ribonuclease 7
The stratum corneum has a high amount of ribonuclease 7 (RNase7), which is constitutively expressed in the epidermis of healthy human skin and mostly generated by keratinocytes. Proinflammatory cytokines, skin damage, and certain infections can all lead to an increase in RNase7 levels (Harder et al. 2010; Simanski et al. 2010; Harder and Schroder 2002). A number of studies have demonstrated that RNase7 is effective against many microorganisms, including S. aureus (Simanski et al. 2010). In addition to its antimicrobial properties, RNase 7 has been implicated in a number of studies as having an immunomodulatory function. While the precise mechanism by which RNase 7 functions as an immunomodulatory mediator is still being investigated, preliminary evidence suggests that it may possess such properties. The immune system contains both resident and transiently migrating cells in healthy human skin. In terms of antimicrobial defense, plasmacytoid dendritic cells (pDCs) are widely recognized as pivotal components of dermal immune system subtypes. Lymph nodes are typical sites of pDC presence and bloodstream circulation. In the presence of inflammation, they have the capability to penetrate the epidermis (Donaghy et al. 2009). pDCs are essential for cellular antimicrobial defense on account of their capacity to secrete an interferon-alpha (IFNα) response that is several times more potent than that of any other cell type (Gilliet et al. 2008). In pDCs, Kopfnagel et al. demonstrated that RNase 7, in conjunction with human self-DNA, induces a potent IFNα response (Kopfnagel et al. 2018a, b). Furthermore, this group examined the impact of RNase 7 on the production of Th2 cytokines by human CD4 + T cells and Th2 cells. They reported that stimulation with RNase 7 substantially decreased the release of the Th2 cytokines IL-4, IL-5, and IL-13. It was discovered that this downregulation of Th2 cytokines was facilitated by a diminished activation of the transcription factor GATA3. It is noteworthy that the ribonuclease activity of RNase 7 was not required to achieve this result. As a result of this particular regulation, the authors hypothesized a receptor-mediated process that remains unknown and requires additional research (Kopfnagel et al. 2017). Research shows that human keratinocytes recognize self-DNA through RNase 7. A pathway mediated by type I interferons led to a significantly enhanced release of the chemokine IP-10 (CXCL10) when keratinocytes were activated by a DNA/RNase 7 complex. Furthermore, keratinocytes might be protected against herpes simplex virus type 1 (HSV-1) infection by inducing an interferon-beta (IFNß) dependent antiviral response through DNA and RNase 7 activation. Extensive evidence suggests that RNase 7 has the ability to promptly activate keratinocytes and pDCs via DNA and bind self-DNA upon its release (Kopfnagel et al. 2020a, b). Thus, RNase 7 may function as an alarmin that transforms released self-DNA into a danger signal in order to detect a breach in the epidermal barrier (Kopfnagel et al. 2020a, b). Given that inflammatory skin diseases, including atopic dermatitis and psoriasis, induce RNase 7 expression, it is probable that RNase 7’s immunomodulatory properties are involved in these conditions. Additionally, it is noteworthy to assess whether the ribonuclease activity of RNase 7 could potentially contribute to the regulation of RNA-mediated inflammation in the context of skin injury by degrading host RNA released from damaged cells.
AMPs and Skin Barrier
Among the many physical, chemical, and viral dangers that humans face, the epidermis stands as the principal physical barrier separating us from the outside world. The intestinal epithelium consists of a single layer of columnar enterocytes, whereas the epidermis of the skin is a stratified squamous epithelium made up of keratinocyte cells that proliferate in the basal layer, move upward, and eventually differentiate into corneocytes. Protein cross-linking mediated by transglutaminase, lamellar body secretion, and nuclear loss are hallmarks of this transition (Mahanty and Setty 2021). Lipids produced by keratinocytes cover the crevices between corneocytes in the outermost layers, forming a strong barrier that prevents water loss through the epidermis and the entrance of foreign substances (Kahraman et al. 2019). The integrity of the skin barrier, the restriction of potentially dangerous compounds, and the transit of necessary molecules are all regulated by tight junction proteins, which are found in other mucosal barrier locations as well (Lee et al. 2018; Brandner 2016). The activation of CRAMP (the murine ortholog of LL-37) and mBD-3 (the murine ortholog of hBD-2) can be seen in response to both acute and chronic disruptions of the physical skin barrier, according to Aberg et al. (Aberg et al. 2008). Furthermore, certain studies have shown that AMP expression in keratinocytes occurs at the same time as the expression of several epidermal structural components that form the skin permeability barrier, such as involucrin, loricrin, keratin-1 and keratin-10, transglutaminase-1, and transglutaminase-3 (Borkowski and Gallo 2011). An essential function of hBD-3 in maintaining homeostasis of the epidermal barrier was discovered (Kiatsurayanon et al. 2014). In addition to lowering paracellular permeability in keratinocyte layers, hBD-3 enhances the production of tight junction (TJ) proteins, specifically claudins, and alters their location at cell-cell borders. For the hBD-3-mediated control of TJ barrier function, it is necessary to activate Rac1, atypical protein kinase C, glycogen synthase kinase-3, and phosphatidylinositol 3 kinase, all of which hBD-3 promotes (Kiatsurayanon et al. 2014). Autophagy-deficient AD animals and AhR-suppressed AD mice did not exhibit hBD-3-mediated enhancement of the TJ barrier, indicating that hBD-3-mediated autophagy may have a role in the control of inflammation and the epidermal barrier in AD (Peng et al. 2022). In addition to controlling differentiation, psoriasin enhances the function of the skin’s TJ barrier by increasing the expression of TJ proteins such as claudins and occludin (Kiatsurayanon et al. 2014). Moreover, an increase in psoriasin-induced accumulation of β-catenin and E-cadherin at the interface of cells results in a decrease in the paracellular permeability of keratinocyte layers. Keratinocytes, the primary cell population of the epidermis, serve as the central sentinels of the skin and not only maintain the physical barrier but also make a substantial contribution to the skin’s defense against bacterial infections. The cells exhibit a diverse array of nucleotide-binding oligomerization domain 2 (NOD2) and Toll-like receptors (TLRs), both of which are critical for the identification of pathogen-associated molecular patterns (PAMPs). The detection of PAMPs activates innate immunity, which involves the production of various cytokines, chemokines, and AMPs that aid the recruitment of immune cells to the site of infection (Krishna and Miller 2012; Nestle et al. 2009). In a recent study, it was discovered that exposure of keratinocytes to HBD2 did not inhibit the activity of protease (V8) in maintaining barrier integrity. Rather, it altered cell function and proteomic profiles, which were linked to increased expression levels of extracellular matrix (ECM) proteins, such as LAMB1. These results demonstrate that HBD2 possesses barrier-protective properties (Shelley et al. 2023).
AMPs and Skin Immune Defense
It has been observed that commensal and pathogenic microorganisms have developed distinct mechanisms to regulate the skin’s innate immunity (Wanke et al. 2011). Through TLR-2, epidermal growth factor receptor (EGFR), and NF-kB activation, skin commensals stimulate the expression of hBD-3 and RNase7 in primary human keratinocytes. In contrast, pathogenic staphylococci activate the phosphatidylinositol 3-kinase/AKT (PI3K/AKT) and mitogen-activated protein kinase (MAPK) signaling pathways and inhibit NF-kB activation. It is noteworthy that commensal bacteria possess the capability to enhance the innate immune response of human keratinocytes towards pathogens through the inhibition of NF-kB suppression and the upregulation of AMP expression. This observation implies that the two activation pathways may operate in conjunction to beneficial effect. Furthermore, in the case of cutaneous infections, AMPs stimulate a robust host response that includes the production of cytokines and chemokines, inflammation, and a cellular reaction (Gallo and Huttner 1998; Namjoshi et al. 2008). The epidermal expression of hBDs was stimulated in both monocytes and lymphocytes subsequent to exposure to molecules derived from microbes (Sørensen et al. 2005). The G protein-coupled receptor (GPCR) and phospholipase C (PLC) signaling pathways activate keratinocytes to produce proinflammatory cytokines (IL-6, IL-10, IP-10) and chemokines (MCP-1, MIP3-α, and RANTES), but hBD-1 does not stimulate keratinocytes (Niyonsaba et al. 2007a, b). On top of that, hBDs activate STAT3 and EGFR, which in turn cause keratinocyte migration and proliferation. Along with endogenous inflammatory mediators, cathelicidin (LL-37) enhances the production of certain inflammatory effectors via a complicated process involving numerous pathways, including GPCR, EGFR, and TLR (Reinholz et al. 2012). Consequently, cathelicidin peptides enhance the passage of activated cells and the release of cytokines (IL-6, IL-8, IL-10, IL-18, and IP-10) and chemokines (MCP-1, MIP3α, and RANTES) (Frohm et al. 1997). Additionally, keratinocytes are stimulated by dermcidin-1 L (DCD-1 L) to produce cytokines (TNF-α, IL-8, and IP-10) and chemokines (MIP3α) via the G protein and p38/MAPK pathways
The pathogen-affinity of AMPs, however, could make infection easier in some cases. Shigella infects the intestinal epithelium via interacting with bacterial surface proteins and human enteric HD5, which increase adhesion and penetration of the epithelium (Xu et al. 2018). Macrophage HD5 can also enhance Shigella phagocytosis, which in turn leads to macrophage cell death and the release of bacteria that infect intestinal epithelial cells by bacterial reproduction (Xu et al. 2019).
Although inflammatory reactions play a crucial role in eliminating harmful invaders and infected cells, when they are overdone or left unchecked, they can cause tissue damage, chronic inflammatory illness, and even cancer. Thus, in order to maintain a microenvironment in a state of homeostasis, it is necessary to regulate the inflammatory response once the level of inflammation exceeds a specific threshold. AMPs’ expression levels in inflammatory locations determine whether they promote or inhibit inflammation (Prasad et al. 2019). In a study conducted by Hosoda et al. (Hosoda et al. 2017), it was discovered that LL-37 had dual effects. Firstly, it released NETs, which inhibited bacterial growth. Secondly, it eased inflammatory responses by reducing cytokines, soluble TREM-1, and danger-associated molecular patterns (DAMPs), which improved the survival of mice with cecal ligation and puncture (CLP) sepsis. Additionally, LL-37 reduces monocyte and neutrophil chemotaxis by mediating the internalization of the chemokine receptor CXCR2 (Hosoda et al. 2017). In addition to promoting intracellular ROS generation and bacterial intracellular ingestion, LL-37 considerably inhibits the release of pro-inflammatory cytokines in LPS-stimulated neutrophils (Alalwani et al. 2010). Furthermore, LL-37 inhibits the growth of Aspergillus fumigatus infections by encasing the fungal hyphae and decreasing macrophage production of inflammatory cytokines (Luo et al. 2019). In order to maintain the microenvironment stable and protect tissues from infection, AMPs have an immunomodulatory function.
AMPs and Skin Diseases
Although AMPs do provide protection for the skin, numerous studies have put forth the hypothesis that they may also play an active role in the development of diverse skin diseases. An expanding body of evidence has already established the involvement of AMPs in the pathophysiology of various conditions, including psoriasis vulgaris, AD, acne vulgaris, hidradenitis suppurativa, rosacea, and wound healing (Fig. 2) There are limited studies or reports available for the vast majority of infectious or inflammatory skin diseases. Significantly, AMPs have the capacity to function as pro- or anti-inflammatory molecules in addition to antimicrobial compounds, which gives them the reputation of a double-edged sword (Rademacher et al. 2021).
Psoriasis
Psoriasis is a prevalent skin disease that occurs due to an autoimmune condition. It is defined by abnormal responses from the innate and adaptive immune systems. T cells, keratinocytes, and dendritic cells (DCs) are the key players in this condition (Hawkes et al. 2017). Injury, infection, or mechanical stimulation may cause psoriasis, particularly in those who are genetically prone to the condition (Wang and Jin 2018). Within this particular situation, several immunological chemicals, such as AMPs, experience a rapid rise in concentration in the adjacent skin area. This prompts the maturation and activation of DCs and T cells, leading to an excessive release of interleukin (IL)-17. Consequently, immune cells infiltrate the area and trigger an inflammatory cascade. The levels of AMPs, such as cathelicidin (LL-37), human β-defensins (hBDs), S100 proteins, and RNase 7, are elevated in the skin lesions and/or blood serum of individuals with psoriasis compared to those of healthy individuals (Fuentes-Duculan et al. 2017; Kolbinger et al. 2017; D’Amico et al. 2016; Bierkarre et al. 2016). Lethal infections are thus extremely rare in psoriatic skin lesions. In the last several decades, the role of AMPs as immunoregulatory molecules in psoriasis has come to light. In a PRR-dependent manner, AMPs activate keratinocytes and innate immune cells such as neutrophils, macrophages, and DCs. This stimulation causes DC maturation, NET formation, and the recruitment of neutrophils and macrophages (Wang and Jin 2018). Additionally, AMPs interact directly with T cells as autoantigens, modulating adaptive immune responses in psoriasis (Lande et al. 2014). Studies have also reported positive associations between AMP expression and specific symptoms such as itch (Aizawa et al. 2019) severity (Wilsmann-Theis et al. 2016), and genetic susceptibility (Mabuchi and Hirayama 2016) of psoriasis. Elevated LL-37 concentrations in psoriatic skin lesions have shown a positive correlation with the severity of the disease (Hwang et al. 2014). Cytokines, including IL-17 A and tumor necrosis factor (TNF)-α, can enhance the production of LL-37 in keratinocytes in psoriasis (Zhang et al. 2016), which is rapidly induced following skin injury (Zhang et al. 2016). Recently, there was a favorable correlation observed between the level of LL-37 and the levels of IFN-γ, IL-17, and IL-22 in the blood. The presence of LL-37 in the bloodstream may control the synthesis of IFN-γ, IL-17, and IL-22 in individuals with psoriasis (Lao et al. 2023). In addition to its ability to form complexes with nucleic acids, LL-37 also directly controls the activity of keratinocytes and immune cells in psoriasis. For instance, LL-37 stimulates the synthesis of cytokines and chemokines, such as IFNs, IL-36γ, and C-X-C motif chemokine ligands (CXCLs), in keratinocytes (Hemshekhar et al. 2018; Li et al. 2014). The inhibition of cell death and the improvement of the skin’s protective barrier are two additional LL-37 regulatory actions on keratinocytes that point to the protein’s role in the onset of psoriasis. Additionally, LL-37 directly activates (Li et al. 2014) (Chamorro et al. 2009) T cells that are important in the maintenance of the IL-23/Th17 axis; this is because LL-37 is an autoantigen (Fuentes-Duculan et al. 2017). Additionally, it has been documented that individuals afflicted with moderate to severe plaque psoriasis might produce skin-homing CD4 + and/or CD8 + T lymphocytes specific to LL-37 (Lande et al. 2014). The psoriasis patients’ blood or skin lesions are invaded by these autoreactive T cells, which then secrete IFN-γ and Th17 cytokines. Additionally, LL-37 controls macrophages through TLR9 and triggers IL-8 secretion by mast cells, in addition to T cells (Nakagawa and Gallo 2015; Yu et al. 2017). LL-37 promotes the differentiation of monocytes into the CD14highCD16 + subpopulation in psoriasis guttate (Qian et al. 2015). This suggests that LL-37 plays a pivotal role in the development of psoriasis by priming either the innate or adaptive immune responses.
Similar to LL-37, hBD-2 and hBD-3 increase the generation of IFN-α and boost the absorption of self-DNA or CpG DNA by pDCs (Tewary et al. 2013). hBD-3 stimulates the activation of myeloid dendritic cells (mDCs) by a mechanism that relies on the presence of Toll-like receptors 1 and 2 (TLR1/2) (Akiyama et al. 2014). Furthermore, both hBD-3 and its mouse counterpart, murine β-defensin-14, stimulate the secretion of IL-23 by epidermal Langerhans cells (LCs) and worsen the inflammation of the skin, resembling the symptoms of psoriasis (Sweeney et al. 2016). From a clinical perspective, a higher number of copies of β-defensin genes in the genome has been linked to a greater likelihood of developing psoriasis (Stuart et al. 2012; Hollox et al. 2008). The balance of the microbiome can be impacted by HBDs released by commensal bacteria (Meade and O’Farrelly 2018). As a result, dysregulated hBD expression may potentially play a role in psoriatic inflammation through the microbiome. For instance, in a way that is dependent on RAGE, the production of cytokines associated with psoriasis, such as IL-1α, IL-23, and MIP-2, in keratinocytes is induced by the mouse ortholog mS100a7a15, which is the primary function of S100 proteins in psoriasis (Wolf et al. 2010). Keratinocytes mediate psoriatic inflammation by expressing IL-8, CXCL1, CXCL2, CCL20, and complement component 3 in response to S100A8/A9 (Nukui et al. 2008; Schonthaler et al. 2013). In psoriasis, hyperkeratosis and parakeratosis are caused by the aberrant differentiation and excessive proliferation of keratinocytes, which are facilitated by these proteins (Granata et al. 2019; Lei et al. 2017; Benoit et al. 2006). Psoriatic arthritis, Crohn’s disease, metabolic syndrome, and cardiovascular problems are among the comorbidities associated with psoriasis that have been linked to S100 proteins. Serum levels of S100A8/S100A9 and S100A12, for instance, are much higher in psoriatic arthritis patients; these two molecules may serve as indicators of the severity of the disease (Ademowo et al. 2016; Aochi et al. 2011). In psoriasis patients, serum S100A7/S100A15 and S100A8/S100A9 levels are favorably linked with intima-media thickness and aortic vascular inflammation, respectively (Awad et al. 2018; Naik et al. 2015). It is noteworthy that the promotion of psoriatic inflammation and the activation of the itch-associated genes transient receptor potential vanilloid type 1 (TRPV1) and transient receptor potential ankyrin 1 (TRPA1) positively regulate the expression of S100A8/S100A9. This suggests that S100 proteins may play a role in the development of psoriatic pruritus (Zhou et al. 2018) (Zhou et al. 2019). IL-17 A, IFN-, and IL-1 are positive regulators of RNase 7 expression; they are inflammatory cytokines (Simanski et al. 2013). It is worth mentioning that RNase 7 is triggered by skin damage at a faster rate than hBD-2 and hBD-3. In psoriasis and atopic dermatitis (AD), skin lesions show elevated levels of RNase 7 (Harder et al. 2010). This discovery disproves the hypothesis that AMP expression is reduced in AD and points to the role of RNase 7 during the acute phase of inflammation (Nakatsuji et al. 2017). Among the many psoriasis triggers, RNase 7 is comparable to LL-37 and hBDs in that it is known to enhance self-DNA sensing by human pDCs and keratinocytes via TLR regulation (Kopfnagel et al. 2018a, b, 2020a, b). Interestingly, apart from its ribonuclease activity, RNase 7 has been found to inhibit the production of Th2 cytokines (IL-13, IL-4, and IL-5) in human CD4 + T cells
In the epidermis affected by psoriasis, hBD-2 and hBD-3, LL-37, and RNase 7 are all induced It is believed that the increased expression of AMPs in the epidermis of individuals with psoriasis is a factor in the low incidence of infections in psoriatic lesions. In addition to their antimicrobial properties, AMPs also exert immunomodulatory effects in the context of psoriasis. In recent years, the role of LL-37 as a modulator of psoriasis progression has received particular attention. In a study conducted by Gambichler et al., it was demonstrated that etanercept, a biologic inhibitor of the pro-inflammatory cytokine TNF-α, reduces LL-37 expression in psoriasis. This finding may indicate that the observed clinical improvement is mediated through this mechanism (Gambichler et al. 2011).
Atopic Dermatitis
Exacerbation of AD is associated with elevated plasma levels of a-defensins 1–3, which have been found to positively correlate with AD severity, itch intensity, serum IL-8, and immunoglobulin E (IgE) levels, according to a prior study. Prior research has demonstrated that hBD-2 and hBD-3 concentrations in AD skin biopsies are lower than those in psoriasis biopsies (Nomura et al. 2003; Ong et al. 2002). The hypothesis suggests that the shortages in hBD were a result of excessive production of Th2-derived cytokines, which suppressed the synthesis of hBD. Additionally, the absence of hBD inducers such as IFNc, IL-17, and IL-22 contributed to this deficiency (Alase et al. 2012; Liew et al. 2010). It was also hypothesized that a reduction in hBD could be a factor in the increased susceptibility of AD patients to infections, particularly hBD-2 and hBD-3, which demonstrated antimicrobial activity against the most prevalent microorganisms that colonize AD skin, S. aureus, herpes simplex virus (HSV), and vaccinia virus (Chieosilapatham et al. 2017; Gallo and Nakatsuji 2011). Conversely, in the lesional AD epidermis, hBD-2 and hBD-3 levels were elevated in comparison to the healthy control skin (Asano et al. 2008). While the association between high or low hBD expression and AD pathogenesis remains debatable, a prior investigation revealed that hBD-1 and hBD-3 improved skin barrier function by reinstating the permeability of disrupted epidermis at tight junctions in AD skin. This discovery has provided support for the potential of hBD-3 as a viable therapeutic intervention for AD (Kiatsurayanon et al. 2014; Hönzke et al. 2016). Cathelicidins have immunomodulatory effects in addition to pro- and anti-inflammatory actions (Harten et al. 2018). There may be a connection between the itching feeling in AD and cathelicidins, which can stimulate mast cell degranulation and downregulate IL-10 in keratinocytes (Kahlenberg and Kaplan 2013). In addition to enhancing the skin barrier, cathelicidins promote cutaneous immunity by increasing the expression and distribution of components of tight junctions on cell membranes (Akiyama et al. 2014; Nguyen et al. 2020). Evidence suggests that cathelicidin levels are lower in lesional AD skin in AD patients and that these levels are connected with both the severity of AD infections and the ability to ward against cutaneous Staphylococcus aureus infections (Ong et al. 2002). LL-37 is linked to all three main pathogenetic characteristics of AD—barrier injury, staphylococcal hypercolonization, and Th2 inflammation—so it is possible that it plays a significant role in the development of the disease. The substantial reductions in LL-37 concentrations observed in the epidermis of AD patients suggest that LL-37 might be a key factor in the development of AD. This raises the possibility that LL-37 could be targeted therapeutically in the future to combat AD (Szabó et al. 2023; Joshi et al. 2023). Psoriasin production can be increased in response to a disruption in the epidermis barrier; in patients with AD, a higher psoriasin level was associated with an increased susceptibility to infections (Harder et al. 2010; Gläser et al. 2009). One study demonstrated an elevation in psoriasin concentration in the stratum granulosum of the epidermis of individuals with AD lesions in comparison to the skin that is healthy. Conversely, another study observed a minimal level of this peptide in the same area (Harder et al. 2010; Gläser et al. 2009; Patra et al. 2018). DCD1L induces the synthesis of IL-4, IL-13, IL-31, and TNF-α by keratinocytes, hence modulating the immune response (Paulmann et al. 2012). The sweat of individuals with AD showed a reduction in dermcidin levels, indicating that the increased vulnerability to certain skin infections and changes in colonization in these patients may be linked to a drop in antimicrobial peptides in the sweat. Nevertheless, immunohistochemical research revealed a more pronounced intensity of the dermcidin antigen in the sweat glands of patients with AD compared to individuals with lichen planus and psoriasis (Burian and Schittek 2015). The incongruous findings suggest that the malfunctioning of sweat glands and delivery systems may contribute to AD (Shiohara et al. 2011). When comparing AD skin samples to those from psoriatic or normal skin, immunohistochemical analysis showed that RNase7 expression was upregulated (Harder et al. 2010). The significance of RNase7 in the pathogenesis of AD is highlighted by the fact that CD4 + T cells from AD patients generated less IL-4, IL-5, and IL-13 when stimulated with RNase7 compared to controls. These Th2 cytokines are crucial to the formation of AD (Kopfnagel et al. 2017).
Impaired expression of AMPs may contribute to the increased susceptibility of AD patients to S. aureus superinfections, according to one hypothesis. hBD-2, hBD-3, and LL-37 expression levels were found to be lower in the epidermis of AD patients compared to those with psoriasis vulgaris, according to research (Nomura et al. 2003; Ong et al. 2002). Reasons for this might include an overabundance of Th2 cytokines (IL-4, IL-10, and IL-13) and the absence of key inducers, including IL-1, IL-17, and IL-22, in AD skin (Gläser et al. 2009). Nevertheless, AMPs in AD were only detected when compared to skin with lesional psoriasis, not healthy skin. Cathelicidin LL-37 expression was greater in lesional atopic skin compared to non-lesional atopic skin, according to Ballardini et al. (Ballardini et al. 2009). This may be consistent with the finding that oral supplementation in AD patients induces LL-37 production in the skin and directly regulates human cathelicidin expression in keratinocytes; UVB phototherapy promotes the healing of eczema lesions via an increase in vitamin D production (Hata et al. 2008).
Rosacea
The skin affected by rosacea has an unusually elevated level of cathelicidin expression (Yamasaki et al. 2007). Patients with rosacea abnormally convert cathelicidin into peptide forms not present in healthy skin, according to mass spectrometry analysis of these cathelicidin peptides. An increase in stratum corneum tryptic enzyme (SCTE/kallikrein 5) was responsible for the post-translational processing aberration observed in these cathelicidin peptides (Hata et al. 2008; Yamasaki et al. 2006). The pathophysiology of this disorder was associated with the significance of these findings, as peptides identified in rosacea induced cytokine secretion from human keratinocytes and caused skin inflammation and telangiectasia in mice but not in normal skin (Yamasaki et al. 2006, 2007). Hence, the combination of increased synthesis of antimicrobial peptides and excessive enzymatic processing offers a rationale for the array of clinical manifestations observed in individuals with this prevalent dermatological condition. This study is the initial documentation indicating that dysfunction in antimicrobial peptides might worsen inflammatory skin conditions. Activation of the intrinsic immune system often results in a regulated elevation of cytokines and antimicrobial compounds in the skin, such as cathelicidin (Patil et al. 2004). Cathelicidin peptides can exhibit dual properties of vasoactivity and proinflammatory activity in some forms (Hoover et al. 2003). An important insight into rosacea was gained by noting that patients with this condition exhibit unusually elevated amounts of cathelicidin in their outermost layer of skin, known as the epidermis (Bastian and Schäfer 2001). Significantly, the cathelicidin peptide types present in individuals with rosacea exhibited both a higher quantity and distinct molecular weights in comparison to those observed in those without the condition. The atypical cathelicidin peptides facilitate and control the movement of leukocytes toward chemical signals, the formation of new blood vessels, and the production of components in the extracellular matrix. Conversely, the cathelicidin peptides typically present on healthy skin mostly act as antibiotics and have minimal to no impact on inflammation (Hoover et al. 2003). LL-37, in its active form, is normally found in neutrophils that have been recruited to investigate skin infections or injuries. Nevertheless, LL-37 appears to be produced in the epidermis of rosacea patients through an aberrant mechanism involving serine proteases. Rabbit models of hind-limb ischemia-induced neovascularization in response to LL-37, which was identified as a potent angiogenic factor when tested in animal models (Hoover et al. 2003). Skin inflammation mimicking pathologic alterations in rosacea was observed in mice when cathelicidin or its production enzymes were injected into their skin, according to Yamasaki and colleagues (Bastian and Schäfer 2001). Excessive amounts of cathelicidin peptides are caused by an abnormal synthesis of the local serine protease KLK5, which breaks down a precursor protein in the epidermis (Hollox et al. 2003). It appears that rosacea sufferers have an unusually high level of protease activity, which leads to an improper conversion of cathelicidin to peptides that cause the skin to become inflamed and develop vascular abnormalities. Rosacea sufferers have higher levels of TLR2 expression in their skin compared to those who are unaffected (Feng et al. 2006). Glucocorticoids have been found to enhance the expression of Toll-like receptor 2 in keratinocytes found in the skin (Conejo-Garcia et al. 2004). Glucocorticoid-induced rosacea-like dermatitis, also known as perioral dermatitis, exhibits symptoms such as redness, pus-filled lesions, and small raised bumps that resemble those observed in rosacea. It is possible that this condition is triggered by TLR2. Muto et al. found that mice lacking mast cells (MCs) due to the KitW-sh mutation did not exhibit rosacea-like symptoms following dermal injection of LL-37. Additionally, they observed that stabilizing mast cells can immediately alleviate skin inflammation in both mice and individuals with rosacea (Wetering et al. 1997). The findings emphasize the significant involvement of mast cells in the inflammatory process following cathelicidin activation and suggest that suppressing activated mast cells might be a potential therapeutic approach for treating rosacea. In an in vivo experiment, Two and colleagues reported that inhibiting KLK5 might potentially enhance the clinical symptoms of rosacea by reducing the synthesis of LL-37 (Niyonsaba et al. 2005). Studies in clinical settings have found that rosacea patients exhibit increased levels of dermcidin, changed profiles of peptides, or genetic abnormalities when compared to those without the condition. Observations have been made on the correlations between dermcidin levels and the severity of the disease, its subtypes, or the response to therapy (Elesawy et al. 2023). Perhaps a turning point has been attained in the treatment of rosacea.
Acne Vulgaris
The pilosebaceous units are chronically inflamed in acne vulgaris, a condition that disproportionately affects young adults and adolescents. Comedones, papules, pustules, nodules, and cysts are hallmarks of this skin condition, which develops due to inflammation and blockage. From what is known, the pathophysiology includes P. acnes colonization, inflammation, excessive sebum production, and follicular hyper-proliferation (Gollnick 2003). The AMPs upregulated in keratinocytes and sebocytes in acne vulgaris are caused by Cutibacterium (previously Propionibacterium) acnes. These AMPs have the potential to either amplify the inflammatory response or have beneficial effects, such as antimicrobial and anti-inflammatory properties (Korting et al. 2012). A significant increase in human defensins (HBD-1) and − 2 in the lesional and perilesional epithelium of acne vulgaris patients is observed. It is highly plausible that P. acnes triggers the expression of HBD-2, which is mediated by the Toll-like receptor (TLR) signaling pathway (Das and Reynolds 2014). While the precise functions of supplementary antimicrobial peptides (AMPs) remain not completely elucidated, it is recognized that psoriasin, produced by sebocytes and keratinocytes, along with defensins and cathelicidin LL-37, possess the capability to eradicate P. acnes bacteria. This makes these AMPs promising targets for future therapeutic interventions targeting the acne pathophysiology. Several AMPs, including LL-37, hBD-2, RNase 7, and psoriasin, have been demonstrated to inhibit its proliferation in vitro. The microbiota of the hair follicle is rich in C. acnes, and the keratinocytes of the hair follicle generate AMPs, such as LL-37, psoriasin, and RNase 7 (Niyonsaba et al. 2007a, b). C. acnes growth regulation in the hair follicle niche may thus be AMP-dependent (Lousada et al. 2021). Acne vulgaris and other dermatological conditions caused by Candida albicans have not yet been proven to have defective AMP-mediated growth regulation of the bacteria (Harder et al. 2013). On the other hand, research has demonstrated that acne patients’ sweat has lower levels of dermcidin (DCD) (Nakano et al. 2015).
One study (Al-Sudany et al. 2019) demonstrated elevated levels of S100a7a in patients with acne vulgaris in both groups prior to therapeutic intervention. This finding aligns with the results reported by Borovaya and Batycka-Baran, who also observed a significant increase in this antimicrobial peptide in chronic inflammatory skin lesions of various dermatoses, attributed to excessive production by epidermal keratinocytes(Borovaya et al. 2014; Batycka-Baran et al. 2015, 2019). However, while the increase in S100a7a peptide has been seen in cases of acne vulgaris, the excessive production of keratinocytes in acne vulgaris has not been shown (Borovaya et al. 2014).
LL-37 demonstrates the capacity to inhibit the growth of P. acnes. LL-37, similar to hBD-2, is present in sebaceous glands. In addition to its pro-inflammatory and anti-inflammatory properties, LL-37 also affects the functionality of TLR. This AMP is capable of LPS neutralization. There is an upregulation of LL-37 gene expression in response to P. acnes (Lee et al. 2010). Furthermore, psoriasin is upregulated in acne vulgaris lesions and is also present in acne-associated sebaceous glands (Borovaya et al. 2014; Ganceviciene et al. 2006). Additionally, RNase 7 exhibits in vitro efficacy against P. acnes and is found in the epithelium of hair follicles (Simanski et al. 2012).
Another potential alternative to using AMP produced by humans is to create synthetic AMP using elements already present in nature, such as those in plants and animals. The development of synthesized AMP as an acne medication has really been the subject of some research. Omiganan, a man-made version of the naturally occurring bovine AMP indolicidine, is one such AMP that shows great promise as an acne treatment (Melo et al. 2006). As a topical solution for the treatment of acne, this chemical had favorable outcomes in five clinical studies and exhibited highly powerful antibacterial activity. In mild to moderate acne, omigiana reduced the severity of the illness and the inflammatory lesion (Wiesner and Vilcinskas 2010).
Hidradenitis Suppurativa (HS)
More recent studies have shed light on the processes that contribute to HS. The HS phenotype is caused by a combination of events, including follicular blockage and rupture, an immunological response specific to foreign bodies, a particular genetic signature, and environmental variables such as microbial colonization (Prens and Deckers 2015). It was demonstrated that AMPs were overexpressed in HS lesions relative to non-lesional epidermis (Schlapbach et al. 2009a, b). The overexpression of psoriasin-mRNA was observed in keratinocytes, while dermal macrophages were the primary producers of hBD-2-mRNA. As a result, HS lesions exhibited a relative deficiency of hBD-2 in the epidermis. Emelianov et al. identified increased levels of the proteins hBD-3, psoriasin, and cathelicidin LL-37 in an immunohistochemical analysis of HS patients (Emelianov et al. 2012a, b). On the contrary, other researchers documented a relative mRNA deficiency of multiple AMPs, which was hypothesized to be associated with a relative deficiency of IL-22 and IL-20 in HS (Wolk et al. 2011). It is still not known how AMP expression patterns in HS work. Most studies that have looked at hBD-1 expression have found that it is downregulated in HS. One study found no change in hBD-1 mRNA expression compared to healthy controls, while many studies show reduced hBD-1 mRNA expression and protein synthesis (Ardon et al. 2021; Bechara et al. 2012). Schlapbach et al. demonstrated a 20-fold upregulation of hBD-2 mRNA in the lesional HS epidermis in comparison to healthy controls (Schlapbach et al. 2009a, b). It is interesting to note that two studies show lower amounts of hBD-2 protein in lesional HS skin, in contrast to the larger levels that were found in one experiment (Ardon et al. 2021; Schlapbach et al. 2009a, b). There is a lack of consensus among the available evidence about the involvement of hBD-3 in HS. According to the findings of four investigations (Emelianov et al. 2012a, b; Giamarellos-Bourboulis et al. 2016), the mRNA and protein levels of hBD-3 were found to be elevated. Hofmann et al. found an increase and induction of hBD-3 at both the transcriptional and protein levels, with the exception of individuals who had severe HS, also known as Hurley stage III (Hofmann et al. 2012). Because these individuals may be more susceptible to bacterial superinfections, the authors speculate that their decreased hBD-3 inducibility may indicate a propensity for the development of severe HS. Another possible immunological explanation for hBD-3’s absence is its inhibitory properties towards tumor necrosis factor (TNF), which would indicate an anti-inflammatory role for the protein (Semple et al. 2010a, b).
S100A7 mRNA and protein expression were shown to be elevated in lesional HS skin, according to Schlapbach et al. (Schlapbach et al. 2009a, b). Several additional studies have confirmed these findings S100A7 overexpression in HS appears to be less severe than in psoriatic skin. Because IL-22 is an important inducer of S100A7, Wolk et al. propose that HS patients do not exhibit the same level of upregulation as psoriasis patients Lesional HS skin had higher S100A7 protein levels than perilesional HS skin, according to Batycha-Baran et al. (Batycka-Baran et al. 2021). It is noteworthy that the serum concentrations of S100A7 protein were considerably diminished in comparison to both healthy controls and patients diagnosed with psoriasis, thereby challenging prior research (Batycka-Baran et al. 2021).
AMPs and Wound Healing
Protection of cell migration and proliferation, facilitation of angiogenesis, and modulation of inflammation are a few of the numerous mechanisms by which human AMPs have been shown to promote cutaneous wound healing (Veith et al. 2019). In addition to regulating cell maturation and migration, defensins also possess immunomodulatory properties. An illustration of this can be seen in the upregulation of keratinocyte cytokine production, cell migration, and proliferation via phosphorylation of the growth factor receptor (EGFR) and signal transducer and activator of transcription (STAT) by keratinocytes at lesion sites, where hBD-2 and hBD-3 are highly expressed (Petkovic et al. 2021). In a porcine model, hBD-3 significantly accelerates the closure of infected cutaneous lesions when applied topically (Takahashi et al. 2021). Additionally, hBD-3 regulates the trafficking of monocytes and attracts macrophages to the site of injury via the chemokine receptor CCR2 (Jin et al. 2010). Furthermore, it suppresses the TLR signaling pathway in immune cells, which is subsequently followed by the in vitro and in vivo expression of proinflammatory genes (Gao et al. 2017). The properties of LL-37 in promoting tissue regeneration have been the subject of extensive research. After transfer to the excision incisions of mice, LL-37 accelerates the reepithelialization and formation of granulation tissue, in addition to promoting the migration of keratinocytes in vitro (Carretero et al. 2008). Additionally, it has been observed that LL-37 promotes angiogenesis and cell proliferation by activating formyl peptide receptor-like 1 (Verjans et al. 2016). In addition, it combats pathogenic microbes indirectly and mediates the anti-inflammatory response. Therefore, by directly impeding the activity of TLR4 (toll-like receptor 4) on dendritic cells, it could impede their maturation and prevent them from presenting a response to lipopolysaccharide (LPS) or other ligands recognized by TLR4 (Zhang et al. 2021a, b). The effectiveness of an engineered LL-37 peptide, 17BIPHE2, in eradicating resistant MRSA in a mouse model of chronic wounds has been demonstrated in terms of therapeutic effects (Miao et al. 2021). Significantly, LL-37 exhibited dose-response activity and was found to be safe in a clinical trial designed to treat venous leg ulcers; however, full lesion healing could not be observed under any conditions (Grönberg et al. 2014). In addition to inducing growth factors such as EGF and VEGF, the LL-37 peptide has been shown to bind to their receptors (Mookherjee and Hancock 2007; Tokumaru et al. 2005). The insufficiency of CRAMP (the murine homolog of LL-37/hCAP-18) during wound restoration in mice demonstrates that the peptide can induce endothelial cell stimulation, leading to an upregulation of proliferation and subsequent enhancement of dermal neovascularization (Koczulla et al. 2003). Moreover, elevated concentrations of hBD-2 and hBD-3 identified in wound sites stimulate the migration and proliferation of keratinocytes, suggesting their participation in the process of re-epithelialization of the epithelium undergoing repair (MacLeod and Mansbridge 2016; Raziyeva et al. 2021). Akt and extracellular signal-regulated kinase (ERK) levels were found to be elevated in a recent study that applied insulin topically to wounds (Lima et al. 2012). ERK is a component of a phosphorylation pathway that initiates gene transcription, which in turn promotes cellular growth. Elevated Akt levels induce angiogenesis by stimulating VEGF signaling (Yoon and Seger 2006; Mouritzen et al. 2018; Somanath et al. 2008). Improved collagen fiber structure, IL-6, and VEGF were observed in wounds treated with LL-37-conjugated gold nanoparticles (Comune et al. 2017). A gene delivery system consisting of ultra-small gold nanoparticles (AuNPs) fused with antimicrobial peptide (LL-37) facilitated accelerated wound closure in chronic diabetic wounds. This was evidenced by the perfusion of newly formed blood vessels into the wounds and a reduction in the bacterial burden. Furthermore, VEGF expression increased, and granulation tissue improved with accelerated re-epithelialization (Wang et al. 2018). Wound medications inspired by LL-37 are effective, but their delivery stability must be ensured via artificially synthesized carriers such as calcium phosphate (CaP) nanoparticles. The LL-37 retains its biological functionality and antimicrobial activity against Gram-positive and Gram-negative bacteria despite being coated with CaP to prevent enzymatic degradation (Tsikourkitoudi et al. 2020). Moreover, the utilization of an LL-37-containing keratin hydrogel was found to improve wound closure, and LL-37 delivery was found to enhance angiogenesis (Jelodari et al. 2023). Nanodefensin (ND), which possesses a dual antimicrobial and immunomodulatory effect, is an additional topical anesthetic derived from human α-defensin 5 (HD5). Locally applied nanodefensin-encased hydrogel (NDEFgel) to the injured surface induced wound regeneration and upregulated the expression of myofibroblasts and GTP-binding protein Rac1, in addition to enhancing the pharmacological stability of the coating (Luo et al. 2021). The existing evidence indicates that defensins play a positive role in promoting the healing of diabetic wounds. This influence is attributed to their antimicrobial properties, immunomodulatory effects, promotion of angiogenesis, regeneration of tissue, and improvement of insulin resistance (Li et al. 2022). Based on the existing research, defensins appear to promote diabetic wound healing via antimicrobial, immunomodulatory, angiogenic, tissue regenerator, and insulin resistance improvement mechanisms.
Numerous studies have provided confirmation of the therapeutic potential of AMPs as modulators of human wound healing. For the local treatment of infected cutaneous lesions, AMPs are promising candidates, potentially surpassing conventional antibiotics on account of their antimicrobial activity (Mangoni et al. 2016). Several studies have documented the potential of LL-37 to promote wound healing in challenging-to-heal venous leg ulcers (Mangoni et al. 2016) by stimulating re-epithelialization and increased vascularization of the injured epidermis (Ramos et al. 2011).
Conclusion
The creation of peptides and the discovery of the many properties of AMPs have both made tremendous strides forward. The advantageous properties sought for the treatment of skin infections and wounds encompass the ability to modulate immunity by enhancing protective responses, mitigating excessive inflammatory reactions, and exerting direct antimicrobial effects. Additionally, the capability to promote wound healing stands as an additional advantage. In order to use AMPs therapeutically and broaden the scope of current skin condition therapies, it is necessary to understand the pathophysiology of AMP-associated skin diseases. AMPs have the potential to be a game-changer in the medical field as active agents for wound healing, immunomodulators, and pathogen-specific antibiotics for skin infections. It is unfortunate that the immense promise of AMPs as a novel class of medicines has not yet been validated.
Data Availability
No datasets were generated or analysed during the current study.
References
Aberg KM, Man MQ, Gallo RL, Ganz T, Crumrine D, Brown BE et al (2008) Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol 128(4):917–925
Ademowo OS, Hernandez B, Collins E, Rooney C, Fearon U, van Kuijk AW et al (2016) Discovery and confirmation of a protein biomarker panel with potential to predict response to biological therapy in psoriatic arthritis. Ann Rheum Dis 75(1):234–241
Agier J, Brzezińska-Błaszczyk E, Żelechowska P, Wiktorska M, Pietrzak J, Różalska S (2018) Cathelicidin LL-37 affects Surface and Intracellular Toll-Like receptor expression in tissue mast cells. J Immunol Res 2018:7357162
Aizawa N, Ishiuji Y, Tominaga M, Sakata S, Takahashi N, Yanaba K et al (2019) Relationship between the degrees of itch and serum Lipocalin-2 levels in patients with psoriasis. J Immunol Res 2019:8171373
Akiyama T, Niyonsaba F, Kiatsurayanon C, Nguyen TT, Ushio H, Fujimura T et al (2014) The human cathelicidin LL-37 host defense peptide upregulates tight junction-related proteins and increases human epidermal keratinocyte barrier function. J Innate Immun 6(6):739–753
Al-Sudany NK, Mohammed NH, Alrifai SB (2019) Downregulation of S100a7a antimicrobial peptide in acne vulgaris patients after isotretinoin therapy. Dermatol Ther 32(6):e13136
Alalwani SM, Sierigk J, Herr C, Pinkenburg O, Gallo R, Vogelmeier C et al (2010) The antimicrobial peptide LL-37 modulates the inflammatory and host defense response of human neutrophils. Eur J Immunol 40(4):1118–1126
Alase A, Seltmann J, Werfel T, Wittmann M (2012) Interleukin-33 modulates the expression of human β-defensin 2 in human primary keratinocytes and may influence the susceptibility to bacterial superinfection in acute atopic dermatitis. Br J Dermatol 167(6):1386–1389
Aldred PM, Hollox EJ, Armour JA (2005) Copy number polymorphism and expression level variation of the human alpha-defensin genes DEFA1 and DEFA3. Hum Mol Genet 14(14):2045–2052
Alford MA, Baquir B, Santana FL, Haney EF, Hancock REW (2020) Cathelicidin Host Defense Peptides and Inflammatory Signaling: striking a balance. Front Microbiol 11:1902
Aochi S, Tsuji K, Sakaguchi M, Huh N, Tsuda T, Yamanishi K et al (2011) Markedly elevated serum levels of calcium-binding S100A8/A9 proteins in psoriatic arthritis are due to activated monocytes/macrophages. J Am Acad Dermatol 64(5):879–887
Ardon C, Wang C, Prens E, van Straalen K (2021) Noninvasive assessment of cytokine and antimicrobial peptide levels in hidradenitis suppurativa using transdermal analysis patches. Br J Dermatol 184(2):343–345
Asano S, Ichikawa Y, Kumagai T, Kawashima M, Imokawa G (2008) Microanalysis of an antimicrobial peptide, beta-defensin-2, in the stratum corneum from patients with atopic dermatitis. Br J Dermatol 159(1):97–104
Awad SM, Attallah DA, Salama RH, Mahran AM, Abu El-Hamed E (2018) Serum levels of psoriasin (S100A7) and koebnerisin (S100A15) as potential markers of atherosclerosis in patients with psoriasis. Clin Exp Dermatol 43(3):262–267
Ballardini N, Johansson C, Lilja G, Lindh M, Linde Y, Scheynius A et al (2009) Enhanced expression of the antimicrobial peptide LL-37 in lesional skin of adults with atopic eczema. Br J Dermatol 161(1):40–47
Bastian A, Schäfer H (2001) Human alpha-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul Pept 101(1–3):157–161
Batycka-Baran A, Hattinger E, Zwicker S, Summer B, Zack Howard OM, Thomas P et al (2015) Leukocyte-derived koebnerisin (S100A15) and psoriasin (S100A7) are systemic mediators of inflammation in psoriasis. J Dermatol Sci 79(3):214–221
Batycka-Baran A, Hattinger E, Marchenkov A, Koziol M, Bieniek A, Szepietowski J et al (2019) Koebnerisin (S100A15): a novel player in the pathogenesis of rosacea. J Am Acad Dermatol 80(6):1753–1755
Batycka-Baran A, Baran W, Nowicka-Suszko D, Koziol-Gałczyńska M, Bieniek A, Matusiak Ł et al (2021) Serum concentration and skin expression of S100A7 (psoriasin) in patients suffering from hidradenitis suppurativa. Dermatology 237(5):733–739
Bauer B, Pang E, Holland C, Kessler M, Bartfeld S, Meyer TF (2012) The Helicobacter pylori virulence effector CagA abrogates human β-defensin 3 expression via inactivation of EGFR signaling. Cell Host Microbe 11(6):576–586
Bechara FG, Sand M, Skrygan M, Kreuter A, Altmeyer P, Gambichler T (2012) Acne inversa: evaluating antimicrobial peptides and proteins. Ann Dermatol 24(4):393–397
Bellotti D, Remelli M (2022) Lights and shadows on the therapeutic use of antimicrobial peptides. Molecules 27(14):4584
Benoit S, Toksoy A, Ahlmann M, Schmidt M, Sunderkötter C, Foell D et al (2006) Elevated serum levels of calcium-binding S100 proteins A8 and A9 reflect disease activity and abnormal differentiation of keratinocytes in psoriasis. Br J Dermatol 155(1):62–66
Bierkarre H, Harder J, Cuthbert R, Emery P, Leuschner I, Mrowietz U et al (2016) Differential expression of antimicrobial peptides in psoriasis and psoriatic arthritis as a novel contributory mechanism for skin and joint disease heterogeneity. Scand J Rheumatol 45(3):188–196
Boman HG (2003) Antibacterial peptides: basic facts and emerging concepts. J Intern Med 254(3):197–215
Borkowski AW, Gallo RL (2011) The coordinated response of the physical and antimicrobial peptide barriers of the skin. J Invest Dermatology 131(2):285–287
Borovaya A, Dombrowski Y, Zwicker S, Olisova O, Ruzicka T, Wolf R et al (2014) Isotretinoin therapy changes the expression of antimicrobial peptides in acne vulgaris. Arch Dermatol Res 306(8):689–700
Brandner JM (2016) Importance of Tight Junctions in Relation to Skin Barrier Function. In: Agner T, editor. Skin Barrier Function. 49: S.Karger AG; p. 0
Brook M, Tomlinson GH, Miles K, Smith RW, Rossi AG, Hiemstra PS et al (2016) Neutrophil-derived alpha defensins control inflammation by inhibiting macrophage mRNA translation. Proc Natl Acad Sci U S A 113(16):4350–4355
Broome AM, Ryan D, Eckert RL (2003) S100 protein subcellular localization during epidermal differentiation and psoriasis. J Histochem Cytochem 51(5):675–685
Büchau AS, Gallo RL (2007) Innate immunity and antimicrobial defense systems in psoriasis. Clin Dermatol 25(6):616–624
Buck CB, Day PM, Thompson CD, Lubkowski J, Lu W, Lowy DR et al (2006) Human alpha-defensins block papillomavirus infection. Proc Natl Acad Sci U S A 103(5):1516–1521
Burian M, Schittek B (2015) The secrets of dermcidin action. Int J Med Microbiol 305(2):283–286
Carretero M, Escámez MJ, García M, Duarte B, Holguín A, Retamosa L et al (2008) In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Invest Dermatol 128(1):223–236
Chamorro CI, Weber G, Grönberg A, Pivarcsi A, Ståhle M (2009) The human antimicrobial peptide LL-37 suppresses apoptosis in keratinocytes. J Invest Dermatol 129(4):937–944
Chen N, Jiang C (2023) Antimicrobial peptides: structure, mechanism, and modification. Eur J Med Chem 255:115377
Chieosilapatham P, Ogawa H, Niyonsaba F (2017) Current insights into the role of human β-defensins in atopic dermatitis. Clin Exp Immunol 190(2):155–166
Chiricozzi A, Guttman-Yassky E, Suárez-Fariñas M, Nograles KE, Tian S, Cardinale I et al (2011) Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol 131(3):677–687
Christophers E, Schröder JM (2022) Evolution of innate defense in human skin. Exp Dermatol 31(3):304–311
Cipáková I, Gasperík J, Hostinová E (2006) Expression and purification of human antimicrobial peptide, dermcidin, in Escherichia coli. Protein Expr Purif 45(2):269–274
Comune M, Rai A, Chereddy KK, Pinto S, Aday S, Ferreira AF et al (2017) Antimicrobial peptide-gold nanoscale therapeutic formulation with high skin regenerative potential. J Control Release 262:58–71
Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ et al (2004) Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med 10(9):950–958
D’Amico F, Skarmoutsou E, Granata M, Trovato C, Rossi GA, Mazzarino MC (2016) S100A7: a rAMPing up AMP molecule in psoriasis. Cytokine Growth Factor Rev 32:97–104
Das S, Reynolds R (2014) Recent advances in Acne Pathogenesis: implications for Therapy. Am J Clin Dermatol. ;15
Di Nardo A, Vitiello A, Gallo RL (2003) Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol 170(5):2274–2278
Donaghy H, Bosnjak L, Harman AN, Marsden V, Tyring SK, Meng TC et al (2009) Role for plasmacytoid dendritic cells in the immune control of recurrent human herpes simplex virus infection. J Virol 83(4):1952–1961
Dong X, Limjunyawong N, Sypek EI, Wang G, Ortines RV, Youn C et al (2022) Keratinocyte-derived defensins activate neutrophil-specific receptors Mrgpra2a/b to prevent skin dysbiosis and bacterial infection. Immunity 55(9):1645–62e7
Eckert RL, Broome A-M, Ruse M, Robinson N, Ryan D, Lee K (2004) S100 proteins in the epidermis. J Invest Dermatology 123(1):23–33
Elesawy F, Mohamed G, Abd EL, Mohammed S (2023) Assessment of serum Dermcidin Level in patients with Rosacea: a Comprehensive Review. Benha J Appl Sci 8(2):161–166
Emelianov VU, Bechara FG, Gläser R, Langan EA, Taungjaruwinai WM, Schröder JM et al (2012a) Immunohistological pointers to a possible role for excessive cathelicidin (LL-37) expression by apocrine sweat glands in the pathogenesis of hidradenitis suppurativa/acne inversa. Br J Dermatol 166(5):1023–1034
Emelianov V, Bechara F, Gläser R, Langan E, Taungjaruwinai W, Schröder J et al (2012b) Immunohistological pointers to a possible role for excessive cathelicidin (LL-37) expression by apocrine sweat glands in the pathogenesis of hidradenitis suppurativa/acne inversa. Br J Dermatol 166(5):1023–1034
Feng Z, Dubyak GR, Lederman MM, Weinberg A (2006) Cutting edge: human beta defensin 3–a novel antagonist of the HIV-1 coreceptor CXCR4. J Immunol 177(2):782–786
Frohm M, Agerberth B, Ahangari G, Stâhle-Bäckdahl M, Lidén S, Wigzell H et al (1997) The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem 272(24):15258–15263
Fuentes-Duculan J, Bonifacio KM, Hawkes JE, Kunjravia N, Cueto I, Li X et al (2017) Autoantigens ADAMTSL5 and LL37 are significantly upregulated in active psoriasis and localized with keratinocytes, dendritic cells and other leukocytes. Exp Dermatol 26(11):1075–1082
Gallo RL, Huttner KM (1998) Antimicrobial peptides: an emerging concept in cutaneous biology. J Invest Dermatol 111(5):739–743
Gallo RL, Nakatsuji T (2011) Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol 131(10):1974–1980
Gallo RL, Ono M, Povsic T, Page C, Eriksson E, Klagsbrun M et al (1994a) Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc Natl Acad Sci U S A 91(23):11035–11039
Gallo RL, Ono M, Povsic T, Page C, Eriksson E, Klagsbrun M et al (1994b) Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proceedings of the National Academy of Sciences. ;91(23):11035-9
Gambichler T, Kobus S, Kobus A, Tigges C, Scola N, Altmeyer P et al (2011) Expression of antimicrobial peptides and proteins in etanercept-treated psoriasis patients. Regul Pept 167(2–3):163–166
Ganceviciene R, Fimmel S, Glass E, Zouboulis CC (2006) Psoriasin and follicular hyperkeratinization in acne comedones. Dermatology 213(3):270–272
Gao W, Xiong Y, Li Q, Yang H (2017) Inhibition of toll-like receptor signaling as a Promising Therapy for Inflammatory diseases: a journey from Molecular to Nano Therapeutics. Front Physiol 8:508
Giamarellos-Bourboulis EJ, Platzer M, Karagiannidis I, Kanni T, Nikolakis G, Ulrich J et al (2016) High copy numbers of β-defensin cluster on 8p23. 1, confer genetic susceptibility, and modulate the physical course of hidradenitis suppurativa/acne inversa. J Invest Dermatology 136(8):1592–1598
Gilliet M, Cao W, Liu YJ (2008) Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol 8(8):594–606
Gläser R, Meyer-Hoffert U, Harder J, Cordes J, Wittersheim M, Kobliakova J et al (2009) The antimicrobial protein psoriasin (S100A7) is upregulated in atopic dermatitis and after experimental skin barrier disruption. J Invest Dermatol 129(3):641–649
Gollnick H (2003) Current concepts of the pathogenesis of Acne. Drugs 63(15):1579–1596
Granata M, Skarmoutsou E, Gangemi P, Mazzarino MC, D’Amico F (2019) S100A7, Jab1, and p27(kip1) expression in psoriasis and S100A7 CRISPR-activated human keratinocyte cell line. J Cell Biochem 120(3):3384–3392
Grönberg A, Mahlapuu M, Ståhle M, Whately-Smith C, Rollman O (2014) Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: a randomized, placebo-controlled clinical trial. Wound Repair Regen 22(5):613–621
Grubman A, Kaparakis M, Viala J, Allison C, Badea L, Karrar A et al (2010) The innate immune molecule, NOD1, regulates direct killing of Helicobacter pylori by antimicrobial peptides. Cell Microbiol 12(5):626–639
Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R (1996) The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem 238(2):325–332
Han F, Zhang H, Xia X, Xiong H, Song D, Zong X et al (2015) Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J Immunol 194(4):1882–1893
Hancock RE, Sahl H-G (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24(12):1551–1557
Harder J, Schroder JM (2002) RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J Biol Chem 277(48):46779–46784
Harder J, Schröder J-M (2005) Psoriatic scales: a promising source for the isolation of human skin-derived antimicrobial proteins. J Leukoc Biol 77(4):476–486
Harder J, Bartels J, Christophers E, Schröder J-M (1997) A peptide antibiotic from human skin. Nature 387(6636):861
Harder J, Dressel S, Wittersheim M, Cordes J, Meyer-Hoffert U, Mrowietz U et al (2010) Enhanced expression and secretion of antimicrobial peptides in atopic dermatitis and after superficial skin injury. J Invest Dermatol 130(5):1355–1364
Harder J, Tsuruta D, Murakami M, Kurokawa I (2013) What is the role of antimicrobial peptides (AMP) in acne vulgaris? Exp Dermatol 22(6):386–391
Hassan M, Kjos M, Nes I, Diep D, Lotfipour F (2012) Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. J Appl Microbiol 113(4):723–736
Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D et al (2008) Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol 122(4):829–831
Hawkes JE, Chan TC, Krueger JG (2017) Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol 140(3):645–653
Hegyi Z, Zwicker S, Bureik D, Peric M, Koglin S, Batycka-Baran A et al (2012) Vitamin D analog calcipotriol suppresses the Th17 cytokine–induced proinflammatory S100 alarmins psoriasin (S100A7) and koebnerisin (S100A15) in psoriasis. J Invest Dermatology 132(5):1416–1424
Hemshekhar M, Choi KG, Mookherjee N (2018) Host defense peptide LL-37-Mediated chemoattractant Properties, but not anti-inflammatory cytokine IL-1RA production, is selectively controlled by Cdc42 rho GTPase via G protein-coupled receptors and JNK mitogen-activated protein kinase. Front Immunol 9:1871
Herman A, Herman AP (2019) Antimicrobial peptides activity in the skin. Skin Res Technol 25(2):111–117
Hilchie AL, Wuerth K, Hancock RE (2013) Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol 9(12):761–768
Hofmann SC, Saborowski V, Lange S, Kern WV, Bruckner-Tuderman L, Rieg S (2012) Expression of innate defense antimicrobial peptides in hidradenitis suppurativa. J Am Acad Dermatol 66(6):966–974
Hollox EJ, Armour JA, Barber JC (2003) Extensive normal copy number variation of a beta-defensin antimicrobial-gene cluster. Am J Hum Genet 73(3):591–600
Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, Rodijk-Olthuis D et al (2008) Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet 40(1):23–25
Hönzke S, Wallmeyer L, Ostrowski A, Radbruch M, Mundhenk L, Schäfer-Korting M et al (2016) Influence of Th2 cytokines on the cornified envelope, tight Junction proteins, and ß-Defensins in filaggrin-deficient skin equivalents. J Invest Dermatol 136(3):631–639
Hoover DM, Wu Z, Tucker K, Lu W, Lubkowski J (2003) Antimicrobial characterization of human beta-defensin 3 derivatives. Antimicrob Agents Chemother 47(9):2804–2809
Horibe K, Nakamichi Y, Uehara S, Nakamura M, Koide M, Kobayashi Y et al (2013) Roles of cathelicidin-related antimicrobial peptide in murine osteoclastogenesis. Immunology 140(3):344–351
Hosoda H, Nakamura K, Hu Z, Tamura H, Reich J, Kuwahara-Arai K et al (2017) Antimicrobial cathelicidin peptide LL–37 induces NET formation and suppresses the inflammatory response in a mouse septic model. Mol Med Rep 16(4):5618–5626
Huan Y, Kong Q, Mou H, Yi H (2020) Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front Microbiol 11:2559
Huang FC (2014) Differential regulation of interleukin-8 and human beta-defensin 2 in Pseudomonas aeruginosa-infected intestinal epithelial cells. BMC Microbiol 14:275
Hwang YJ, Jung HJ, Kim MJ, Roh NK, Jung JW, Lee YW et al (2014) Serum levels of LL-37 and inflammatory cytokines in plaque and guttate psoriasis. Mediators Inflamm 2014:268257
Inomata M, Horie T, Into T (2020) Effect of the Antimicrobial peptide LL-37 on Gene expression of chemokines and 29 toll-like receptor-Associated proteins in human gingival fibroblasts under stimulation with Porphyromonas gingivalis Lipopolysaccharide. Probiotics Antimicrob Proteins 12(1):64–72
Jelodari S, Daemi H, Mohammadi P, Verdi J, Al-Awady J, Ai M (2023) J, et al. Assessment of the efficacy of an LL-37-Encapsulated keratin hydrogel for the treatment of full-thickness wounds. ACS Applied Bio Materials
Ji P, Zhou Y, Yang Y, Wu J, Zhou H, Quan W et al (2019) Myeloid cell-derived LL-37 promotes lung cancer growth by activating Wnt/β-catenin signaling. Theranostics 9(8):2209–2223
Jin G, Weinberg A (eds) (2019) Human antimicrobial peptides and cancer. Seminars in cell & developmental biology. Elsevier
Jin G, Kawsar HI, Hirsch SA, Zeng C, Jia X, Feng Z et al (2010) An antimicrobial peptide regulates Tumor-Associated macrophage trafficking via the chemokine receptor CCR2, a model for Tumorigenesis. PLoS ONE 5(6):e10993
Jinquan T, Vorum H, Larsen CG, Madsen P, Rasmussen HH, Gesser B et al (1996) Psoriasin: a novel chemotactic protein. J Invest Dermatology 107(1):5–10
Johansen C, Bertelsen T, Ljungberg C, Mose M, Iversen L (2016) Characterization of TNF-Α–and IL-17A–mediated synergistic induction of DEFB4 gene expression in human keratinocytes through IκBζ. J Invest Dermatology 136(8):1608–1616
Joshi AA, Vocanson M, Nicolas J-F, Wolf P, Patra V (2023) Microbial derived antimicrobial peptides as potential therapeutics in atopic dermatitis. Front Immunol 14:1125635
Kahlenberg JM, Kaplan MJ (2013) Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol 191(10):4895–4901
Kahraman E, Kaykın M, Şahin Bektay H, Güngör S (2019) Recent advances on topical application of Ceramides to restore barrier function of skin. Cosmetics 6(3):52
Kandler K, Shaykhiev R, Kleemann P, Klescz F, Lohoff M, Vogelmeier C et al (2006) The anti-microbial peptide LL-37 inhibits the activation of dendritic cells by TLR ligands. Int Immunol 18(12):1729–1736
Kiatsurayanon C, Niyonsaba F, Smithrithee R, Akiyama T, Ushio H, Hara M et al (2014) Host defense (Antimicrobial) peptide, human β-defensin-3, improves the function of the epithelial tight-junction barrier in human keratinocytes. J Invest Dermatol 134(8):2163–2173
Koczulla R, Von Degenfeld G, Kupatt C, Krötz F, Zahler S, Gloe T et al (2003) An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Investig 111(11):1665–1672
Koeninger L, Armbruster NS, Brinch KS, Kjaerulf S, Andersen B, Langnau C et al (2020) Human β-Defensin 2 mediated Immune Modulation as treatment for experimental colitis. Front Immunol 11:93
Kolbinger F, Loesche C, Valentin MA, Jiang X, Cheng Y, Jarvis P et al (2017) β-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol 139(3):923–32e8
Koo HB, Seo J (2019) Antimicrobial peptides under clinical investigation. Pept Sci 111(5):e24122
Kopfnagel V, Wagenknecht S, Brand L, Zeitvogel J, Harder J, Hofmann K et al (2017) RN ase 7 downregulates TH 2 cytokine production by activated human T cells. Allergy 72(11):1694–1703
Kopfnagel V, Wagenknecht S, Harder J, Hofmann K, Kleine M, Buch A et al (2018a) RNase 7 strongly promotes TLR9-mediated DNA sensing by human plasmacytoid dendritic cells. J Invest Dermatology 138(4):872–881
Kopfnagel V, Wagenknecht S, Harder J, Hofmann K, Kleine M, Buch A et al (2018b) RNase 7 strongly promotes TLR9-Mediated DNA sensing by human plasmacytoid dendritic cells. J Invest Dermatol 138(4):872–881
Kopfnagel V, Dreyer S, Baumert K, Stark M, Harder J, Hofmann K et al (2020a) RNase 7 promotes sensing of self-DNA by human keratinocytes and activates an antiviral immune response. J Invest Dermatology 140(8):1589–1598 e3
Kopfnagel V, Dreyer S, Baumert K, Stark M, Harder J, Hofmann K et al (2020b) RNase 7 promotes sensing of Self-DNA by Human keratinocytes and activates an antiviral Immune response. J Invest Dermatol 140(8):1589–98e3
Korting HC, Schöllmann C, Stauss-Grabo M, Schäfer-Korting M (2012) Antimicrobial peptides and skin: a paradigm of translational medicine. Skin Pharmacol Physiol 25(6):323–334
Krishna S, Miller LS (eds) (2012) Innate and adaptive immune responses against Staphylococcus aureus skin infections. Seminars in immunopathology. Springer
Kurpet K, Chwatko G (2022) S100 proteins as novel therapeutic targets in psoriasis and other autoimmune diseases. Molecules 27(19):6640
Lai Y, Gallo RL (2009) AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol 30(3):131–141
Lai YP, Peng YF, Zuo Y, Li J, Huang J, Wang LF et al (2005) Functional and structural characterization of recombinant dermcidin-1L, a human antimicrobial peptide. Biochem Biophys Res Commun 328(1):243–250
Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C et al (2014) The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun 5:5621
Laneri S, Brancaccio M, Mennitti C, De Biasi MG, Pero ME, Pisanelli G et al (2021) Antimicrobial peptides and physical activity: a Great Hope against COVID 19. Microorganisms. ;9(7)
Lao J, Xie Z, Qin Q, Qin R, Li S, Yuan Y (2023) Serum LL-37 and inflammatory cytokines levels in psoriasis. Immun Inflamm Dis 11(3):e802
Larrick JW, Lee J, Ma S, Li X, Francke U, Wright SC et al (1996) Structural, functional analysis and localization of the human CAP18 gene. FEBS Lett 398(1):74–80
Le C-F, Fang C-M, Sekaran SD (2017) Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob Agents Chemother 61(4):02340–02316. https://doi.org/10.1128/aac
Lee SE, Kim JM, Jeong SK, Jeon JE, Yoon HJ, Jeong MK et al (2010) Protease-activated receptor-2 mediates the expression of inflammatory cytokines, antimicrobial peptides, and matrix metalloproteinases in keratinocytes in response to Propionibacterium acnes. Arch Dermatol Res 302(10):745–756
Lee B, Moon KM, Kim CY (2018) Tight Junction in the intestinal epithelium: its Association with diseases and Regulation by Phytochemicals. J Immunol Res 2018:2645465
Lei H, Wang Y, Zhang T, Chang L, Wu Y, Lai Y (2017) TLR3 activation induces S100A7 to regulate keratinocyte differentiation after skin injury. Sci China Life Sci 60(2):158–167
Li M, Rigby K, Lai Y, Nair V, Peschel A, Schittek B et al (2009) Staphylococcus aureus mutant screen reveals interaction of the human antimicrobial peptide dermcidin with membrane phospholipids. Antimicrob Agents Chemother 53(10):4200–4210
Li N, Yamasaki K, Saito R, Fukushi-Takahashi S, Shimada-Omori R, Asano M et al (2014) Alarmin function of cathelicidin antimicrobial peptide LL37 through IL-36γ induction in human epidermal keratinocytes. J Immunol 193(10):5140–5148
Li G, Wang Q, Feng J, Wang J, Wang Y, Huang X et al (2022) Recent insights into the role of defensins in diabetic wound healing. Biomed Pharmacother 155:113694
Liang S, Guo XK, Ou J, Huang R, Xue Q, Zhang B et al (2019) Nutrient sensing by the intestinal epithelium orchestrates mucosal Antimicrobial Defense via Translational Control of Hes1. Cell Host Microbe 25(5):706–18e7
Liang H, Li J, Zhang K (2023) Pathogenic role of S100 proteins in psoriasis. Front Immunol 14:1191645
Liew FY, Pitman NI, McInnes IB (2010) Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol 10(2):103–110
Lima MH, Caricilli AM, de Abreu LL, Araújo EP, Pelegrinelli FF, Thirone AC et al (2012) Topical insulin accelerates wound healing in diabetes by enhancing the AKT and ERK pathways: a double-blind placebo-controlled clinical trial. PLoS ONE 7(5):e36974
Lin B, Hung A, Li R, Barlow A, Singleton W, Matthyssen T et al (2022) Systematic comparison of activity and mechanism of antimicrobial peptides against nosocomial pathogens. Eur J Med Chem 231:114135
Lousada MB, Lachnit T, Edelkamp J, Rouillé T, Ajdic D, Uchida Y et al (2021) Exploring the human hair follicle microbiome. Br J Dermatol 184(5):802–815
Luo XL, Li JX, Huang HR, Duan JL, Dai RX, Tao RJ et al (2019) LL37 inhibits aspergillus fumigatus infection via directly binding to the Fungus and preventing excessive inflammation. Front Immunol 10:283
Luo G, Sun Y, Zhang J, Xu Z, Lu W, Wang H et al (2021) Nanodefensin-encased hydrogel with dual bactericidal and pro-regenerative functions for advanced wound therapy. Theranostics 11(8):3642
Mabuchi T, Hirayama N (2016) Binding Affinity and Interaction of LL-37 with HLA-C*06:02 in Psoriasis. J Invest Dermatol 136(9):1901–1903
MacLeod AS, Mansbridge JN (2016) The innate immune system in acute and chronic wounds. Adv Wound care 5(2):65–78
Madsen P, Rasmussen HH, Leffers H, Honoré B, Dejgaard K, Olsen E et al (1991) Molecular cloning, occurrence, and expression of a novel partially secreted protein psoriasin that is highly up-regulated in psoriatic skin. J Invest Dermatology 97(4):701–712
Mahanty S, Setty SRG (2021) Epidermal Lamellar Body Biogenesis: insight into the roles of Golgi and Lysosomes. Front Cell Dev Biology. ;9
Mangoni ML, McDermott AM, Zasloff M (2016) Antimicrobial peptides and wound healing: biological and therapeutic considerations. Exp Dermatol 25(3):167–173
Marr AK, Gooderham WJ, Hancock RE (2006) Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol 6(5):468–472
Meade KG, O’Farrelly C (2018) β-Defensins: farming the Microbiome for Homeostasis and Health. Front Immunol 9:3072
Melo MN, Dugourd D, Castanho MA (2006) Omiganan pentahydrochloride in the front line of clinical applications of antimicrobial peptides. Recent Pat Antiinfect Drug Discov 1(2):201–207
Miao F, Li Y, Tai Z, Zhang Y, Gao Y, Hu M et al (2021) Antimicrobial peptides: the promising therapeutics for Cutaneous Wound Healing. Macromol Biosci 21(10):2100103
Mookherjee N, Hancock RE (2007) Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci 64(7–8):922–933
Mouritzen MV, Abourayale S, Ejaz R, Ardon CB, Carvalho E, Dalgaard LT et al (2018) Neurotensin, substance P, and insulin enhance cell migration. J Pept Sci 24(7):e3093
Murakami M, Ohtake T, Dorschner RA, Schittek B, Garbe C, Gallo RL (2002) Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J Invest Dermatol 119(5):1090–1095
Mustafa S, Balkhy H, Gabere MN (2018) Current treatment options and the role of peptides as potential therapeutic components for Middle East Respiratory Syndrome (MERS): a review. J Infect Public Health 11(1):9–17
Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T et al (2015) Severity of Psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol 35(12):2667–2676
Nakagawa Y, Gallo RL (2015) Endogenous intracellular cathelicidin enhances TLR9 activation in dendritic cells and macrophages. J Immunol 194(3):1274–1284
Nakano T, Yoshino T, Fujimura T, Arai S, Mukuno A, Sato N et al (2015) Reduced expression of dermcidin, a peptide active against propionibacterium acnes, in sweat of patients with acne vulgaris. Acta Derm Venereol 95(7):783–786
Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T et al (2017) Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. ;9(378)
Namjoshi S, Caccetta R, Benson HA (2008) Skin peptides: biological activity and therapeutic opportunities. J Pharm Sci 97(7):2524–2542
Nestle FO, Di Meglio P, Qin J-Z, Nickoloff BJ (2009) Skin immune sentinels in health and disease. Nat Rev Immunol 9(10):679–691
Nguyen HLT, Trujillo-Paez JV, Umehara Y, Yue H, Peng G, Kiatsurayanon C et al (2020) Role of antimicrobial peptides in skin barrier repair in individuals with atopic dermatitis. Int J Mol Sci. ;21(20)
Niyonsaba F, Ushio H, Nagaoka I, Okumura K, Ogawa H (2005) The human beta-defensins (-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J Immunol 175(3):1776–1784
Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K et al (2007a) Antimicrobial peptides human β-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatology 127(3):594–604
Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K et al (2007b) Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol 127(3):594–604
Niyonsaba F, Suzuki A, Ushio H, Nagaoka I, Ogawa H, Okumura K (2009) The human antimicrobial peptide dermcidin activates normal human keratinocytes. Br J Dermatol 160(2):243–249
Niyonsaba F, Kiatsurayanon C, Chieosilapatham P, Ogawa H (2017) Friends or foes? Host defense (antimicrobial) peptides and proteins in human skin diseases. Exp Dermatol 26(11):989–998
Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF et al (2003) Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol 171(6):3262–3269
Nukui T, Ehama R, Sakaguchi M, Sonegawa H, Katagiri C, Hibino T et al (2008) S100A8/A9, a key mediator for positive feedback growth stimulation of normal human keratinocytes. J Cell Biochem 104(2):453–464
Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T et al (2002) Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 347(15):1151–1160
Patil A, Hughes AL, Zhang G (2004) Rapid evolution and diversification of mammalian alpha-defensins as revealed by comparative analysis of rodent and primate genes. Physiol Genomics 20(1):1–11
Patra V, Mayer G, Gruber-Wackernagel A, Horn M, Lembo S, Wolf P (2018) Unique profile of antimicrobial peptide expression in polymorphic light eruption lesions compared to healthy skin, atopic dermatitis, and psoriasis. Photodermatol Photoimmunol Photomed 34(2):137–144
Paulmann M, Arnold T, Linke D, Özdirekcan S, Kopp A, Gutsmann T et al (2012) Structure-activity analysis of the dermcidin-derived peptide DCD-1L, an anionic antimicrobial peptide present in human sweat. J Biol Chem 287(11):8434–8443
Peng G, Tsukamoto S, Ikutama R, Nguyen HLT, Umehara Y, Trujillo-Paez JV et al (2022) Human β-defensin-3 attenuates atopic dermatitis–like inflammation through autophagy activation and the aryl hydrocarbon receptor signaling pathway. J Clin Investig. ;132(17)
Petkovic M, Mouritzen MV, Mojsoska B, Jenssen H (2021) Immunomodulatory Properties of Host Defence Peptides in Skin Wound Healing. Biomolecules. ;11(7)
Pirtskhalava M, Gabrielian A, Cruz P, Griggs HL, Squires RB, Hurt DE et al (2016) DBAASP v.2: an enhanced database of structure and antimicrobial/cytotoxic activity of natural and synthetic peptides. Nucleic Acids Res 44(D1):D1104–D1112
Prasad SV, Fiedoruk K, Daniluk T, Piktel E, Bucki R (2019) Expression and function of host defense peptides at inflammation sites. Int J Mol Sci. ;21(1)
Prens E, Deckers I (2015) Pathophysiology of hidradenitis suppurativa: an update. J Am Acad Dermatol 73(5 Suppl 1):S8–11
Qian L, Chen W, Sun W, Li M, Zheng R, Qian Q et al (2015) Antimicrobial peptide LL-37 along with peptidoglycan drive monocyte polarization toward CD14(high)CD16(+) subset and may play a crucial role in the pathogenesis of psoriasis guttata. Am J Transl Res 7(6):1081–1094
Rademacher F, Gläser R, Harder J (2021) Antimicrobial peptides and proteins: Interaction with the skin microbiota. Exp Dermatol 30(10):1496–1508
Ramos R, Silva JP, Rodrigues AC, Costa R, Guardão L, Schmitt F et al (2011) Wound healing activity of the human antimicrobial peptide LL37. Peptides 32(7):1469–1476
Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A (2021) Immunology of acute and chronic wound healing. Biomolecules 11(5):700
Reinholz M, Ruzicka T, Schauber J, Cathelicidin (2012) LL-37: an antimicrobial peptide with a role in inflammatory skin disease. Ann Dermatol 24(2):126–135
Rieg S, Steffen H, Seeber S, Humeny A, Kalbacher H, Dietz K et al (2005) Deficiency of dermcidin-derived antimicrobial peptides in sweat of patients with atopic dermatitis correlates with an impaired innate defense of human skin in vivo. J Immunol 174(12):8003–8010
Saito-Sasaki N, Sawada Y (2023) S100 proteins in the pathogenesis of Psoriasis and atopic dermatitis. Diagnostics 13(20):3167
Schittek B, Paulmann M, Senyurek I, Steffen H (2008) The role of antimicrobial peptides in human skin and in skin infectious diseases. Infectious disorders-drug targets (formerly current drug targets-infectious disorders). 8(3):135–143
Schlapbach C, Yawalkar N, Hunger RE (2009a) Human beta-defensin-2 and psoriasin are overexpressed in lesions of acne inversa. J Am Acad Dermatol 61(1):58–65
Schlapbach C, Yawalkar N, Hunger RE (2009b) Human β-defensin-2 and psoriasin are overexpressed in lesions of acne inversa. J Am Acad Dermatol 61(1):58–65
Schonthaler HB, Guinea-Viniegra J, Wculek SK, Ruppen I, Ximénez-Embún P, Guío-Carrión A et al (2013) S100A8-S100A9 protein complex mediates psoriasis by regulating the expression of complement factor C3. Immunity 39(6):1171–1181
Schutte BC, McCray PB Jr (2002) β-defensins in lung host defense. Annu Rev Physiol 64(1):709–748
Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE (2002) The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol 169(7):3883–3891
Semple F, Webb S, Li HN, Patel HB, Perretti M, Jackson IJ et al (2010a) Human beta-defensin 3 has immunosuppressive activity in vitro and in vivo. Eur J Immunol 40(4):1073–1078
Semple F, Webb S, Li HN, Patel HB, Perretti M, Jackson IJ et al (2010b) Human β-defensin 3 has immunosuppressive activity in vitro and in vivo. Eur J Immunol 40(4):1073–1078
Senyürek I, Paulmann M, Sinnberg T, Kalbacher H, Deeg M, Gutsmann T et al (2009) Dermcidin-derived peptides show a different mode of action than the cathelicidin LL-37 against Staphylococcus aureus. Antimicrob Agents Chemother 53(6):2499–2509
Seyfi R, Kahaki FA, Ebrahimi T, Montazersaheb S, Eyvazi S, Babaeipour V et al (2020) Antimicrobial peptides (AMPs): roles, functions and mechanism of action. Int J Pept Res Ther 26:1451–1463
Shelley JR, McHugh BJ, Wills J, Dorin JR, Weller R, Clarke DJ et al (2023) A mechanistic evaluation of human beta defensin 2 mediated protection of human skin barrier in vitro. Sci Rep 13(1):2271
Shiohara T, Doi T, Hayakawa J (2011) Defective sweating responses in atopic dermatitis. Curr Probl Dermatol 41:68–79
Simanski M, Dressel S, Gläser R, Harder J (2010) RNase 7 protects healthy skin from Staphylococcus aureus colonization. J Invest Dermatol 130(12):2836–2838
Simanski M, Köten B, Schröder JM, Gläser R, Harder J (2012) Antimicrobial RNases in cutaneous defense. J Innate Immun 4(3):241–247
Simanski M, Rademacher F, Schröder L, Schumacher HM, Gläser R, Harder J (2013) IL-17A and IFN-γ synergistically induce RNase 7 expression via STAT3 in primary keratinocytes. PLoS ONE 8(3):e59531
Soehnlein O, Kai-Larsen Y, Frithiof R, Sorensen OE, Kenne E, Scharffetter-Kochanek K et al (2008) Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages. J Clin Invest 118(10):3491–3502
Somanath PR, Chen J, Byzova TV (2008) Akt1 is necessary for the vascular maturation and angiogenesis during cutaneous wound healing. Angiogenesis 11:277–288
Sørensen OE, Thapa DR, Rosenthal A, Liu L, Roberts AA, Ganz T (2005) Differential regulation of beta-defensin expression in human skin by microbial stimuli. J Immunol 174(8):4870–4879
Steffen H, Rieg S, Wiedemann I, Kalbacher H, Deeg M, Sahl HG et al (2006) Naturally processed dermcidin-derived peptides do not permeabilize bacterial membranes and kill microorganisms irrespective of their charge. Antimicrob Agents Chemother 50(8):2608–2620
Stuart PE, Hüffmeier U, Nair RP, Palla R, Tejasvi T, Schalkwijk J et al (2012) Association of β-defensin copy number and psoriasis in three cohorts of European origin. J Invest Dermatol 132(10):2407–2413
Sun L, Finnegan CM, Kish-Catalone T, Blumenthal R, Garzino-Demo P, La Terra Maggiore GM et al (2005) Human beta-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J Virol 79(22):14318–14329
Sweeney CM, Russell SE, Malara A, Kelly G, Hughes R, Tobin AM et al (2016) Human ß-Defensin 3 and its mouse Ortholog Murine ß-Defensin 14 Activate Langerhans cells and exacerbate Psoriasis-Like skin inflammation in mice. J Invest Dermatol 136(3):723–727
Szabó L, Kapitány A, Somogyi O, Alhafez I, Gáspár K, Palatka R et al (2023) Antimicrobial peptide loss, except for LL-37, is not characteristic of atopic dermatitis. Acta Dermato-Venereologica. ;103
Takahashi M, Umehara Y, Yue H, Trujillo-Paez JV, Peng G, Nguyen HLT et al (2021) The antimicrobial peptide human β-Defensin-3 accelerates Wound Healing by promoting Angiogenesis, Cell Migration, and Proliferation through the FGFR/JAK2/STAT3 signaling pathway. Front Immunol 12:712781
Tam JP, Wang S, Wong KH, Tan WL (2015) Antimicrobial peptides from plants. Pharmaceuticals (Basel) 8(4):711–757
Tewary P, de la Rosa G, Sharma N, Rodriguez LG, Tarasov SG, Howard OM et al (2013) β-Defensin 2 and 3 promote the uptake of self or CpG DNA, enhance IFN-α production by human plasmacytoid dendritic cells, and promote inflammation. J Immunol 191(2):865–874
Thorey IS, Roth J, Regenbogen J, Halle JP, Bittner M, Vogl T et al (2001) The Ca2+-binding proteins S100A8 and S100A9 are encoded by novel injury-regulated genes. J Biol Chem 276(38):35818–35825
Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y et al (2005) Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol 175(7):4662–4668
Tsikourkitoudi V, Karlsson J, Merkl P, Loh E, Henriques-Normark B, Sotiriou GA (2020) Flame-made calcium phosphate nanoparticles with high drug loading for delivery of biologics. Molecules 25(7):1747
Vaezi Z, Bortolotti A, Luca V, Perilli G, Mangoni ML, Khosravi-Far R et al (2020) Aggregation determines the selectivity of membrane-active anticancer and antimicrobial peptides: the case of killerFLIP. Biochimica et Biophysica Acta (BBA). - Biomembr 1862(2):183107
van Harten RM, van Woudenbergh E, van Dijk A, Haagsman HP (2018) Cathelicidins: Immunomodulatory Antimicrobials. Vaccines (Basel). ;6(3)
Van Wetering S, Mannesse-Lazeroms SP, Van Sterkenburg MA, Daha MR, Dijkman JH, Hiemstra PS (1997) Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am J Physiol 272(5 Pt 1):L888–L896
Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB (2019) Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev 146:97–125
Verjans E-T, Zels S, Luyten W, Landuyt B, Schoofs L (2016) Molecular mechanisms of LL-37-induced receptor activation: an overview. Peptides 85:16–26
Wang WM, Jin HZ (2018) Skin microbiome: an actor in the pathogenesis of Psoriasis. Chin Med J (Engl) 131(1):95–98
Wang S, Yan C, Zhang X, Shi D, Chi L, Luo G et al (2018) Antimicrobial peptide modification enhances the gene delivery and bactericidal efficiency of gold nanoparticles for accelerating diabetic wound healing. Biomater Sci 6(10):2757–2772
Wang C, Wang S, Li D, Wei DQ, Zhao J, Wang J (2020) Human intestinal defensin 5 inhibits SARS-CoV-2 Invasion by cloaking ACE2. Gastroenterology 159(3):1145–7e4
Wanke I, Steffen H, Christ C, Krismer B, Götz F, Peschel A et al (2011) Skin commensals amplify the innate immune response to pathogens by activation of distinct signaling pathways. J Invest Dermatol 131(2):382–390
Wiesner J, Vilcinskas A (2010) Antimicrobial peptides: the ancient arm of the human immune system. Virulence 1(5):440–464
Wilsmann-Theis D, Wagenpfeil J, Holzinger D, Roth J, Koch S, Schnautz S et al (2016) Among the S100 proteins, S100A12 is the most significant marker for psoriasis disease activity. J Eur Acad Dermatol Venereol 30(7):1165–1170
Wolf R, Mascia F, Dharamsi A, Howard OM, Cataisson C, Bliskovski V et al (2010) Gene from a psoriasis susceptibility locus primes the skin for inflammation. Sci Transl Med 2(61):61ra90
Wolk K, Warszawska K, Hoeflich C, Witte E, Schneider-Burrus S, Witte K et al (2011) Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol 186(2):1228–1239
Xu D, Liao C, Zhang B, Tolbert WD, He W, Dai Z et al (2018) Human enteric α-defensin 5 promotes Shigella infection by enhancing bacterial adhesion and invasion. Immunity 48(6):1233–1244 e6
Xu D, Liao C, Xiao J, Fang K, Zhang W, Yuan W et al (2019) Human enteric defensin 5 promotes Shigella Infection of Macrophages. Infect Immun. ;88(1)
Yamasaki K, Gallo RL (2008) Antimicrobial peptides in human skin disease. Eur J Dermatol 18(1):11–21
Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM et al (2006) Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. Faseb j 20(12):2068–2080
Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A et al (2007) Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med 13(8):975–980
Yoon S, Seger R (2006) The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 24(1):21–44
Yu Y, Zhang Y, Zhang Y, Lai Y, Chen W, Xiao Z et al (2017) LL-37-induced human mast cell activation through G protein-coupled receptor MrgX2. Int Immunopharmacol 49:6–12
Zhang L-j, Gallo RL (2016) Antimicrobial peptides. Curr Biol 26(1):R14–R9
Zhang C, Yang M (2022) Antimicrobial peptides: from design to clinical application. Antibiotics 11(3):349
Zhang LJ, Sen GL, Ward NL, Johnston A, Chun K, Chen Y et al (2016) Antimicrobial peptide LL37 and MAVS Signaling Drive Interferon-β production by epidermal keratinocytes during skin Injury. Immunity 45(1):119–130
Zhang Q-Y, Yan Z-B, Meng Y-M, Hong X-Y, Shao G, Ma J-J et al (2021a) Antimicrobial peptides: mechanism of action, activity and clinical potential. Military Med Res 8(1):48
Zhang Q-Y, Yan Z-B, Meng Y-M, Hong X-Y, Shao G, Ma J-J et al (2021b) Antimicrobial peptides: mechanism of action, activity and clinical potential. Military Med Res 8:1–25
Zhang K, Lian S, Shen X, Zhao X, Zhao W, Li C (2022) Recombinant porcine beta defensin 2 alleviates inflammatory responses induced by Escherichia coli in IPEC-J2 cells. Int J Biol Macromol 208:890–900
Zhou Y, Follansbee T, Wu X, Han D, Yu S, Domocos DT et al (2018) TRPV1 mediates inflammation and hyperplasia in imiquimod (IMQ)-induced psoriasiform dermatitis (PsD) in mice. J Dermatol Sci 92(3):264–271
Zhou Y, Han D, Follansbee T, Wu X, Yu S, Wang B et al (2019) Transient receptor potential ankyrin 1 (TRPA1) positively regulates imiquimod-induced, psoriasiform dermal inflammation in mice. J Cell Mol Med 23(7):4819–4828
Acknowledgements
The authors are grateful to the Researchers Supporting Project No. (RSP2024R161), King Saud University, Riyadh, Saudi Arabia.
Funding
The current study did not receive any funding.
Author information
Authors and Affiliations
Contributions
A.B.C.D.E.F wrote the main manuscript text and A.B.C edit manuscript. All authors reviewed the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hsu, CY., Yousif, A.M., Abullah, K.A. et al. Antimicrobial Peptides (AMPs): New Perspectives on Their Function in Dermatological Diseases. Int J Pept Res Ther 30, 33 (2024). https://doi.org/10.1007/s10989-024-10609-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s10989-024-10609-7