Abstract
Antimicrobial peptides (AMPs) are a crucial part of innate immunity that exist in the most of living organisms. In fact, AMPs have ability to incite the innate immune response and combat with a broad range of microbes, including bacteria, virus, parasite and fungi. Moreover, recent studies indicated that, the small cationic peptides have ability to target cancer cells and can be used as the cancer therapeutic agents. AMPs are the very tiny macromolecules, commonly in the range of 6 to 100 amino acids. During last decades with the growing antibiotic resistance, AMPs have gained considerable attention because of potential application to combat multidrug-resistant microorganisms. Therefore, herein we aimed to review the features of antibacterial peptides, including their classification, structure, source, mechanism of action and clinical application. Furthermore, problems in the production of recombinant peptides and also newest researches in the clinical developments of AMPs for treatment of crucial diseases; particularly cancers will be reviewed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Several decades ago, peptides have been isolated and purified from bacteria, fungi, algae, insect herbs such as miltinin, morrisin secroupin, ponaretoxin, mastoparan, scorpion, frogs and mammals. The most attention are focused about their features, especially targeting different ion channels and cell membrane components (Hmed et al. 2013). Over than 750 eukaryotic antimicrobial peptides (AMPs) have been reported until now (Hancock et al. 2016) that are collected according to their sequence homology, functional similarities and three-dimensional structure (Payandeh et al. 2019; Peravali et al. 2013). AMPs form the important part of the immune system (Brogden et al. 2016), as the activity of them creates a perturbation in the plasma membrane which causes cell lysis (Peravali et al. 2013). Therefore, it seems that these peptides with spread-spectrum emerge can be as a new potential therapeutic agents (Brogden et al. 2016).
Despite the existence of the majority antibiotics, AMPs seem to act as a safety regulator, enhancing immunity that is the unique properties of these peptides (Li 2011). Considering high biological activity and low cost of the AMPs, they can be considered as a good candidates for drug synthesis by many pharmaceutical researchers (Hurdle et al. 2011; Midura-Nowaczek and Markowska 2014). In this review, we first give an introduction of antibacterial peptides, and then discuss about their source, structure and mechanism of action. In the following, we focus on the problems of recombinant peptides production and finally, we consider the recent researches in clinical developments of AMPs for treatment of important diseases; particularly cancers.
Classification of Antibacterial Peptides
The peptides from scorpion sources are classified into two main groups including non-disulfide-bridged peptides (NDBPs) with multiple activity (Table 1) (Almaaytah and Albalas 2014; Zhang et al. 2015) and peptides with disulfide bridges. The NDBPs can be divided into two subgroups consisting of cationic peptides and acidic peptides. The number of amino acids are varied from 13 to 56 (Santibáñez-López and Possani 2015). As regards, the high biological activities of these peptides such as lysis feature, anticancer effect, immune regulatory function, bradykinin-potentiating factor and significant antimicrobial activity lead to study about NDBPs (Ramírez-Carreto et al. 2015). The peptides with disulfide bridges usually target the membrane boundaries of ion channels. They were applied in the treatment of diseases such as epilepsy, glioma, autoimmune diseases, neurological diseases and chronic pain (Pahlavan et al. 2019; Zhang et al. 2015). The majority of these peptides are classified into four different subgroup based on targeting ion channels (Hmed et al. 2013) and used as the modulatory agent or blocking activity of the ion channels (Santibáñez-López and Possani 2015). Recently reports indicated that fungi as the widespread family of eukaryotes can produce peptides with antimicrobial properties (Table 2). They are a new generation of antibiotics which can be a good alternative of new biological molecules. Many of these have anti-tumor activity and can cause the mortality of many cancer cells by prevention of their growing with less detectable mechanisms. Diphencin is a group of peptides that are responsible for protecting the host. The similarity in structure of fungal AMPs involves the discontinuity of β-plates which stabilized by disulfide bonds however, α helix observed in some homelands of these peptides (Kastin 2013). Antifungal peptides are another type of peptides which are often produced by filamentary fungi. They are very small and rich in cysteine for producing three or four disulfide bridges. Some of antifungal peptides isolated from the fungi and fungi sensitive to these peptides are listed in Table 3. These peptides are often made in the form of adult prephenate peptides so they are inactive and protected in the host (Sathoff et al. 2019; Shah 2019).
Another classification of antibacterial peptides are based on the structure of their amino acids (Hmed et al. 2013). Antibacterial peptides are usually between 12 and 50 amino acids in length (Huang et al. 2010) containing positively charged amino acids, such as arginine, lysine or histidine which provide an acidic environments along with 50% of hydrophobic area (Mojsoska et al. 2015). Moreover, antibacterial peptides can be classified into two another categories including; synthetic non-ribosomal peptides and synthetic ribosomal peptides or natural (Keymanesh et al. 2009; Zheng et al. 2017). The first group mostly is produced by bacteria while the latter one is produced by all animals and bacteria (Martin et al. 2017).
Ultimately, electrostatic charge is as an important feature for classification of AMPs (Moreno-Montoro et al. 2017). According to this characteristic, the antibacterial peptides are divided into two major categories. A large number of peptides are cationic peptide having positive charge while the other groups include non-cationic peptides (Ashby et al. 2017) which are scarce. Interestingly, in the scientific literature, the “antibacterial peptides” term only refers to its cationic type (Sudheendra et al. 2015).
Structure of Antibacterial Peptides
The structure of antibacterial peptides are in four styles, including α-helices, β-sheet, extended and loop forms (Fig. 1) (Narayana and Chen 2015; Nguyen et al. 2011). Natural derived antibacterial peptide divided into peptides with β-sheet, α-helix, cyclic peptides and peptides with long lengths. β-Sheet and α-helix are more abundant in nature (Harmouche et al. 2017a). Most of linear antibacterial peptides lose their structure in solution, while cyclic peptide form β-sheets due to the presence of one or more disulfide bond ‘cysteine-cysteine’ (Teixeira et al. 2012). The most of AMPs have less than 100 amino acid residue that are essential for antimicrobial activity (Fjell et al. 2012). Hydrophobic property such as α-helices, β-sheets and polyprolin helices are increased during the formation of secondary structure (Khurshid et al. 2016; Poluri and Gulati 2017).
Action Mechanism of Antibacterial Peptides
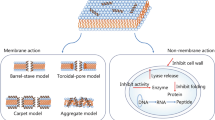
AMP’s mechanism is dependent on the number of physicochemical properties including the sequence of amino acids, charge, amphipathic property, structure especially secondary structure, and etc. (Mojsoska et al. 2015). These peptides have different mechanisms for disrupting the membrane (Mahlapuu et al. 2016). Despite of having suitable characteristics of AMPs (Bayer et al. 2017), many researchers believe that the ability of AMPs for binding to membranes of bacteria has an important role in the development of them (Bayer et al. 2017; Juretić et al. 2017). Some mechanisms of binding AMPs to membranes of bacteria include barrel-stave, toroidal pore worm hole, carpet and detergent like model as shown in Fig. 2 (Kumar et al. 2018). In the barrel-stave model, peptides with a special direction are placed between membrane and joined together to form an ion channel (Harder and Schröder 2016; Krauson 2011). In the toroidal pore worm hole model, peptides are placed in parallel direction with double layer membrane. In this orientation, spiral hydrophilic head is placed toward hydrophilic region of lipids and the aqueous phase is outside of membrane while hydrophobic head is placed in the hydrophobic center of membrane (Gaspar and Castanho 2016) and finally, in the carpet model, peptides mounted on the membrane surface to create disorder and chaos (Harder and Schröder 2016). Accumulation of peptides in an appropriate concentration increases the curvature of the membrane which leads to increase formation of membrane spiral pores (Gaspar and Castanho 2016; Wang et al. 2016). In this mechanism, peptides cover the carpet, forming membrane surface that resemble to early stages of a “spiral hole” (Iglic and Kulkarni 2014). It works like a detergent and breaks membrane into the small pieces (Lohner 2017). After this step, peptides aggregate together and increase local concentration. According to their amphipathic property, pieces are like micelles (Andreev et al. 2016; Lohner 2017).
The mechanism of peptides activity in bacteria. a The barrel-stave model. b The toroidal-pore model. c The carpet model. d Detergent “like” model (Kumar et al. 2018)
Moreover, the other insertion models of host defense peptide such as molecular electroporation and sinking raft model are shown in Fig. 3 (Bechinger 2011). In the sinking raft and molecular electroporation models unstable holes are formed in membrane, changing the electric charge on the two sides of the membrane and ultimately develops holes (Miao et al. 2015). Plant and mammalian membranes such as red blood cells membrane consist of neutral phospholipids and large amounts of cholesterol while, bacterial cell membranes is rich of acidic phospholipids such as phosphatidylglycerol and cardiolipin (Kornspan and Rottem 2012). Electrostatic interaction between negatively charged phospholipids and positively charged AMP’s as well negative bacterial membranes is a major driving force for cellular communication (Huang et al. 2014; Paiva and Breukink 2013). Figure 4 shows the outer parts of the plant and mammalian membranes has no charge, because the negatively charged membranes lipids are on the inner surface of the plasma membrane. In fact, the outer surface of the membrane usually was made from phosphatidylcholine and sphingomyelin with a small portion of some ganglioside (Hemler et al. 1995) so, the interaction between amphipathic AMPs with the plant and mammalian membranes are very week. While the effective interaction is present between the hydrophobic part of amphipathic AMPs and the outer parts of bacteria membranes due to existence of negative charge phospholipids (Del Vecchio and Stahelin 2018).
Molecular basis of cell selectivity of antimicrobial peptides in plant and mammalian in comparison with bacterial cells (Gerner and Raffatellu 2018)
Sources of Antibacterial Peptides
Antibacterial peptides are synthesized by most organisms including bacteria, fungi, algae, plants, insects (Melittin, Moricin, Cecropin, Poneratoxin, Mastoparan) frogs and mammals (Ingber et al. 2013). Animal AMPs were first discovered in invertebrates and later found in vertebrates. These peptides have shown different sequences, structures and goals (Li et al. 2012). Toxins from animals and plants are natural resources and rich in biological molecules (de la Salud Bea et al. 2015). AMPs also are obtained from milk peptides (Dziuba and Dziuba 2014; Théolier et al. 2014). In addition to natural resources, these peptides can be produced synthetically (Fjell et al. 2012). Since, hitherto synthetic peptides have made and sold commercially (Uhlig et al. 2014). AMPs are produced by four chemical methods, including culture of industrial microorganisms, genetically modified organisms, enzymatic hydrolysis of proteins and separation from natural sources. With protein engineering, the discovered species are also continuously improving. Compared to separation protocol of natural sources and chemical synthesis, it can be suggested that recombinant methods to be better method in regard to production scale through profitable methods (Li 2011).
Recombinant Antibacterial Peptides
AMPs are one of the compounds commonly used instead of antibiotics, displaying pathogenic bacterial resistance. These protein-like molecules often contain some depleted or modified amino acids that are not found in polypeptides made by ribosomes (Bondaryk et al. 2017). Based on their biosynthesis mechanism, these compounds classified in two groups consist of bacteriosins and peptide antibiotics. Bacteriosins are ribosomal synthesized compounds which produce by bacteria and act against the other close kinship bacteria. Unlike Bacteriosins, the second group of recombined AMPs are synthesized by stepwise and compression reactions which associated by non-ribosome peptide syntheses (NPRS; Thoendel et al. 2010). The main obstacle in the use of antibacterial peptides, as antibiotics, is their ability to lyse eukaryotic cells especially red blood cells. Indeed, for application of them, they should have low hemolytic activity and have high antimicrobial activity. Recent studies have shown that the high hydrophobicity and amphipathic property of AMPs to associate with hemolytic activity (Wang et al. 2015). Therefore, the range of selection of these peptides should be improved to lower hemolysis or other toxicity, in this case, modification of current methods for designing or identifying of AMPs can be helpful to achieve these goals. In order to apply AMPs in therapeutic application, production of small AMPs by recombinant DNA methods is a challenge approach to discuss. Escherichia coli (E. coli) is the most commonly used host for expressing AMPs due to high growth rate. However, some problems such as low crop yields, proteolytic decomposition of the hybrid protein and toxicity of the expressed product on the host should be considered about AMPs (Li et al. 2017). Various ways are presented for overcoming these problems, for instance use of hybridization, production of expression vectors containing multiple linked genes or addition of anionic fragment to counteract and neutralize the cationic peptides. Positive AMPs are loaded with acidic components that can be neutralized and significantly reduce their toxicity effects on host cells. Bioreactor studies have been identified that production of antibiotics is strongly dependent on the scale of work and more importantly, is related with dissolved oxygen concentration, pH environment, glucose consumption and cell differentiation. These peptides are now mainly produced from extraction of host organisms or in solid-phase peptide synthesis (SPPS). SPPS has become the dominant method for peptide production (Mehrlatifan et al. 2016; Yazdani et al. 2019). However, both of them are expensive and produce low yield. It seems the recombinant DNA technology is useful for production of various prokaryotic and eukaryotic proteins (Gaglione et al. 2019). In fact, the recombinant DNA technology has opened a new window for production of these compounds cheaper than the previous systems. For a long time, E. coli strains are a good host for heterologous proteins production. In general, the bacterial peptides can be produced easily in the cytoplasm of the bacterium. Multiple host-carrier systems have been used to produce AMPs via recombinant DNA technology using E. coli (Baird 2017). One of the major problems when working with peptides is to obtain sufficient materials with determined structural and functional properties (Mehrlatifan et al. 2016). This problem becomes harder when they combined with peptides rich in disulfide bonds, as they may produce non-native disulfide-bond isoforms. For example, a peptide with 3 disulfide bonds can be 15 and 1 peptide with 4 disulfide bonds can form 105 different disulfide isomers, respectively. There are several methods for expressing cytoplasm in E. coli to produce peptides rich in disulfide bands. One way to overcome this problem is bypassing the cytoplasm which secretes the recombinant protein to the bacterial periplasm where the endogenous protein forms a disulfide bond. The recombinant peptides with an annual growth of 15%, account for a large amount of market for pharmaceutical products. The combination of recombinant DNA technology and mass production process, makes it possible to commercially produce them (Harrison et al. 2015; Uhlig et al. 2014). Over the past several years, extensive research has been conducted on the production of multiple recombinant peptides including human growth hormone, interferon gamma, GM-CSF, G-CSF, interleukin-2, streptokinase, and etc. Although, a lot of peptides have been introduced as novel drugs however, their high flexibility cause limitation for application of them as antibacterial drugs. Peptides are hydrophilic compounds so they cannot cross the brain barrier. In addition, the short-lived half-lives and high cost of formulation are another disadvantage in comparison with the other novel drugs (Abarghooi et al. 2012). The production system of E. coli has several benefits, including rapid proliferation rate, presence of various strains of bacteria, existence of highly expressed genetic systems for the production of alien peptides, development of fermentation methods, proper genetic stability and low mutation rates in the genome. However, there are some problems such as; high levels of endotoxin, insoluble intracellular masses due to high expression speeds that need to be rearranged, lack of post-translational reforming systems (glycosylation, ending amines, sulfation, etc.), degradation alien peptide by cell proteases, lack of disulfide bonds in bacterial cytoplasm resuscitation and so on (Dehaghani et al. 2010; Ebrahimi et al. 2010; Jafari et al. 2014; Mohtashami and Ashtiani 2010; Morowvat et al. 2014a). Extremely high expression of the recombinant peptide in the cell, leads to accumulation of them in an abnormal structure. Creation of ankylosing spondylitis is one of the major problems in production of peptide as a cytoplasmic form (Babaeipour et al. 2008; Bakhtiari et al. 2014; Kahaki et al. 2014; Morowvat et al. 2014b). The expression of heterologous genes is influenced by several factors including stability and copy number of plasmid, promoter power, mRNA stability, ribosomal availability, the efficiency of transcription and translational processes, post translational modifications, stability and solubility of recombinant protein and culture conditions (Cao et al. 2017; Umenhoffer et al. 2017). In addition of E. coli, many antibacterial peptides have been expressed in various expression systems (Table 4). In all systems, hydrodynamic condition of immersion fermentation can be improved by changing and modifying the bioreactor structure with an emphasis on aeration optimization and agitation systems (Gaur et al. 2017; Jones et al. 2016; Jossen et al. 2017; Maghsoudi et al. 2009; Morowvat et al. 2015; Oikonomopoulos et al. 2015; Ranjbari et al. 2015). For the first time, Conde et al. (2000) isolated a new peptide was known as scorpin from the scorpion venom. This peptide has an antimicrobial activity and contains 75 amino acids. They found that scorpin has a unique amino acid sequence in which the amino terminus has similarity some of the zirconine and its carboxyl terminus is similar to some diffusers (Conde et al. 2000). Moreover, the gene of scorpion was cloned from a cDNA library. Ev37 is the first known gene in the Euscorpiidae family which has encoded as the new scorpin pseudo-peptide codon and cloned from the cDNA library of the scorpion venom. Ev37 peptide contains 78 amino acids. Recombinant peptide Ev37 and two peptides (Ev37-N and Ev37-C) in E. coli were used for functional studies (Feng et al. 2013; Maghsoudi et al. 2009; Mohseni et al. 2009; Saleh et al. 2008). Although, the use of recombinant AMPs very limited because of the uncontrolled genetic alterations which make through random mutagenesis. However, it appears recombinant AMPs can be promising candidates in the heterologous production in bacteria and other organisms as a cost-effective method.
Application of Antibacterial Peptides
AMPs or host defense peptides have been used in different fields such as therapeutic applications, agriculture, aquaculture, food industry and etc. (Meng et al. 2010). These peptides have several biological activities such as anticancer, immune-modulatory, anti-parasitic, antiviral, anti-biofilm, anti-inflammatory, angiogenesis and wound healing activities (Hilchie et al. 2019). Cancer as one of the main causes of morbidity and mortality in the world which become as a serious health concern for both developed and developing countries (Beaglehole et al. 2011; Siegel et al. 2013). Despite dramatic improvements in the treatment of cancer, resistance to anti-cancer drugs remains a major problem, leading to efforts in development of novel anti-cancer agents with new performance (Cardoso et al. 2018). Although it is not clear why some host defense peptides are capable of destroy cancer cells while the others do not (Fakheri and Jabbari 2014; Huang et al. 2015; Wang 2014). Electrostatic attraction between the cationic peptides and cell membrane anionic components is the most important factor in selective function of AMPs (Harder and Schröder 2016; Sadredinamin et al. 2016). Due to increased expression of anionic molecules, cancer cells membrane contain negative charges, so it can be assumed that these peptides may destroy cancer cells (Polyansky et al. 2010). Cationic AMPs are one of the most prominent classes of membrane active peptides. These peptides have a potential as a cancer inhibitory effect and cause biomembrane lysis (Araste et al. 2018). Amphipathic AMPs destroy membrane integrity, leading to membrane leakage and death of cancer cells which mediated by the hydrophobic and electrostatic interactions (Guo et al. 2016). Positive net charge of AMPs (+ 4 to + 6) causes to interact with the negatively charged phosphate head groups of cancer cell membrane and hydrophobic region. Some of AMPs have dual activity, including as an antimicrobial agent and as a selective anticancer peptides (ACPs) by lytic activities against membrane, however some of them penetrate inside cells and act on intracellular targets (Sani and Separovic 2016). Multiple α-helical ACPs display an anticancer activity via necrosis and apoptosis mechanism (Huang et al. 2015). Polybia-MPI, Temporin-1CEa, LTX-302, Anoplin, Cecropin A/ B and A12L/A20L exhibit specific anticancer activity against various cancer cells and disrupt the cell membrane, leading to necrosis of cancer cells. Mellitin from honey bee venom with specific activity causes disruption of cells with necrosis mechanism (Huang et al. 2015). LL-37, HPRP-A1, Pardaxin, BMAP-28 and RGD-tachyplesin can induce apoptosis in some of cancer cells. Magainin II (MG2) and A9K have anticancer activity by several different mechanisms of action (Huang et al. 2015). Cecropin B has a potential anticancer activity against leukemia cells, stomach cancer, and lung cancer. A novel modified peptide CB1a as an ACP derived from cecropin B showed apoptosis activity in leukemia and carcinoma cells (Qin et al. 2019). Several amphibian AMPs such as magainins, aureins, tryptophyllins, dermaseptins, phylloseptins, bombinins (BLP-7) and bombinin (H-BO) showed antiproliferative activity (Zhou et al. 2018). Furthermore, some peptides like bacteriocin have the cytotoxicity activity against various human cancer cells. Bacteriocin enterocin-B and the heterodimer of bacteriocins (enterocin-A + B) as antibacterial agents have the growth inhibitory effect of against HeLa, HT-29, AGS, Caco2 and MCF-7 cancer cells (Ankaiah et al. 2018). Antiproliferative activity of some plant-derived antimicrobial polypeptides was assayed against some of cancerous cells lines like Hela, Bt-549 and Neuro-2a. These peptides showed significant cytotoxicity with the highest IC50 against Neuro-2a (Al Saiqali et al. 2018). Another possible inducing cause of planned death in cancer cells is arrival of AMPs in the cytoplasm, causing turbulence in the mitochondrial membrane (Chen et al. 2015).
Assessment of commercial development of AMPs in different countries were shown utilization of peptide drugs in various stages of treatment such as catching an infection, diabetic foot ulcers, acne, mucus membrane infections, gingivitis and meningitis (Rzepiela et al. 2010; Strandberg et al. 2009). Furthermore, one of the most important advantages of these peptides as drugs is that they have no side effects (Tieleman 2017; Woo and Wallqvist 2011). Since peptides are natural substrates that can stick to a large number of enzymes and receptors, it seems that their utilization in drug synthesis can be a successful approach. However, for usage of these peptides as a medicine, manipulation of these compounds particularly in their properties such as metabolic stability, high tendency and specificity for a particular enzyme or receptor should be considered (Harmouche et al. 2017b). Other usage of these peptides is application in agriculture which includes control of post-harvest corrupt and insect as plant pests. Regarding the control of post-harvest corruption, the use of chemical fungicides has become increasingly restricted due to resistance to them (Vetchinkina et al. 2016). In the case of aquaculture, the use of vaccines and antibiotics in the aquatic environment has increased concern about the occurrence of resistance, so using AMPs is a good solution for overcoming this problem. Another application of these peptides is in the food industry. Indeed, adding preservatives is the usual way to prevent or reduce microbial growth in food products, so AMPs are a good choice as preservatives. Among these peptides, the bacteriocin is the most prominent group due to effectiveness against gram-positive bacteria, the main cause of food poisoning (Stern Bauer and Hayouka 2018). Multi-functional activities of AMPs, including antibacterial, antiviral, anti-fungal, anti-parasitic and protease properties have led researchers to pay attention to the application of them in the food industry, agriculture and medicine sciences in recent years.
Conclusion
AMPs are an important component of innate host defense which have anti-microbial, anti-viral, anti-cancer and contraceptive activities. They can be a good alternative to antibiotics and chemical preservatives. These peptides contain different chemical structures, sequences and transcripts, having antimicrobial activity. In the near future, it is expected to witness significant progress in using AMPs instead of antibiotics. On the other hand, a large number of AMPs not be used properly. Therefore, more efforts are needed to examine the applications of these compounds, especially in the new sectors of food and medicine. For example, there is an increasing awareness of the role of AMPs in reducing the incidence of certain cancers, especially clonal cancer. The exact mechanism of this phenomenon is not identified, it is probably due to control of mutagenic compounds by direct binding to carcinogens or inhibition of microbes that produce these agents. Therefore, there is a high potential for use of AMPs and further research is in demand to have significant effects in the food and medicine industry.
References
Abarghooi F, Babaeipour V, Memary HR, Mofid M (2012) Overproduction bacteriorhodopsin in E. coli as pharmacological targets. Res Pharm Sci 7:473
Akef HM (2019) Anticancer and antimicrobial activities of scorpion venoms and their peptides. Toxin Rev 38:41–53
Al Saiqali M, Tangutur AD, Banoth C, Bhukya B (2018) Antimicrobial and anticancer potential of low molecular weight polypeptides extracted and characterized from leaves of Azadirachta indica. Int J Biol Macromol 114:906–921
Alex JM et al (2019) Calixarene-mediated assembly of a small antifungal protein. IUCrJ 6:238–247
Almaaytah A, Albalas Q (2014) Scorpion venom peptides with no disulfide bridges: a review. Peptides 51:35–45
Andreev K et al (2016) Cyclization improves membrane permeation by antimicrobial peptoids. Langmuir 32:12905–12913
Ankaiah D, Palanichamy E, Antonyraj CB, Ayyanna R, Perumal V, Ahamed SIB, Arul V (2018) Cloning, overexpression, purification of bacteriocin enterocin-B and structural analysis, interaction determination of enterocin-A, B against pathogenic bacteria and human cancer cells. Int J Biol Macromol 116:502–512
Araste F, Abnous K, Hashemi M, Taghdisi SM, Ramezani M, Alibolandi M (2018) Peptide-based targeted therapeutics: focus on cancer treatment. J Control Release 292:141–162
Arias M, Hilchie AL, Haney EF, Bolscher JG, Hyndman ME, Hancock RE, Vogel HJ (2016) Anticancer activities of bovine and human lactoferricin-derived peptides. Biochem Cell Biol 95:91–98
Ashby M, Petkova A, Gani J, Mikut R, Hilpert K (2017) Use of peptide libraries for identification and optimization of novel antimicrobial peptides. Curr Top Med Chem 17:537–553
Babaeipour V, Shojaosadati SA, Khalilzadeh R, Maghsoudi N, Tabandeh F (2008) A proposed feeding strategy for the overproduction of recombinant proteins in Escherichia coli. Biotechnol Appl Biochem 49:141–147
Babič J et al (2019) Correction to: SPEXOR: design and development of passive spinal exoskeletal robot for low back pain prevention and vocational reintegration. SN Appl Sci 1:454
Baird P (2017) The role of genetics in population health. Why are some people healthy and others not? Routledge, New York, pp 133–160
Bakhtiari N, Mirshahi M, Babaeipour V, Maghsoudi N, Tahzibi A (2014) Down regulation of ackA-pta pathway in Escherichia coli BL21 (DE3): a step toward optimized recombinant protein expression system. Jundishapur J Microbiol 7:e8990
Bayer A et al (2017) The antimicrobial peptide human beta-defensin-3 is induced by platelet-released growth factors in primary keratinocytes. Mediat Inflamm 2017:6157491
Beaglehole R, Bonita R, Magnusson R (2011) Global cancer prevention: an important pathway to global health and development. Public Health 125:821–831
Bechinger B (2011) Insights into the mechanisms of action of host defence peptides from biophysical and structural investigations. J Pept Sci 17:306–314
Bondaryk M, Staniszewska M, Zielińska P, Urbańczyk-Lipkowska Z (2017) Natural antimicrobial peptides as inspiration for design of a new generation antifungal compounds. J Fungi 3:46
Brogden KA, Bates AM, Fischer CL (2016) Antimicrobial peptides in host defense: functions beyond antimicrobial activity. Antimicrobial peptides. Springer, Cham, pp 129–146
Cao Y, Hinkle GJ, Slater SC, Chen X, Goldman BS (2017) Expression of microbial proteins in plants for production of plants with improved properties. US Patent App. 15/612,580
Cardoso MH, Oshiro KG, Rezende SB, Cândido ES, Franco OL (2018) The structure/function relationship in antimicrobial peptides: what can we obtain from structural data? Advances in protein chemistry and structural biology, vol 112. Elsevier, Amsterdam, pp 359–384
Chang C et al (2017) Human β-defensin 2 in primary sclerosing cholangitis. Clin Transl Gastroenterol 8:e80
Chen H-L, Su P-Y, Kuo S-C, Lauderdale T-LY, Shih C (2018) Adding a C-terminal cysteine (CTC) can enhance the bactericidal activity of three different antimicrobial peptides. Front Microbiol 9:1440
Chen Q et al (2019) Increased gene copy number of DEFA1/DEFA3 worsens sepsis by inducing endothelial pyroptosis. Proc Natl Acad Sci USA 116:3161–3170
Chen W, Cheng X, Zhang X, Zhang Q, Sun H, Huang W, Xie Z (2015) The expression features of serum Cystatin C and homocysteine of Parkinson’s disease with mild cognitive dysfunction. Eur Rev Med Pharmacol Sci 19:2957–2963
Cid-Uribe JI, Santibáñez-López CE, Meneses EP, Batista CV, Jiménez-Vargas JM, Ortiz E, Possani LD (2018) The diversity of venom components of the scorpion species Paravaejovis schwenkmeyeri (Scorpiones: Vaejovidae) revealed by transcriptome and proteome analyses. Toxicon 151:47–62
Conde R, Zamudio FZ, Rodrı́guez MH, Possani LD (2000) Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett 471:165–168
de la Salud Bea R, Petraglia AF, de Johnson LEL (2015) Synthesis, antimicrobial activity and toxicity of analogs of the scorpion venom BmKn peptides. Toxicon 101:79–84
Dehaghani SA, Babaeipour V, Mofid M, Divsalar A, Faraji F (2010) An efficient purification method for high recovery of recombinant human granulocyte colony stimulating factor from recombinant E. coli. Int J Environ Sci Dev 1:111–114
Del Vecchio K, Stahelin RV (2018) Investigation of the phosphatidylserine binding properties of the lipid biosensor, Lactadherin C2 (LactC2), in different membrane environments. J Bioenerg Biomembr 50:1–10
Doğan T, İğci N, Biber A, Gerekci S, Hüsnügil HH, Izbirak A, Özen C (2018) Peptidomic characterization and bioactivity of Protoiurus kraepelini (Scorpiones: Iuridae) venom. Turk J Biol 42:490–497
Dziuba B, Dziuba M (2014) New milk protein-derived peptides with potential antimicrobial activity: an approach based on bioinformatic studies. Int J Mol Sci 15:14531–14545
Ebrahimi F, Rasaee MJ, Mousavi SL, Babaeipour V (2010) Production and characterization of a recombinant chimeric antigen consisting botulinum neurotoxin serotypes A, B and E binding subdomains. J Toxicol Sci 35:9–19
Fakheri BA, Jabbari M (2014) Small but potent killers
Feng J et al (2013) Expression and characterization of a novel scorpine-like peptide Ev37, from the scorpion Euscorpiops validus. Protein Expr Purif 88:127–133
Fjell CD, Hiss JA, Hancock RE, Schneider G (2012) Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov 11:37–51
Gaglione R et al (2019) Cost-effective production of recombinant peptides in Escherichia coli. N Biotechnol 51:39–48
Gaspar D, Castanho MA (2016) Anticancer peptides: prospective innovation in cancer therapy. Host defense peptides and their potential as therapeutic agents. Springer, Cham, pp 95–109
Gaur R, Singh A, Tripathi A, Singh R (2017) Bioreactors. Principles and applications of environmental biotechnology for a sustainable future. Springer, Singapore, pp 233–272
Gerner RR, Raffatellu M (2018) A worm’s gut feelings: neuronal muscarinic and epithelial canonical Wnt pathways promote antimicrobial defense. Immunity 48:839–841
Guo Z, Peng H, Kang J, Sun D (2016) Cell-penetrating peptides: possible transduction mechanisms and therapeutic applications. Biomed Rep 4:528–534
Hancock RE, Haney EF, Gill EE (2016) The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol 16:321
Harder J, Schröder J-M (2016) Antimicrobial peptides. Springer, Cham
Harmouche N et al (2017a) Solution and solid-state nuclear magnetic resonance structural investigations of the antimicrobial designer peptide GL13K in membranes. Biochemistry 56:4269–4278
Harmouche N et al (2017b) Solution and solid-state NMR structural investigations of the antimicrobial designer peptide GL13K in membranes. Biochemistry 56:4269–4278
Harrison RG, Todd P, Rudge SR, Petrides DP (2015) Bioseparations science and engineering. Topics in chemical engineering. Oxford University Press, Oxford
Hemler ME, Weitzman JB, Pasqualini R, Kawaguchi S, Kassner PD, Berdichevsky FB (1995) Structure, biochemical properties, and biological functions of integrin cytoplasmic domains. In: Integrins: the biological problems, pp 1–35. https://doi.org/10.1201/9780203711644-1
Hilchie A, Hoskin D, Coombs MP (2019) Anticancer activities of natural and synthetic peptides. Antimicrobial peptides. Springer, Cham, pp 131–147
Hmed B, Serria HT, Mounir ZK (2013) Scorpion peptides: potential use for new drug development. J Toxicol 2013:958797
Huang Y, Feng Q, Yan Q, Hao X, Chen Y (2015) Alpha-helical cationic anticancer peptides: a promising candidate for novel anticancer drugs. Mini Rev Med Chem 15:73–81
Huang Y, He L, Li G, Zhai N, Jiang H, Chen Y (2014) Role of helicity of α-helical antimicrobial peptides to improve specificity. Protein Cell 5:631–642
Huang Y, Huang J, Chen Y (2010) Alpha-helical cationic antimicrobial peptides: relationships of structure and function. Protein Cell 1:143–152
Hulett MD, Lay FT (2017) Plant defensins and use in the treatment of proliferative diseases. Google Patents
Hurdle JG, O’Neill AJ, Chopra I, Lee RE (2011) Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol 9:62–75
Iglic A, Kulkarni CV (2014) Advances in planar lipid bilayers and liposomes, vol 20. Elsevier, New York
Ingber D, Super M, Leslie DC, Didar T, Watters AL, Berthet JB, Waterhouse A (2013) Modification of surfaces for simultaneous repellency and targeted binding of desired moieties. Google Patents
Jacob B, Rajasekaran G, Kim EY, Park I-S, Bang J-K, Shin SY (2016) The stereochemical effect of SMAP-29 and SMAP-18 on bacterial selectivity, membrane interaction and anti-inflammatory activity. Amino Acids 48:1241–1251
Jafari S et al (2014) Recombinant production of mecasermin in E. coli expression system. Res Pharm Sci 9:453
Jones JA et al (2016) Experimental and computational optimization of an Escherichia coli co-culture for the efficient production of flavonoids. Metab Eng 35:55–63
Jossen V, Eibl R, Pörtner R, Kraume M, Eibl D (2017) Stirred bioreactors: current state and developments, with special emphasis on biopharmaceutical production processes. Current developments in biotechnology and bioengineering. Elsevier, New York, pp 179–215
Juretić D, Vukičević D, Tossi A (2017) Tools for designing amphipathic helical antimicrobial peptides. Methods Mol Biol 1548:23–34
Kahaki FA, Babaeipour V, Memari HR, Mofid MR (2014) High overexpression and purification of optimized bacterio-opsin from Halobacterium salinarium R1 in E. coli. Appl Biochem Biotechnol 174:1558–1571
Kastin A (2013) Handbook of biologically active peptides. Academic, Amsterdam
Kautz L, Aschemeyer S, Gabayan V, Ganz T, Nemeth E (2016) Erythroferrone regulates hepcidin expression independently of matriptase 2. Am J Hematol 92:E61–E63
Keymanesh K, Soltani S, Sardari S (2009) Application of antimicrobial peptides in agriculture and food industry. World J Microbiol Biotechnol 25:933–944
Khurshid Z, Naseem M, Sheikh Z, Najeeb S, Shahab S, Zafar MS (2016) Oral antimicrobial peptides: types and role in the oral cavity. Saudi Pharm J 24:515–524
Kolyada V (2019) On Cèsaro and Copson norms of nonnegative sequences. Ukr’ kyi Mat Z 71:220–229
Kornspan JD, Rottem S (2012) The phospholipid profile of mycoplasmas. J Lipids. https://doi.org/10.1155/2012/640762
Kovács R et al (2019) In vivo applicability of Neosartorya fischeri antifungal protein 2 (NFAP2) in treatment of vulvovaginal candidiasis. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.01777-18
Krauson AJ (2011) Mechanisms of pore formation in membranes. Tulane University
Kumar P, Kizhakkedathu J, Straus S (2018) Antimicrobial peptides: diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 8:4
Li J-F et al (2017) Identification of a cyclodextrin inclusion complex of antimicrobial peptide CM4 and its antimicrobial activity. Food Chem 221:296–301
Li Y (2011) Recombinant production of antimicrobial peptides in Escherichia coli: a review. Protein Expr Purif 80:260–267
Li Y, Xiang Q, Zhang Q, Huang Y, Su Z (2012) Overview on the recent study of antimicrobial peptides: origins, functions, relative mechanisms and application. Peptides 37:207–215
Lohner K (2017) Membrane-active antimicrobial peptides as template structures for novel antibiotic agents. Curr Top Med Chem 17:508–519
Ma JL et al (2019) Effects of dietary supplementation of recombinant plectasin on growth performance, intestinal health and innate immunity response in broilers. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-019-9515-2
Maghsoudi N, Bakhtiari N, Mirshahi M, Babaeepour V (2009) Effect of antisense nucleotide against acetate production on recombinant beta interferon production by E. coli. N Biotechnol. https://doi.org/10.1016/j.nbt.2009.06.451
Mahlapuu M, Håkansson J, Ringstad L, Björn C (2016) Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol 6:194
Manchisi J, Mhandu TJ, Kane J, Ndlovu S (2019) A hybrid leaching process to enhance the dissolution of cupriferous micas in the Chingola refractory ore. Hydrometallurgy 186:151–161
Mander L, Liu H-W (2010) Comprehensive natural products II: chemistry and biology, vol 1. Elsevier, Oxford
Martin GE et al (2017) Sphingosine’s role in epithelial host defense: a natural antimicrobial and novel therapeutic. Biochimie 141:91–96
Medeiros-Silva J, Jekhmane S, Breukink E, Weingarth M (2019) Towards the native binding modes of Lipid II targeting antibiotics. ChemBioChem 20:1731–1738
Mehrlatifan S, Mirnurollahi SM, Motevalli F, Rahimi P, Soleymani S, Bolhassani A (2016) The structural HCV genes delivered by MPG cell penetrating peptide are directed to enhance immune responses in mice model. Drug Deliv 23:2852–2859
Meng S, Xu H, Wang F (2010) Research advances of antimicrobial peptides and applications in food industry and agriculture. Curr Protein Pept Sci 11:264–273
Miao J et al (2015) iTRAQ-based quantitative proteomic analysis of the antimicrobial mechanism of peptide F1 against Escherichia coli. J Agric Food Chem 63:7190–7197
Midura-Nowaczek K, Markowska A (2014) Antimicrobial peptides and their analogs: searching for new potential therapeutics. Perspect Med Chem 6:73
Mohseni SS, Babaeipour V, Vali AR (2009) Design of sliding mode controller for the optimal control of fed-batch cultivation of recombinant E. coli. Chem Eng Sci 64:4433–4441
Mohtashami M, Ashtiani FZ (2010) The effect of microwave heating on the yield and quality of soy hull pectin. In: Proceedings of 2010 international conference on biotechnology and food science, Bangalore, India, 9–10 February
Mojsoska B, Zuckermann RN, Jenssen H (2015) Structure-activity relationship study of novel peptoids that mimic the structure of antimicrobial peptides. Antimicrob Agents Chemother 59:4112–4120
Moreno-Montoro M et al (2017) Antioxidant, ACE-inhibitory and antimicrobial activity of fermented goat milk: activity and physicochemical property relationship of the peptide components. Food Funct 8:2783–2791
Morowvat MH, Babaeipour V, Memari HR, Vahidi H (2015) Optimization of fermentation conditions for recombinant human interferon beta production by Escherichia coli using the response surface methodology. Jundishapur J Microbiol 8:e16236
Morowvat MH, Babaeipour V, Rajabi-Memari H, Vahidi H (2014) Metabolic changes of recombinant Escherichia coli BL21 (DE3) during overexpression of recombinant human interferon beta in HCDC. Int J Biosci 4:131–138
Morowvat MH, Babaeipour V, Rajabi-Memari H, Vahidi H, Maghsoudi N (2014) Overexpression of recombinant human beta interferon (rhINF-β) in periplasmic space of Escherichia coli. Iran J Pharm Res 13:151
Narayana JL, Chen J-Y (2015) Antimicrobial peptides: possible anti-infective agents. Peptides 72:88–94
Nguyen LT, Haney EF, Vogel HJ (2011) The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol 29:464–472
Oikonomopoulos A, Van Deen WK, Manansala A-R, Lacey PN, Tomakili TA, Ziman A, Hommes DW (2015) Optimization of human mesenchymal stem cell manufacturing: the effects of animal/xeno-free media. Sci Rep 5:16570
Oudart J-B et al (2015) Plasmin releases the anti-tumor peptide from the NC1 domain of collagen XIX. Oncotarget 6:3656
Pahlavan Y, Kahroba H, Samadi N, Karimi A, Ansarin K, Khabbazi A (2019) Survivin modulatory role in autoimmune and autoinflammatory diseases. J Cell Physiol 234:19440–19450
Paiva AD, Breukink E (2013) Antimicrobial peptides produced by microorganisms. Antimicrobial peptides and innate immunity. Springer, Basel, pp 53–95
Payandeh Z, Rajabibazl M, Mortazavi Y, Rahimpour A, Taromchi AH, Dastmalchi S (2019) Affinity maturation and characterization of the ofatumumab monoclonal antibody. J Cell Biochem 120:940–950
Peravali J, Kotra S, Sobha K, Nelson R, Rajesh K, Pulicherla K (2013) Antimicrobial peptides: an effective alternative for antibiotic therapy. Mintage J Pharm Med Sci 2:1–7
Poluri KM, Gulati K (2017) World of proteins: structure–function relationships and engineering techniques. Protein engineering techniques. Springer, Singapore, pp 1–25
Polyansky AA, Ramaswamy R, Volynsky PE, Sbalzarini IF, Marrink SJ, Efremov RG (2010) Antimicrobial peptides induce growth of phosphatidylglycerol domains in a model bacterial membrane. J Phys Chem Lett 1:3108–3111
Prens E, Deckers I (2015) Pathophysiology of hidradenitis suppurativa: an update. J Am Acad Dermatol 73:S8–S11
Qin Y et al (2019) From antimicrobial to anticancer peptides: the transformation of peptides. Recent Pat Anti-cancer Drug Discov 14:70–84
Rajanbabu V, Chen J-Y, Wu J-L (2015) Antimicrobial peptides from marine organisms. Springer handbook of marine biotechnology. Springer, Berlin, pp 747–758
Ramírez-Carreto S, Jiménez-Vargas JM, Rivas-Santiago B, Corzo G, Possani LD, Becerril B, Ortiz E (2015) Peptides from the scorpion Vaejovis punctatus with broad antimicrobial activity. Peptides 73:51–59
Ranjbari J, Moghimi H, Vahidi H, Babaeipour V, Alibakhshi A, Arezumand R (2015) Effect of Chitosan on production of insulin-like growth factor 1 protein in Escherichia coli. Int J Biosci 6:180–187
Richmond-Rakerd LS et al (2019) Common genetic contributions to high-risk trauma exposure and self-injurious thoughts and behaviors. Psychol Med 49:421–430
Roy AC, Wilson GG, Edgell DR (2016) Perpetuating the homing endonuclease life cycle: identification of mutations that modulate and change I-TevI cleavage preference. Nucleic Acids Res 44:7350–7359
Rzepiela AJ, Sengupta D, Goga N, Marrink SJ (2010) Membrane poration by antimicrobial peptides combining atomistic and coarse-grained descriptions. Faraday Discuss 144:431–443
Sadredinamin M, Mehrnejad F, Hosseini P, Doustdar F (2016) Antimicrobial peptides (AMPs). Nov Biomed 4:70–76
Saleh M, Reza VA, Valiollah B (2008) Designing new controller for fed-batch cultivation of recombinant E. coli. In: 2008 27th Chinese control conference, 2008. IEEE, pp 207–211
Sani M-A, Separovic F (2016) How membrane-active peptides get into lipid membranes. Acc Chem Res 49:1130–1138
Santibáñez-López CE, Possani LD (2015) Overview of the Knottin scorpion toxin-like peptides in scorpion venoms: insights on their classification and evolution. Toxicon 107:317–326
Sathoff AE, Velivelli S, Shah DM, Samac DA (2019) Plant defensin peptides have antifungal and antibacterial activity against human and plant pathogens. Phytopathology 109:402–408
Shah D (2019) Antifungal plant proteins, peptides, and methods of use. Google Patents
Shishodia SK, Tiwari S, Shankar J (2019) Resistance mechanism and proteins in Aspergillus species against antifungal agents. Mycology 10:1–15
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Stern Bauer T, Hayouka Z (2018) Random mixtures of antimicrobial peptides inhibit bacteria associated with pasteurized bovine milk. J Pept Sci 24:e3088
Strandberg E, Tremouilhac P, Wadhwani P, Ulrich AS (2009) Synergistic transmembrane insertion of the heterodimeric PGLa/magainin 2 complex studied by solid-state NMR. Biochim Biophys Acta Biomembr 1788:1667–1679
Sudheendra U, Dhople V, Datta A, Kar RK, Shelburne CE, Bhunia A, Ramamoorthy A (2015) Membrane disruptive antimicrobial activities of human β-defensin-3 analogs. Eur J Med Chem 91:91–99
Teixeira V, Feio MJ, Bastos M (2012) Role of lipids in the interaction of antimicrobial peptides with membranes. Prog Lipid Res 51:149–177
Théolier J, Fliss I, Jean J, Hammami R (2014) MilkAMP: a comprehensive database of antimicrobial peptides of dairy origin. Dairy Sci Technol 94:181–193
Thoendel M, Kavanaugh JS, Flack CE, Horswill AR (2010) Peptide signaling in the staphylococci. Chem Rev 111:117–151
Tieleman D (2017) Antimicrobial peptides in the cross hairs of computer simulations. Biophys J 113:1–3
Uhlig T et al (2014) The emergence of peptides in the pharmaceutical business: from exploration to exploitation. EuPA Open Proteomics 4:58–69
Umenhoffer K et al (2017) Genome-wide abolishment of mobile genetic elements using genome shuffling and CRISPR/Cas-assisted MAGE allows the efficient stabilization of a bacterial chassis. ACS Synth Biol 6:1471–1483
Uzair B, Bint-e-Irshad S, Khan BA, Azad B, Mahmood T, Rehman MU, Braga VA (2018) Scorpion venom peptides as a potential source for human drug candidates. Protein Pept Lett 25:702–708
Vetchinkina E, Komakhina V, Vysotskii D, Zaitsev D, Smirnov A, Babakov A, Komakhin R (2016) Expression of plant antimicrobial peptide pro-SmAMP2 gene increases resistance of transgenic potato plants to Alternaria and Fusarium pathogens. Russ J Genet 52:939–951
Wang G (2014) Human antimicrobial peptides and proteins. Pharmaceuticals 7:545–594
Wang G, Li X, Wang Z (2015) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 44:D1087–D1093
Wang Y, Chen CH, Hu D, Ulmschneider MB, Ulmschneider JP (2016) Spontaneous formation of structurally diverse membrane channel architectures from a single antimicrobial peptide. Nat Commun 7:13535
Woo H-J, Wallqvist A (2011) Spontaneous buckling of lipid bilayer and vesicle budding induced by antimicrobial peptide magainin 2: a coarse-grained simulation study. J Phys Chem B 115:8122–8129
Yazdani P et al (2019) Layered double hydroxide nanoparticles as an appealing nanoparticle in gene/plasmid and drug delivery system in C2C12 myoblast cells. Artif Cells Nanomed Biotechnol 47:436–442
Zhang J, Liu Y, Li Y, Su N, Zhou Y, Xiang J, Sun Y (2018) Biological function of a gC1qR homolog (EcgC1qR) of Exopalaemon carinicauda in defending bacteria challenge. Fish Shellfish Immunol 82:378–385
Zhang L et al (2015) Unique diversity of the venom peptides from the scorpion Androctonus bicolor revealed by transcriptomic and proteomic analysis. J Proteomics 128:231–250
Zheng Z et al (2017) The synergistic efficacy of Aedes aegypti antimicrobial peptide cecropin A2 and tetracycline against Pseudomonas aeruginosa. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.00686-17
Zhou B, Wang P, Zhang K, Jin F-s, Li Y-f, Zhang J, Sun Z-y (2015) Reduction of fertility in male mice immunised with pSG.SS.C3d3.YL.Bin1b recombinant vaccine. Eur J Contracept Reprod Health Care 20:372–378
Zhou C et al (2018) Discovery of two bombinin peptides with antimicrobial and anticancer activities from the skin secretion of Oriental fire-bellied toad, Bombina orientalis. Chem Biol Drug Des 91:50–61
Acknowledgements
This work was supported by Molecular Medicine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran and Shahid Beheshti University of Medical Sciences, Tehran.
Author information
Authors and Affiliations
Contributions
The core idea of this study came from R. S., F. A. K., and V.T. They also directed the other authors and analyzed the collected papers. R. S., F. A. K., T. E., S. E., S. M., V. B., and V. T. wrote the manuscript in collaboration with V. B. and V. T.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Seyfi, R., Kahaki, F.A., Ebrahimi, T. et al. Antimicrobial Peptides (AMPs): Roles, Functions and Mechanism of Action. Int J Pept Res Ther 26, 1451–1463 (2020). https://doi.org/10.1007/s10989-019-09946-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-019-09946-9