Abstract
A global public health crisis has been created by the emergence of serious infectious illnesses, the rising prevalence of infections that are impenetrable to antibiotics (AMR), and an inadequate supply of novel antibiotics. The expansion of antimicrobial drugs with unique molecular scaffolds is a time-consuming and cost-effective approach at the recent trend. On the other hand, the long-term exploitation of conventional antibiotics sparked the emergence of “superbugs” that are multi-drug resistant and are responsible for more deaths than HIV. Antimicrobial peptides (AMPs) are short peptides of natural innate defensive role against invading pathogens. Due to their broad spectrum of activity and low tendency to bacterial resistance, it can be recognized as one of the most potential antimicrobials. AMPs are opposed to conventional antibiotics, and it has their own benefits. AMPs use a wide range of mechanisms to exert their effects, including cell membrane damage, DNA fragmentation, impair macromolecule synthesis, damage to cell organelles, enzyme inhibition, and potential antimicrobial activity through immune regulation mechanisms. The main drawback of employing AMPs is the natural occurrence and are less stable and more toxic. Hence, to maintain this short-lived peptide’s stability and multifunctional action, small-molecule antibiotic-peptide conjugates (APCs) are crucial. This review focuses on the types, mechanisms, pharmaceutical applications, and APCs approach to treat infections with multiple drug resistance and concludes the potential of AMPs in biomedical practice and in drug delivery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the World Health Organization (WHO), antimicrobial resistance is a significant worldwide health issue, and it poses the greatest threat to human health today. The top priority is to find broad-spectrum antibiotics with little potential for bacterial resistance development. Antimicrobial peptides (AMPs) are appealing new antibacterial alternatives to traditional antibiotics for combating multidrug-resistant microorganisms (Falanga et al. 2016). Prokaryotes and eukaryotes are capable of producing AMPs (Bednarska et al. 2017). The 1980s saw the initial discovery of AMPs, which were short peptides produced by living organisms in nature, including bacteria, fungus and plants. Promising AMPs are identified from Bacillus spp, which show broad spectrum inhibitory activity against a panel of pathogens (Mora et al. 2011; Baindara et al. 2013). Similarly, an AMP has been isolated from Propionibacterium jensenii (Dziuba and Nalepa 2012). As of now, 3791 AMPs are reported from six kingdoms (Kang et al. 2019a). These groups of AMPs are classified based on size, net charge, structural properties, stability and solubility (Elias and Choi 2005). AMPs have broad spectrum activity against a wide range of bacteria (Bahar and Ren 2013; Zanetti 2004). Compared to classical antibiotics, the mode of action of AMPs is distinct, particularly in terms of membrane permeability (Lei et al. 2019). There are diverse models that can be explained by the mechanisms of action of these peptides (Som et al. 2008). The amphiphilic nature of peptides enabled them to destabilize the bacterial membrane barrier via interacting with bacterial cell membrane (Chen et al. 2007). Nowadays, AMPs are used to treat bacterial infection, inflammation, and wound healing (Costa et al. 2019; Mahlapuu et al. 2016). Among all, are taken to AMPs clinical trials for cancer treatment (Zhang et al. 2021a). However, the natural peptides are not stable, and its toxic effect consistently persist. To open for potential clinical applications, it is essential to synthesize and optimize AMPs to overcome the disadvantage of natural peptides and generate therapeutic regimens more efficiently. On the other hand, antibiotics substances are more effective against a range of pathogenic bacteria (Kościuczuk et al. 2012; Moravej et al. 2018). Furthermore, antibiotic abuse and overuse result in the loss of antibiotic efficacy over time and the emergence of multidrug resistance (Lau et al. 1860). These AMPs are more stable when they undergo chemical modification such as cyclization, hybridization, isomerization, sequence alteration, peptidomimetics and nanoformulations and maintain their biological activity during inimical conditions (Mahlapuu et al. 2016; Yang et al. 2021). For instance, different nanoparticles and AMPs with various combinations can exert strong biological activity and stability against protease containing environment (Yang et al. 2021; Thapa et al. 2020). Designing novel AMPs and combining them with currently available small molecules (antibiotics) is one potential approach for addressing the complex AMR challenge. In contrast to conventional, expensive, and time-consuming screening of new antimicrobial agents, small molecule-peptide conjugates have excellent mechanisms to eliminate the emergence and spread of current antimicrobial-resistant threats. The main topics of this review are the origin, types, structural characteristics, stability, and mechanism of action (MOA) in relation to small molecules. Furthermore, it discusses the combination of AMPs with antibiotics due to their stability and broad-spectrum activity for more clinical usages.
Antimicrobial Peptide Sources
AMPs are found in prokaryotic and eukaryotic organisms, such as microorganisms, plants, amphibians, birds, mammals, and invertebrates (Yazici et al. 2018; Moyer et al. 2019; Braun et al. 2018; Wang et al. 2020; Li et al. 2019a; Lee et al. 2019). It can be categorized based on the sources (natural origin) as per APD3 (manually listed database of AMPs) (Wang 2022). Quite interestingly, there are 335 peptides from bacterial origin, 13 peptides from fungi, 8 peptides from protists, 4 peptides from archaea, 342 peptides from plants, and 2200 peptides from animals, along with synthetic peptides. In total, 5000 AMPs have been discovered or synthesized so far.
AMPs from Mammalian Source
Mammalian AMPs are identified in humans, cattle and other vertebrates. Amongst, defensins and cathelicidins are the major type of AMPs in the mammalian system (Huan et al. 2020). Defensin, a type of AMPs, has been identified in the leukocytes of rabbits (Hirsch 1956). Cathelicidins are another type of AMPs with distinct peptide length, amino acid residue and conserved domain (Harten et al. 2018). This group of peptides is obtained in neutrophils and macrophages in an inactive form and activated via the processing of leukocytes (Ageitos et al. 2017). Lactoferrin, a dairy AMP has been identified from cow milk (Groves et al. 1965), and other AMPs are obtained by enzymatic hydrolysis of milk (Huan et al. 2020). AMPs derived from human milk play a crucial role in breastfeeding to eshew infant morbidity and mortality. It is quite Interesting to note that, Casein 201 derived from β-casein has been found in human colostrum (preterm and term) (Zhang et al. 2017). Furthermore, human host defensive peptide (HDPs) lysosomes were derived from Human leukocytes (Zeya and Spitznagel 1963) and AMPs consisting of low molecular weight which are derived from the female genital tracts (Sharma et al. 2011).
AMP Derived from Microbial Origin
AMPs are also obtained from microorganisms such as bacteria and fungi. For instance, a wide variety of Bacillus strains secreting AMPs with potential broad spectrum activity against a panel of pathogens; Salmonella, Shigella, Staphylococcus aureus and E.coli (Mora et al. 2011; Baindara et al. 2013; Naimah et al. 2017). Similarly, Propionibacterium jensenni produced AMPs a propionicin are identified (Holo et al. 2002), and antibacterial battacin a cyclic AMPs are discovered from Paenibacillus tianmuensis (Oshiro et al. 2019). Nisin is the famous AMP identified from bacteria, originally synthesized by the host defense system to suppress other bacterial growth to compete nutrients in the ecological niches (Mattick and Hirsch 1947). Recently, nisin has broadly been used as an antiseptic in the food industry (Gharsallaoui et al. 2016; Shin et al. 2016; Kitagawa et al. 2019). It can be seen from the literature that intestinal microbiota is a fruitful origin of AMPs. Recent reports show that intestinal microbiota identified as a fruitful origin of AMPs (Garcia-Gutierrez et al. 2019; Pushpanathan et al. 2019). Daptomycin and vancomycin are well-known AMPs produced from actinomycetes species. Pargamicins types B, C and D are derived from Amycolatopsis sp. ML1-hF4 (Hashizume et al. 2017). Similarly, a novel lipopeptide arylomycin A6 produced by soil derived Streptomyces parvus (Rao et al. 2013). Recently, temperature stable small peptide glycocin (an unusual property) is derived from thermophilic bacterium (Kaunietis et al. 2019). In addition to this, fungi have also been found to produce a unique AMP (Yazici et al. 2018). Interestingly, the mushroom (Coprinopsis cinerea) secretes copsin, a peptide that exhibits excellent activity against Gram-positive bacteria (Enterococcus faecalis and Listeria monocytogenes) by inhibiting their cell wall mechanism. (Essig et al. 2014).
AMPs from Marine Sources
Besides, marine derived AMPs are more attractive natural peptides with promising bioactivities (Huan et al. 2020). Discodermin A, cyclic AMPs derived from marine sea sponge and invertebrates mainly depends on innate immunity which is used as front line host defense mechanism to protect entry of pathogens (Gourbal et al. 2018). These AMPs primarily occur in the hemolymph, skin mucosa, and other body tissues. A potential wider spectrum of active AMP cecropins are isolated from hemolymph of Hyalophora cecropia (Wu et al. 2018). Marine derived AMPs are grouped under four categories based on biochemical nature and structure without accounting their mode of action (Erdem Büyükkiraz and Kesmen 2022). Thus, marine derived AMPs are structurally and functionally different from soil AMPs due to the extreme environment conations (salinity, tidal forces, pressure, etc.) (Falanga et al. 2016).
AMPs Derived from Plant
Plants also produce AMPs to show natural defence against pathogen invasion in the soil and air. Plant derived AMPs are grouped based on their amino acid residues, number of cysteine, identity and spacing (Lay and Anderson 2005). Based on the charge plant, AMPs are further classified as anionic and cationic peptides (Barbosa Pelegrini et al. 2011). There are diverse family of AMPs including defensins, thionins and cyclotide from plant origin (Srivastava et al. 2021). Amongst, Thionins are most abundant in seeds, leaves, roots and stem of the plants (Höng et al. 2021). Plant defensins have been isolated form leaves, flowers, tubers and pods (Ghag et al. 2012; Lay et al. 2003; Terras et al. 1995). Cysteine rich residues (CRPs) of plants based AMPs play an important role in structural stabilization via participation in multiple disulphide bridges (Tang et al. 2018). The primary function of the CRP is defence system in response to pathogen invasion and it shows activity against insect-larvae and enzyme inhibitory activity (Zhu et al. 2017; Colgrave and Craik 2004). Recent reports show that more than 3300 types of AMPs have been identified from various range of biological sources. AMPs from natural resources are summarized in Table 1.
Classification of Peptide
AMPs are classified into four major groups based of common secondary structures such as α helical, β sheet, α β mixed, and non-αβ peptides (Nguyen et al. 2011).
α-Helical Peptides
One of the largest class of AMPs is α helical peptide, which is well studied (Yan et al. 2022). Usually, AMPs consist of 12 to 54 amino acids residues with cationic properties at physiological pH (Mookherjee et al. 2020).
Moth cecropin, frog magainin, human cathelicidin LL-37 and melittin from bees are prominent examples of cationic peptide which belongs to amphipathic α helical peptides (Andersson et al. 2003; Agerberth et al. 1995). α helix peptides in aqueous solvent exhibit in irregular shape, but on the cell membranes, they undergo folded conformation. Generally, 50% of amino acid residues are hydrophobic, which is involve in amphipathic conformation and supports target membrane interaction (Hollmann et al. 2018). This peptide also contains disulfide bridges, in which N-terminal regions are amphipathic α helix and C –terminal regions have disulfide bridges, namely brevinin 2 and gaegurin (Almeida et al. 2012). These groups of peptides are more affinity towards cell membranes, which collapse the membrane organization through pore formation (Shai 1999). The antibacterial activity is impaired when α helix undergoes amino acid substitutions (Tossi et al. 1994). In the inactive form, AMPs do not exhibit α helical conformation, e.g. Magainin. Besides the separate activity of α helix, it also synergizes the antibacterial and cytotoxicity (Pino-Angeles et al. 2016).
β-Sheet Peptide
The second group of peptides exhibit β sheet conformation stabilized by two or more disulfide bridges with pairs of cysteine residues (Yan et al. 2022; Hollmann et al. 2018). Disulfide bridges in peptides can maintain overall stability and biological activity under various physiological conditions such as pressure, temperature, and serum (Osborn et al. 1995; Panteleev et al. 2015). Like, α helical peptides, the non-polar and polar groups exist in clusters on structurally unpaired surfaces of the peptide. Based on the structural characteristics, they are further sub-grouped into β hairpin, α, β and cyclic θ defensins (Yan et al. 2022). β hairpin structure can be formed via antiparallel β sheet linked by the turn of 3 to 4 amino acids e.g. Hepcidins (Edwards et al. 2016). A few AMPs, namely tachyplesin-1, polyphemusin-1, gomesin, arenicin-3, consist of β hairpin structure with two disulfide bridges (Erdem Büyükkiraz and Kesmen 2022). AMPs, such as lactoferricins, bactenecins, and arenicins, have single disulfide bonds. The primary effect of these disulfide bonds is maintaining structural stability and protecting the AMPs from degradation (Panteleev et al. 2015). These AMPs with no helical conformations are found in fungi, insects, plants and vertebrates (Weerden et al. 2013). Defensins are the most important members of this family of AMPs, and the disulfide bond connectivity of cysteine residues further determines the class of defensins. They are α, β, and cyclic θ defensins, all of which contain a β sheet structure. Among them, θ defensins are cyclic peptides with a cysteine ladder (Cyclic) conformation, which provides antimicrobial activity and also participates in the structure and overall stability of the cyclic scaffold (Conibear et al. 2014). The planar features and strong stability of cyclic peptides can facilitate the development of peptide drugs with wider bioactivity and specificity (Falanga et al. 2017).
Mixed (α, β) and Non (α, β) Peptides
Several groups of AMPs acquire α-helix and β-sheet conformations, which are stabilized by three or four disulfide bonds. The cysteine-stabilized α and β structure consist of single α helix and β stands (2 or 4 antiparallel fashion) has been first discovered from insects defensins and neurotoxins from scorpion (Hill et al. 1991). The mixed αβ AMPs architecture has been constructed by three β sheet and short helix in the N-terminus, e.g. human β defensin-3 (Dhople et al. 2006). Interestingly, cysteine stabilized mixed αβ (CS-αβ) from plants derived defensins show good activity against fungi instead of antibacterial active insect defensins. However, CS-αβ- motif is unclear, but this type AMPs have common mode of action, such as inhibition of cell wall biosynthesis and interaction with lipids II (Dias Rde and Franco 2015). In addition, Non- αβ AMPs are another class of peptides lacking true secondary structure (Yan et al. 2022). These classes of peptides are called residue rich peptides namely, trptophan (Indolicidin), serine, glycin, proline (arasin 1), threonine and histidine (histatin) and they show flexibility in aqueous solution (Yan et al. 2022; Helmerhorst et al. 2005; Paulsen et al. 2013). They possess membrane invasion characteristics by interacting with the cationic choline head group of the lipid bilayer through the formation of hydrogen bonding of lipid bilayer components (Chan et al. 2006). Among these, Indolicidin peptide derived from bovine neutrophils inhibits DNA replication and promotes the filamentation of bacteria via strong interaction with the negatively charged phosphate backbone of DNA (Hsu et al. 2005). Other than this class of AMPs, a short fragment also exists from antimicrobial proteins that are naturally occurring with wider antibacterial activity (Starling 2017; Ragland and Criss 2017; Zhang et al. 2018). It contains 133 residues with α helix, and β sheet in the protein architecture. Recently, arginine-rich NEMURI protein encoded by fruit flies genome has been identified as immune-modulatory effect with potential antibacterial activity (Toda et al. 2019). Moreover, some peptides have metal ions (Cu2+ and Ni2+) (ATCUN) binding motif, with high bind affinity (Portelinha et al. 2021). Interestingly, Cu2+ (ATCUN) can participate in ROS generation (Wende and Kulak 2015; Heinrich et al. 2019).
Mechanism of Action
The antimicrobial effect of the AMPs mainly depends on two mechanisms such as membrane targeting (Loss of membrane integrity) and non-membrane targeting (inhibition of DNA and other biomolecule synthesis) (Erdem Büyükkiraz and Kesmen 2022). Due to the cationic nature of AMPs, they bind via weak vanderwaals interactions with anionic charged bacterial cell membrane (Hollmann et al. 2018). The negative charge of the bacterial membrane is maintained by biomolecules like lipoteichoic acid and lipopolysaccharides (Scott et al. 1999) whereas animal contains a zwitterionic phospholipids (Posphatidylcholine, sphingomyelin and cholesterol, which facilitates AMPs to interact with the bacterial membrane (Matsuzaki 1999; Gaspar et al. 2013). Unlike conventional antibiotics, AMPs have a unique MOA that inhibits the further development of bacterial resistance (Pfalzgraff et al. 2018). Based on the MOA, this can be further divided into three types they are viz membrane disruption, interference of metabolic reactions and immunomodulation (Brogden 2005; Liang and Diana 2020). In the membrane disruption mechanism, AMPs initially attach to the cell membrane of pathogens through electrostatic forces, leading to membrane permeabilization. Subsequently, cell membrane integrity is lost, resulting in drastic changes in membrane potential, leakage of intracellular constituents, and ultimately leading to cell death (Costa et al. 2015)-(Reinhardt and Neundorf 2016). Generally, the action of AMPs on cell membrane disruption has been suggested by three models such as barrel-stave, toroidal-pore, and carpet models (Mookherjee et al. 2020; Brogden 2005).
Barrel-Stave Model
In the barrel-stave model, AMPs form bundles attached to the cell membrane to form hydrophilic pores and lipid interaction by hydrophobic residues. As a result, hydrophilic residues face the pore channels (Yan et al. 2022; Kang et al. 2014; Luca et al. 2014). In extreme conditions, AMPs trigger cell membrane damage, resulting in cell death (Lohner and Prossnigg 2009). Alamethicin is the best example of pore-forming AMP.
Toroidal-Pore Model
In the toroidal-pore model, AMPs aggregate on the surface of the membrane, which promote the membrane to bend substantially via pore. As a result, pore build-up takes place by the interaction of peptides and lipids. This mechanism is similar to the barrel-stave model but lipid–peptide interaction instead of peptide-peptide (Nguyen et al. 2011; Yan et al. 2022). These peptide conformations induce localized membrane deflection, occupying an equal proportion of peptides and phospholipids due to the formation of a ‘toroidal pore’ (Shahmiri et al. 2016). For instance, magainin 2, arenicin, and lacticin Q were used in this model to illustrate the MOA. Additionally, some positively charged peptides, such as TC19, TC84, and BP2, undergo membrane arbitration by initiating fluid domains (Omardien et al. 1860).
Carpet-Like Model
In the Carpet-like model, AMPs enfold in an entire membrane surface, as the name suggested, without forming pores (Bechinger 1999; Pouny et al. 1992; Lee et al. 2016). They may induce tension on the bacterial membrane, leads to membrane disorganization and micelle formation (Shai 1999; Ladokhin and White 2001). Neither pore formation nor penetration of peptides into the (hydrophobic) membrane occurs. This action can induce membrane lysis either partially or completely, eventually leading to cell death consequently (Zhang et al. 2021b). Cathelicidin LL-37, derived from the human body, exerts activity using this mechanism, and β-sheet plays an important role in this model (Shenkarev et al. 2011; Corrêa et al. 2019).
Aggregate Model
Besides these models, the aggregate model is also proposed in which peptides strongly interact with the anionic cell membrane, facilitating the formation of complex lipid-peptide micelle (Hale and Hancock 2007) (Fig. 1). This transmembrane aggregates AMPs, lipids, and water, unlike the carpet model. It can promote ions and intracellular elements leakage through channel formation, resulting in cell death. These channels also help AMPs move further into the cytoplasm and interact with other molecular targets based on killing action. This model provides further insight into cationic peptide translocation across the cell membrane and their activity with intracellular molecules (Hancock and Patrzykat 2002). Other than the proposed membrane model, some AMPs disrupt cell membranes by electroporation and alteration of membrane component distribution (Gan et al. 2021; Nayab et al. 2022).
The AMP counteracts with the bacteria either directly through membrane bound action or intracellularly by transporting across the cell membrane. The AMP works membrane bounded through four different ways: a accumulation of parallelly arranged AMP over cell membrane in a carpet like manner having their hydrophobic region facing the phospholipid and the hydrophilic region facing the aqueous content. Increased accumulation of AMP causes disruption and damage to the cell membrane in detergent-like action. b In the barrel-stave model the AMP aggregate together in a barrel-like manner arranged parallel to the phospholipid bilayer and create an ion channel which causes cytoplasmic leakage and cell death. c The toroidal pore model/wormhole models the AMP embedded into the cell membrane and forms a toroidal pore complex through lipid-AMP interaction. d In case of cationic AMP form lipid-peptide micelle complex which creates a channel and causes leakage of all intracellular content and leading to cell death. This mainly helps in the transport of AMP across the cell membrane to interact with cytoplasmic contents. The intracellular AMP action happens by inhibition of protein synthesis, inhibition of nucleic acid synthesis and inhibition of cell division or cell wall formation
Non-membrane Mechanism of AMPs
Instead of membrane action, some AMPs interact with targets at intracellular levels (Erdem Büyükkiraz and Kesmen 2022). AMPs translocate across the cell membrane through a binding process with intracellular targets. They subsequently disrupt important metabolic processes such as nucleic acid (DNA, RNA) synthesis, inactivation of specific enzymes, protein synthesis, and cell wall synthesis (Brogden 2005). Proline-rich AMPs exhibit intracellular activity by preventing bacterial protein biosynthesis in the protein synthesis inhibitory mechanism. For instance, AMPs bactenecin 7 and Tur1A show translation inhibitor action by arresting the transition from initiation to elongation (Mardirossian et al. 2018). Additionally, a few AMPs have different targets. For example, the insect-derived AMP apidaecin inhibits protein biosynthesis by preventing a releasing factor on the ribosome (Florin et al. 2017). Another peptide, namely DM3, can target crucial intracellular pathways of the translation process (Le et al. 2016). PrAMPs, pyrrhocoricin, and drosocin hinder protein folding pathways by binding specifically (DnaK, HSP) and nonspecifically (GroEL) to bacterial chaperonin (Le et al. 2017; Wrońska and Boguś 2020).
Several AMPs possess antimicrobial activity by interacting with DNA and RNA. The buforin II AMP, derived from frogs, translocates across the membrane and prevents nucleic acid biosynthesis (Park et al. 1998). Likewise, Indolicidin inhibits DNA biosynthesis without causing membrane permeability (Cardoso et al. 2019). Additionally, a few AMPs indirectly inhibit bacterial DNA replication and RNA synthesis (Kang et al. 2018; Wu et al. 2019). Many AMPs participate in metabolic inhibitory activity. Peptide eNAP-2 inhibits elastase and serine proteases of microbial origin (Le et al. 2017). Cathelicidin-BF, from the venom of Bungarus fasciatus hampers thrombin-mediated platelet aggregation and inactivation of protease-activated receptor 4 (Shu et al. 2019). Many AMPs block cell division by preventing DNA replication and SOS response (DNA damage). This disrupts the cell cycle, or chromosome segregation (Lutkenhaus 1990). MciZ, a short residue, is reported as an effective bacterial cell division Z-ring formation and localization inhibitor (Cruz et al. 2020). Additionally, AMPs also affect bacterial adhesion through the adsorption of biomaterial surfaces and they are involved in the prohibition of cell-to-cell communication, and down-regulate the essential genes for biofilm formation (Batoni et al. 1858; Brackman and Coenye 2015; Fuente-Núñez et al. 2012; Pletzer et al. 2016). Some AMPs have immune-modulatory effects as host defense peptides that protect from pathogen invasion by activating various immunological responses (Hancock et al. 2016; Mack and Kim 2016; Kang et al. 2019b). AMPs can regulate different cell receptors, such as cytokine receptors, chemokine receptors, and Toll-like receptors (Zhang et al. 2021a). AMPs are part of the immune system, associated with immune cells to suppress the growth of pathogens and control infection. The immune cell-mediated action of AMPs is extremely complex (Fruitwala et al. 2019). Hence, immuno-modulatory AMPs also stabilize cellular homeostasis by preventing infection-mediated tissue damage (Zhang et al. 2021a). Recent reports reveal that amyloids and amyloidogenic peptide-mediated direct co-aggregation are the foremost antibacterial mechanisms of AMPs (Kurpe et al. 2020).
Therapeutic Potential of AMPs
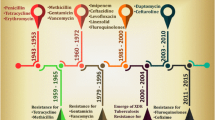
AMPs exhibit various bioactivities such as, antibacterial, antiviral, antifungal, immunomodulatory, tumor modulatory and antiparasitic activities (Liang and Diana 2020; Mokoena 2017; Liang et al. 2020; Struyfs et al. 2021; Kückelhaus et al. 2009). AMPs are considered as potential alternatives for existing antibiotics, due to their low tendency to develop drug resistance. These therapeutic activities support AMPs as a potential drug candidates for pharmaceutical application (Kang et al. 2017). They may be used alone or with other small molecules like antibiotics to achieve good action against pathogens (Gordon et al. 2005). Gramicidin A, is first AMP derived from gram positive Bacillus subtilis and was commercialized in the 1940s (Herrell and Heilman 1941). Even today, the AMPs used to treat topical issues like wounds, eye, nose and throat infections. However, Gramicidin A cannot be used internally due to their severe hemolytic toxicity (Subbalakshmi and Sitaram 1998). In 1953, Nisin was commercially marketed as an antimicrobial compound which can be used as a food preservative (Gharsallaoui et al. 2016).
The potential activities have also expanded for multidrug resistance pathogens, oral infections, and cancers, providing good results when combined with other drugs (Shin et al. 2016). The US food and drug administration (FDA) approved a cyclic peptide Daptomycin in 2003 against multi drug resistance pathogens for topical application (Carpenter and Chambers 2004). Similarly, FDA-approved semisynthetic peptides, Dalbayacin, telavancin and Oritavancin are derivatives of vancomycin between 2009 and 2014 for skin-associated infections. Other peptides, such as polymyxins (Colistins), a cyclic lipopeptide approved in the 1950s for treating MDR Gram-negative bacterial infections (Erdem Büyükkiraz and Kesmen 2022; Yan et al. 2022). As of now, more than 60 peptides have been approved FDA and more than 400 peptides are under clinical trials (Mousavi Maleki et al. 2022).
Synthetic analogue of cationic peptide indolicidine, Omiganen pentahydrochloride -MBI-226 were examined against 1437 clinical isolates and 214 clinical yeasts (Sader et al. 2004). Phase III clinical trials have been successfully completed for rosacea treatment. Hence, exposure to AMP with low concentration after long time facilitates bacterial resistance. High concentration has been recommended for stable bactericidal activity (Rodríguez-Rojas et al. 2021). The AMP also exerts antifungal activity (Lucca and Walsh 1999). Antifungal AMPs interact with fungal cell surfaces and target intracellular components, leading to the development of reactive oxygen species (ROS), mitochondrial dysfunction, apoptosis, and impairment of cell cycles (Struyfs et al. 2021). AMPs, named VLL-28, possess antifungal activity against Candida species, both biofilm and planktonic cells (Roscetto et al. 2018). Some AMPs containing histidine-rich residues have potential antifungal activity. These peptides enter fungal through cells either membrane receptors or transmembrane mechanism. These peptide groups target intracellular organelles, such as mitochondria, without damaging cells, inhibiting mitochondrial respiration, and subsequent cell death were occurred (Li et al. 2019a; Dhir et al. 2018). P113 is an AMP in saliva, which has strong activity against Candida albicans and other bacterial pathogens (Haney et al. 2021; Yu et al. 2017). This peptide is also used for treating oral Candidiasis in HIV patient’s treatment. Furthermore, AMPs have also shown strong antiviral potential with low toxicity, making them helpful in treating viral infections through either host -targeting or virus-targeting mechanisms (Erdem Büyükkiraz and Kesmen 2022; Castel et al. 2011). The epidemic and pandemic scenario induced by viral strains pose a notorious and growing threat in the world (Erdem Büyükkiraz and Kesmen 2022). Bovine antimicrobial peptide-13 exhibits activity against enveloped viruses. These AMPs potentially reduce the transmissible gastroenteritis viral multiplication by inhibiting gene expression and protein synthesis (Liang et al. 2020). The AMPs LL-37 exert activity against different types of enveloped viruses such as Human immunodeficiency virus (HIV), dengue virus (DENV), and Zika virus (ZIKV) through disruption of viral membrane and DNA replication process (Ahmed et al. 2019; Barlow et al. 2011; Tripathi et al. 2013). Likewise, various studies have examined Anti-SARS-COV response for heptad repeat (HR) peptides against these viruses (Outlaw et al. 2020; Xia et al. 2020). OC43-HR2P peptide derivative, EK1, exerts wider activity against multiple human coronaviruses (HCoVs). On the other hand, the HRC domain of SARS-Cov-2S, when conjugated with tetra-ethylene glycol-cholesterol synthesis lipopeptide derivative, impaired virus-mediated cell–cell fusion and halted new infection (Outlaw et al. 2020). In another study, DP7 (VQWRIRVAVIRK) peptide antiviral properties has been demonstrated (Zhang et al. 2021a). The study depicts that DP7 has a strong inhibitory effect on SARS-CoV and SARS-CoV-2 viruses through ACE2 receptor mediated action. Zhang et al. also shows that a concentration of (104 µg/mL and 73.625 µg/mL concentration of DP7) is needed (50% inhibitory concentration) to suppress SARS-CoV and SARS-CoV-2 pseudovirus (Zhang et al. 2021a).
AMPs have demonstrated the ability to selectively kill cancer cells by interacting with the cancer cell membrane (Kang et al. 2017; Nel et al. 2009). Anionic lipids on the outer leaflet of cancer cell membranes facilitate the binding specificity of cationic AMPs on these surfaces (Portelinha et al. 2021). In tumor therapy, classical chemotherapeutic agents often kill cancer and normal cells, leading to severe side effects in patients. However, cationic AMPs exhibit more specific action against target cancer cells such as cell membrane, mitochondria, nucleus lysosome and chromosomal DNA, while sparing normal cells (Rathinakumar et al. 2009; Wang et al. 2017). Numerous studies have shown that AMPs are sensitive to cancer cell but harmless to normal cells (Wang et al. 2008). AMPs can target tumour-specific cells and it can be utilized for current and future cancer treatments (Jin and Weinberg 2019). These peptides strongly attach to the acidic phospholipids on the outer surfaces of the tumour cells, leading to drastic metabolic changes in the tumor cells, such as alteration membrane potential, and cytoskeleton. It results in cell death through the formation of pores and leakage of intracellular components (Mahlapuu et al. 2016; Jäkel et al. 2012). AMPs have emerged as attractive agents for tumor therapy. For instance, the HPRP-A1 peptide derived from Helicobacter pylori has exhibited anticancer activity (Zhao et al. 2013). Moreover, combining HPRP-A1 with a homing peptide (iRGD) has improved anticancer activity, where the homing peptide aids the penetration of HPRP-A1 into A549 MCS (Hu et al. 2018). Similarly, the peptide (L-K6) kills MCF-7 type cancer cells through nuclear convulsion rather than cell surface-mediated mechanism (Hancock et al. 2016).
Certain AMPs also protect hosts from bacterial infection through the immune modulatory effects (Hancock et al. 2016; Kang et al. 2019b; Zasloff 2019). The role of AMPs in immune regulation process is more complex (Zhang et al. 2021a). Numerous cationic peptides have been examined and it found to have potent immunomodulatory responses with two rationales; the ability to induce chemokines and suppress the liberation of pro-inflammatory cytokines (Haney et al. 2015; Reffuveille et al. 2014). Different ways have been associated with the immunomodulation process, including reduced endotoxin-induced inflammatory response, synthesis of pro-inflammatory factors, adaptive immunity response, synthesis of cytokines, and involvement of macrophages to exhibit immune modulatory action (Moravej et al. 2018; Haney et al. 2019). For example, clavanin MO, a derivative of clavanin A peptide, incorporates a hydrophobic amino acid into the conserved oligopeptide FLPII, resulting in dual properties of antimicrobial and immune-modulatory effects in the same peptide (Silva et al. 2016; Sultana et al. 2021). AMPs such as clavanin-MO have been shown to elevate the synthesis of IL-10 and decrease IL-12 in exposed cells, indicating the ability to harmonize the innate immune system by requiring more leukocytes (Silva et al. 2016). Therefore, AMPs can have either pro- inflammatory or anti-inflammatory activity based on their production levels at the sites of inflammation (Prasad et al. 2019). Additionally, AMPs and their immuno modulatory actions inhibit infection-mediated tissue damage and maintain cellular homeostasis (Sultana et al. 2021). AMPs also exhibit a strong anti-inflammatory response. For example, the anti-inflammatory response of Melectin AMPs extracted from M. albifrons was examined by qRT-PCR (Ko et al. 2020).
Apart from these activities, AMPs have exhibited various other activities, including participation in natural immunity, phagocytosis stimulation, promoting the cell cycle of epithelial and fibroblasts cells (Moravej et al. 2018; Aisenbrey et al. 2019). AMPs have been found to stimulate the growth of wound granulation tissues and enhance wound healing properties (Mahlapuu et al. 2016; Taniguchi et al. 2019). The wound-healing activity of recombinant P-LL37 has been reported in dexamethasone-treated mice (Ramos et al. 2011; Silva et al. 2015). Furthermore, AMPs can induce human lymphocytes successfully to remove infected cells caused by viruses, bacteria, and cancer cells. They also play a significant role in chronic inflammation and aid in developing helper T cells, chemokine synthesis, elevation of the antibody IgG level, and stimulation of lymphocytes to eliminate infected cells (Zasloff 2019; Taniguchi et al. 2019; Lande et al. 2014). Some AMPs also play an important role in skin barrier and function, with the potential to treat skin-associated problems, such as acne, psoriasis, diabetic foot ulcer (Nguyen et al. 2020; Alencar-Silva et al. 2018). Additionally, AMPs have been linked to the occurrence and development of diabetes (Conlon et al. 2018; Zainab et al. 2019). Moreover, AMPs also play a key role in supporting colon homeostasis, tissue repair and preserving the colon micro biota (Yoshimura et al. 1950; Rathinam and Chan 2018; Zhang et al. 2019). Besides their therapeutic potential, AMPs have various other applications such as food industry as food additives, animal husbandry, aquaculture, and plant protection applications, to replace the chemical agents (Erdem Büyükkiraz and Kesmen 2022). For instance, Streptomyces albulus produced AMPs (ε-polylysinea homoplymer of l-lysine) has been approved by the FDA for food precautionary applications (GRAS) status due to their broad spectrum active potential (Luz et al. 2018). To maintain the scope of review we highlighted only therapeutic application of AMPs, and other applications were briefly described.
Antibiotics and Peptide Conjugates
AMPs are attractive and promising alternatives to classical antibiotics due to their potency, low tendency to develop resistance, and potential as biomolecule drug delivery vehicles (Splith and Neundorf 2011). However, certain limitations are associated with AMPs in clinical applications, such as low residence time in the bloodstream, toxicity, immunogenicity, sensitivity to host proteases, and other side effects (Moravej et al. 2018). On the other hand, naturally derived peptides are mostly unstable, and long-term clinical use of these AMPs may result in significant toxicity in mammalian cells (Starr et al. 2018). Hence, design of peptides or modification of natural peptides with another type of biomolecules enhance their antibacterial activity with different mode of action, resulting in functionalized AMPs and AMP conjugates (Brogden and Brogden 2011). AMPs are functionalized to synthesise peptide conjugates by attaching substances via wider coupling methodologies. A common concept is to merge the modification at the α amino group to the carboxylic terminal, providing an amide linkage. Chemoselective coupling concept has been adopted to spawn conjugates for free peptide in solution where amino-reactive N-hydroxy succinimides or maleimide (thiol reactive) can be activated (Reinhardt and Neundorf 2016). This review highlights recent advances in synthesizing antibiotics coupled with AMP conjugates and novel activities. The important strategy is that existing antibiotics coupled with AMPs (covalently) can also induce synergistic effects on a wide range of bacteria. This combination strategy enhances antibacterial activity, reduces adverse effects, and requires lower doses (Yamauchi et al. 2022). The synergistic activity of APCs has been reported to overcome bacteria’s existing antibiotics and AMP resistance (David et al. 2018). For example, Vancomycin-magainin conjugates have increased activity against only vancomycin-resistant Enterococci with better minimal inhibitory concentrations than antibiotic alone (Arnusch et al. 2012). These reports suggest that the length of amino acid sequences promotes the MOA on the bacterial membrane, in which shorter fragments adopt the carpet model mode, while longer peptides undergo to form transmembrane pores based on the mode of action (Arnusch et al. 2012). Similar to Vancomycin-peptide conjugates, Telvancin and Dalbavancin APCs are also approved by the FDA for antimicrobial therapy (Etayash et al. 2021). Levofloxacin conjugated with indolicidin (rich hydrophobic residues) via an amide bond has been tested to determine whether this combination impacts activity (Ghaffar et al. 2015). In this study, no improved activity has been observed by chosen linkage, whereas the activity is increased through a physical mixture of levofloxacin and indolicidin. This observation denotes that the amide linkage reduces the activity of levofloxacin (Ghaffar et al. 2015). Recently, ubiquicidin (UBI) a cationic peptide variant linked to chloramphenicol, which exhibits antibacterial activity against E.coli and Staphylococcus aureus (Chen et al. 2015a). The conjugation of chloramphenicol into UBI has been achieved via a glutaraldehyde linker. Many reports revealed that short peptide motif along with antibiotic drugs show positive antibacterial activity. For example, unique polycationic lysine-like substances are produced from modified neomycin B variants that enhance the antibacterial activity and also promote RNA binding affinity (Bera et al. 2011). Neomycin B consist of a hydroxyl residue (OH) at C5 position, which facilitates thr coupling of a short motif (Tryp-Tryp-Lys). This conjugation approach has obtained new antimicrobial activity (Zhang et al. 2008). In addition to this, amphiphilic nanostructures consist of hydrophilic surface and hydrophobic core molecule are derived from when neomycin is modified with short dehydropeptides (Yadav et al. 2014). Overall, these examinations reveal that the increased activity is achieved by physical mixture of AMP and antibiotic, whereas decreased activity is observed through the covalent conjugation of peptide and antibiotics (Ghaffar et al. 2015). Hence, the amalgamated approach itself is very effective. While preparing covalent conjugates, extra consideration should be taken to avoid toxicity and enhance activity. To achieve this, linker sites can be found within the structures of antibiotics to avoid the loss of antibiotics’ activity while combined with AMPs. On the other hand, to get better activity of AMPs in terms of bacterial membrane interaction, it can be modified by a lipidation process with fatty acids (Chu-Kung et al. 2010). d-Amino acid instead of l-amino acid in AMPs can maintain more stability against proteases, which increase pharmacokinetic properties and improve their bioactivity (Li et al. 2016; Zhang and Yang 2022; Luong et al. 2020). In addition, structural engineering approaches such as terminal acetylatin, amidation, cyclization, and l to d amino acid residue substitution inhibits the proteolysis by various proteases and improve the bioactivity (Lu et al. 2020; Jia et al. 2017; Li et al. 2019b). Furthermore to achieve for better stability and target selectivity, nanotechnologies including electrospun nanofibers, liposomes or metal to AMPs, strengthen their overall properties (Tang et al. 2021; Biswaro et al. 2018; Rajchakit and Sarojini 2017). Combining of antibiotics and AMPs is still promising strategy to enhance various bioactivities by adopting suitable conjugation methodologies.
AMPs-Polymer Nano-structures for Drug Delivery
Nanotechnology furnishes the stability and sustained delivery of AMP to enhance the target selectivity. Nano-delivery cargo can improve the pharmacokinetics, bioavailability, and antibacterial efficacy of AMP (Radaic et al. 2020). Various types of nanostructures have been used for AMP delivery, such as carbon nanotubes, metal nanoparticles, liposome-based nanostructures, and polymeric substances (Carratalá et al. 2020). Generally, surfactant, lipid, and polymer-mediated drug delivery are well-developed.
Inorganic Materials
To increase the AMPs’ efficacy, numerous delivery systems have been developed in which peptides are incorporated either covalently or non-covalently (Singh et al. 2016; Sandreschi et al. 2016). Nowadays, organic nanomaterials such as metal oxides, metals, mesoporous materials, and nano clays are attractive and effective drug delivery systems for peptides, proteins, and other biomolecules (Malmsten 2013). Organic nanomaterials also aid protection from enzymatic and chemical degradation, inhibition of aggregation, conformational changes, sustained drug release, promote bioavailability, and reduce toxicity. A quite few nanomaterials show antimicrobial activity, facilitating synergistic effects in combination with AMPs (Huh and Kwon 2011; Hajipour et al. 2012). Mesoporous materials are fascinating for the sustained release of drug molecules and can form mesoporous structures with a wide range of nanoparticles. The effect of nanoparticle porosity and charges, including drug loading and release of AMP LL-37, has been investigated on cell membrane disruption and antimicrobial activity (Braun et al. 2016). In addition, metal nanoparticles such as Au, Pt, Ag, and Cu afford greater response for drug delivery. With special reference to metal nanoparticles as an AMP drug delivery system, the nanodots (AuNDs) prepared by etching and co-precipitation of hybridized ligands and an AMP (Surfactin) and 1-dodecanethiol on Au nanoparticles result in enhanced antimicrobial activity against MDR strains when compared to surfactin alone (Chen et al. 2015b).
Hence, numerous quantum dots show cell toxicity (Malmsten 2013), limiting the usage of drug delivery vehicles. Nowadays, several types of quantum dots are of special interest as AMP delivery vehicles. For instance, ZnO quantum dot, AMP (UB129-41), and MPA based nanoparticle composites are increasing antibacterial activity with less cytotoxicity (Chen et al. 2015b). Carbon-based nanomaterials, namely graphene and carbon nanotubes (CNT), are of charming interest as drug delivery vehicles based on their tendency for aggregation in solution and stabilization via appropriate surface modification (Malmsten 2013). For example, when conjugated with graphene oxide membranes, nisin, a pore-forming AMP, results in 100% MRSA-killing (100%) activity that can be used for water disinfection (Kanchanapally et al. 2015).
Polymeric Substances
Polymers afford greater scope as drug delivery systems for various antimicrobial therapies due to their biocompatibility and potential synergistic activity (Kenawy et al. 2007). Polymers of repeated monomeric units are frequently used in medicinal applications. AMP polymeric nanostructures provide numerous benefits, including stability and bioactivities (Rai et al. 2022). RBRBR, a short peptide exerts enhanced antimicrobial effects against MDR, antibiofilm potential and stability, when it is conjugated in chitosan-based nano particles (Cleophas et al. 2014; Almaaytah et al. 2017). Wu et al. reported the formation of nisin incorporation in nanoparticles by self-assembly of chitosan and poly-gamma-glutamic acid. In-vitro antibacterial study of the chitosan-containing composite show potent activity against E.coli and Listeria monocytogenes compared to nisin/gamma-PGA or nisin alone (Wu et al. 2016). In addition to polymer-based nanoparticles, nanofibrous materials derived from natural or synthetic polymers are promising wound dressing substances based on unique properties like high surface area, porosity, and lower adverse effects (Andreu et al. 2015). Natural and synthetic polymers have exhibited their own merits and demerits (Maftoonazad et al. 2019; Topuz et al. 2021; Hamdan et al. 2021). When compared to natural polymers, synthetic polymers have several advantages such as processability and offer good mechanical properties, for example, Poly (ε-caprolactone) (PCL), poly-lactide (PLA), poly-glycolide (PGA), Polyvinyl alcohol (PVA), etc. (Maftoonazad et al. 2019). Nowadays, researchers focus on a new attractive methodology to enhance the antibacterial efficacy of AMPs coated into biocompatible polymers that extensively combat multi-drug resistance. To improve the physiochemical property of nanofibers, electrospinning technology has been widely used (Mouro et al. 2021; Deng et al. 2018; Mirzaeei et al. 2021). It is a cost-effective approach extensively used in biological and medical practice. Specific nanofibers are generated by applying a strong electric field (Zare et al. 2021). As a result, the electrospun polymeric nanofibers exert special properties like shape, size, and surface interactions. These enhance the therapeutic activity and are utilized as a drug cargo for other natural antibiotics. The unique nature of nanofibers enabled the creation of drug-filled nanofibers with a stable release of drug at the infection site (Pan et al. 2021; Topuz and Uyar 2018; Pankajakshan et al. 2016; Contardi et al. 2017; Ashbaugh et al. 2016; Chen et al. 2014).
Proline-rich peptide APO loaded into PVA (polyvinyl alcohol) nanofibers has been reported as a solid patch dressing application (Sebe et al. 2016). Microgel/nanogels are another type of AMP delivery vehicle. For example, incorporating novicidin into modified hyaluronic acid results in greater encapsulation (up to 70%) and a peptide load above 36%. It has strong stability (colloidal) and exhibited constant peptide release for two weeks (Water et al. 2015). AMP CysHHC10 into a polyethylene glycol (PEG) hydrogel matrix formed on polyethylene terephthalate by thiol-ene reaction resulted in hydrogels with antimicrobial action against gram-positive bacteria (S. aureus and S. epidermidis). The combination of PVA and Chitosan recently provided heat resistance for nanofibers to encapsulate gentamicin for curbed release of up to 72 h (Hamdan et al. 2021). Likewise, PEG polymer covalently attached with HHC10 AMPs shows strong resistance against proteolysis and increased activity against MDR pathogens (Almaaytah et al. 2017). Giram et al. reported that fabricated Eudragit L-100 nanofibers encapsulated with Moxifloxacin to accelerate drug delivery. The antibiotic-loaded nanofibers exhibit potent antimicrobial activity against gram-positive S.aureus and negative E.coli (Giram et al. 2018). Synthetic and natural polymers are used to fabricate nanofibers because of their biocompatibility, processability and biodegradability (Rofeal et al. 2022; Cruz-Maya et al. 2019). Biopolymer-based nanofibrous wound dressing are extensively reliable and easily biodegradable (Parham et al. 2022). On the other hand, the major setback of using natural or designed AMPs could be instability. Hence, it is essential to stabilize the long-acting peptides with the help of biopolymers to retain their stability and multifunctional activity. Nanofibers are widely used as a carriers for curative agents, namely, antibiotics, peptides, and natural extracts. Polyacrylic acid/polyvinyl alcohol nanofibers coated with nisin show superior antimicrobial activity against S.aureus up to 14 days (Santiago-Morales et al. 2016).
Polymer multilayers provide a great opportunity for developing surface coatings, capsules, or particles. For instance, poly methacrylic acid (PMAA) and polyvinylpyrrolidone (PVP) encapsulated with cationic peptide L5 by layer-by-layer deposition has been reported to completely inhibit S. epidermidis growth (Pavlukhina et al. 2010). Polymer conjugates are the best tool to enhance the activity of peptides, proteins, and drug delivery systems. In this context, further studies are also needed to relate to the importance of antimicrobial effects after pathogen exposure. Additionally, AMP-containing nanoparticle systems remain elusive in nanotoxicology, and AMP drug delivery has the potential for complete fulfilment. Overall, investigations reveal that the utilization of drug cargo promotes the transformation of AMPs from the laboratory research level into clinical trials and further moves forward to a wide range of clinical use.
Antibiotics and AMPs Conjugates for Future Clinical Applications
Antibiotic substances are more efficient against pathogenic microorganisms (Kościuczuk et al. 2012; Moravej et al. 2018). However, due to abuse and excessive use of antibiotics, their efficacy is lost over time, leading to multidrug resistance (Lau et al. 1860). The global expansion of drug-resistant bacteria and the competitive scramble between weak pipelines of innovative antibiotics are the main causes of antimicrobial resistance. The antibiotic crisis is a severe problem that has slowed down all aspects of national growth, including healthcare systems (Lim et al. 2016). Hence, creating new chemical entities requires more effort and resources, and it is particularly difficult to re-isolate old compounds. An innovative and unconventional therapeutic approach can be used to combat AMR. AMPs are short peptides ubiquitous in nature produced by various organisms, including bacteria, fungi, and plants, first discovered in the 1980s. The MOA of AMPs is unique, especially regarding membrane permeability, which is a potential and recognized mechanism compared to conventional antibiotics (Lei et al. 2019). However, natural peptides are not stable, and toxic effects consistently persist. Synthetic AMPs become more stable once chemically modified and maintain their biological activity during unfavourable conditions (Mahlapuu et al. 2016). To extend clinical use, developing strategies and prospects of long-lasting AMPs via chemical modification by conjugating potential antibiotics are necessary to overcome the problems associated with the cell membrane barrier of the superior chemical entities. AMPs are also called as antibiotic adjuvants, or delivery vehicles for other chemical entities (Schafer et al. 2021). Outer cell membrane- penetrating AMPs are synthesized and conjugated to existing small organic molecules to carry biomolecules across cell membranes and deliver various other drugs and compounds (Schafer et al. 2021). Therefore, joined armaments can eliminate AMR more efficiently through various mechanisms of action, especially cell-penetrating and host defence mechanisms (Lei et al. 2019; Lim et al. 2016). Recently, promising broad-spectrum antimicrobial activity has been obtained by conjugating AMPs with d-amino acid residues linked to fatty acids (Zhong et al. 2020). For instance, vancomycin conjugated with arginine enables the influx of vancomycin and inhibits the cell wall synthesis of Gram-negative bacteria (Antonoplis et al. 2019). Thus, the consolidated peptide-antibiotic conjugates approach improves antimicrobial efficacy by adding existing antibiotics to treat antimicrobial resistance- associated infectious diseases.
Conclusion
Genetic changes or mutations can lead to the natural emergence of antimicrobial resistance over time. Pathogens can spread rapidly between individuals, enabling bacteria to evade the effects of existing antibiotics, potentially leading to superbugs. Overcoming these infections is now the most challenging step. The discovery of novel antimicrobials is laborious, expensive, and risky, requiring significant resources. Recently, researchers have focused on prospective replacements with distinct AMP interfacial actions to increase efficacy against various infections without causing AMR to improve further. However, a significant barrier to stop bacterial infections which remain due to natural peptides’ short life and toxicity. To overcome this limitation and create more effective treatment regimens, it is crucial to synthesize and improve AMPs for clinical applications. Dual therapy using small molecule-peptide conjugates is an excellent alternative for defeating against AMR. These combinatorial therapies preserve the unique properties of peptides and enable other small biomolecules to penetrate the outer cell membrane barrier of vital bacterial (gram-negative) infections. By adopting these integrated techniques, activity is improved, and the transition from targeted to broad-spectrum therapeutic actions is accelerated without furthering the emergence of antibiotic resistance. Most importantly, preserving the effectiveness of currently available antibiotics helps addressing issues with nosocomial infections linked to AMR and hospital-acquired infections. Utilizing a combination of AMPs and small molecule conjugates provide a potential strategy to address the threat of drug-resistant superbugs.
References
Abry MF, Kimenyi KM, Masiga D, Kulohoma BW (2017) Comparative genomics identifies male accessory gland proteins in five Glossina species. Wellcome Open Res 2:73
Ageitos JM, Sánchez-Pérez A, Calo-Mata P, Villa TG (2017) Antimicrobial peptides (AMPs): ancient compounds that represent novel weapons in the fight against bacteria. Biochem Pharmacol 133:117–138
Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, Gudmundsson GH (1995) FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA 92:195–199
Ahmed A, Siman-Tov G, Hall G, Bhalla N, Narayanan A (2019) Human antimicrobial peptides as therapeutics for viral infections. Viruses 11:704
Aisenbrey C, Marquette A, Bechinger B (2019) The mechanisms of action of cationic antimicrobial peptides refined by novel concepts from biophysical investigations. Adv Exp Med Biol 1117:33–64
Alencar-Silva T, Braga MC, Santana GOS, Saldanha-Araujo F, Pogue R, Dias SC, Franco OL, Carvalho JL (2018) Breaking the frontiers of cosmetology with antimicrobial peptides. Biotechnol Adv 36:2019–2031
Almaaytah A, Mohammed GK, Abualhaijaa A, Al-Balas Q (2017) Development of novel ultrashort antimicrobial peptide nanoparticles with potent antimicrobial and antibiofilm activities against multidrug-resistant bacteria. Drug Des Dev Ther 11:3159–3170
Almeida PF, Pokorny A (2012) 5.10 Interactions of antimicrobial peptides with lipid bilayers. In: Egelman EH (ed) Comprehensive biophysics. Elsevier, Amsterdam, pp 189–222
Andersson M, Boman A, Boman HG (2003) Ascaris nematodes from pig and human make three antibacterial peptides: isolation of cecropin P1 and two ASABF peptides. Cell Mol Life Sci (CMLS) 60:599–606
Andreu V, Mendoza G, Arruebo M, Irusta S (2015) Smart dressings based on nanostructured fibers containing natural origin antimicrobial, anti-inflammatory, and regenerative compounds. Materials (Basel, Switzerland) 8:5154–5193
Antonoplis A, Zang X, Wegner T, Wender PA, Cegelski L (2019) Vancomycin-arginine conjugate inhibits growth of carbapenem-resistant E. coli and targets cell-wall synthesis. ACS Chem Biol 14:2065–2070
Arakawa K, Yoshida S, Aikawa H, Hano C, Bolormaa T, Burenjargal S, Miyamoto T (2016) Production of a bacteriocin-like inhibitory substance by Leuconostoc mesenteroides subsp. dextranicum 213M0 isolated from Mongolian fermented mare milk, airag. Anim Sci J Nihon Chikusan Gakkaiho 87:449–456
Araújo C, Muñoz-Atienza E, Poeta P, Igrejas G, Hernández PE, Herranz C, Cintas LM (2016) Characterization of Pediococcus acidilactici strains isolated from rainbow trout (Oncorhynchus mykiss) feed and larvae: safety, DNA fingerprinting, and bacteriocinogenicity. Dis Aquat Org 119:129–143
Arnusch CJ, Pieters RJ, Breukink E (2012) Enhanced membrane pore formation through high-affinity targeted antimicrobial peptides. PLoS ONE 7:e39768
Ashbaugh AG, Jiang X, Zheng J, Tsai AS, Kim W-S, Thompson JM, Miller RJ, Shahbazian JH, Wang Y, Dillen CA, Ordonez AA, Chang YS, Jain SK, Jones LC, Sterling RS, Mao H-Q, Miller LS (2016) Polymeric nanofiber coating with tunable combinatorial antibiotic delivery prevents biofilm-associated infection in vivo. Proc Natl Acad Sci USA 113:E6919–E6928
Atrih A, Rekhif N, Moir AJG, Lebrihi A, Lefebvre G (2001) Mode of action, purification and amino acid sequence of plantaricin C19, an anti-Listeria bacteriocin produced by Lactobacillus plantarum C19. Int J Food Microbiol 68:93–104
Babasaki K, Takao T, Shimonishi Y, Kurahashi K (1985) Subtilosin A, a new antibiotic peptide produced by Bacillus subtilis 168: isolation, structural analysis, and biogenesis. J Biochem 98:585–603
Bahar AA, Ren D (2013) Antimicrobial peptides. Pharmaceuticals 6:1543–1575
Baindara P, Mandal SM, Chawla N, Singh PK, Pinnaka AK, Korpole S (2013) Characterization of two antimicrobial peptides produced by a halotolerant Bacillus subtilis strain SK.DU.4 isolated from a rhizosphere soil sample. AMB Express 3:2–4
Barbosa Pelegrini P, Del Sarto RP, Silva ON, Franco OL, Grossi-de-Sa MF (2011) Antibacterial peptides from plants: what they are and how they probably work. Biochem Res Int 2011:250349–51
Barlow PG, Svoboda P, Mackellar A, Nash AA, York IA, Pohl J, Davidson DJ, Donis RO (2011) Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE 6:e25333
Batoni G, Maisetta G, Esin S (1858) Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochem Biophys Acta 2016:1044–1060
Baxter AA, Lay FT, Poon IKH, Kvansakul M, Hulett MD (2017) Tumor cell membrane-targeting cationic antimicrobial peptides: novel insights into mechanisms of action and therapeutic prospects. Cell Mol Life Sci (CMLS) 74:3809–3825
Bechinger B (1999) The structure, dynamics and orientation of antimicrobial peptides in membranes by multidimensional solid-state NMR spectroscopy. Biochem Biophys Acta 1462:157–183
Bednarska NG, Wren BW, Willcocks SJ (2017) The importance of the glycosylation of antimicrobial peptides: natural and synthetic approaches. Drug Discov Today 22:919–926
Belmadani A, Semlali A, Rouabhia M (2018) Dermaseptin-S1 decreases Candida albicans growth, biofilm formation and the expression of hyphal wall protein 1 and aspartic protease genes. J Appl Microbiol 125:72–83
Ben Braïek O, Morandi S, Cremonesi P, Smaoui S, Hani K, Ghrairi T (2018) Biotechnological potential, probiotic and safety properties of newly isolated enterocin-producing Enterococcus lactis strains. LWT 92:361–370
Bera S, Zhanel GG, Schweizer F (2011) Synthesis and antibacterial activity of amphiphilic lysine-ligated neomycin B conjugates. Carbohydr Res 346:560–568
Berrocal-Lobo M, Segura A, Moreno M, López G, García-Olmedo F, Molina A (2002) Snakin-2, an antimicrobial peptide from potato whose gene is locally induced by wounding and responds to pathogen infection. Plant Physiol 128:951–961
Bhat SG (2018) Modelling and computational sequence analysis of a bacteriocin isolated from Bacillus licheniformis strain BTHT8. Int J Comput Biol (IJCB) 7:29–34
Biswaro LS, da Costa Sousa MG, Rezende TMB, Dias SC, Franco OL (2018) Antimicrobial peptides and nanotechnology, recent advances and challenges. Front Microbiol 9:855
Brackman G, Coenye T (2015) Quorum sensing inhibitors as anti-biofilm agents. Curr Pharm Des 21:5–11
Braun K, Pochert A, Lindén M, Davoudi M, Schmidtchen A, Nordström R, Malmsten M (2016) Membrane interactions of mesoporous silica nanoparticles as carriers of antimicrobial peptides. J Colloid Interface Sci 475:161–170
Braun MS, Sporer F, Zimmermann S, Wink M (2018) Birds, feather-degrading bacteria and preen glands: the antimicrobial activity of preen gland secretions from turkeys (Meleagris gallopavo) is amplified by keratinase. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiy117
Brillet-Viel A, Pilet M-F, Courcoux P, Prévost H, Leroi F (2016) Optimization of growth and bacteriocin activity of the food bioprotective Carnobacterium divergens V41 in an animal origin protein free medium. Front Mar Sci 3(128):1–13
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238–250
Brogden NK, Brogden KA (2011) Will new generations of modified antimicrobial peptides improve their potential as pharmaceuticals? Int J Antimicrob Agents 38:217–225
Cardoso MH, Meneguetti BT, Costa BO, Buccini DF, Oshiro KGN, Preza SLE, Carvalho CME, Migliolo L, Franco OL (2019) Non-lytic antibacterial peptides that translocate through bacterial membranes to act on intracellular targets. Int J Mol Sci 20:4877
Carpenter CF, Chambers HF (2004) Daptomycin: another novel agent for treating infections due to drug-resistant gram-positive pathogens. Clin Infect Dis 38:994–1000
Carratalá JV, Serna N, Villaverde A, Vázquez E, Ferrer-Miralles N (2020) Nanostructured antimicrobial peptides: the last push towards clinics. Biotechnol Adv 44:107603
Castel G, Chtéoui M, Heyd B, Tordo N (2011) Phage display of combinatorial peptide libraries: application to antiviral research. Molecules (Basel, Switzerland) 16:3499–3518
Chan DI, Prenner EJ, Vogel HJ (2006) Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochem Biophys Acta 1758:1184–1202
Chen Y, Guarnieri MT, Vasil AI, Vasil ML, Mant CT, Hodges RS (2007) Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob Agents Chemother 51:1398–1406
Chen M, Li Y-F, Besenbacher F (2014) Electrospun nanofibers-mediated on-demand drug release. Adv Healthc Mater 3:1721–1732
Chen H, Liu C, Chen D, Madrid K, Peng S, Dong X, Zhang M, Gu Y (2015a) Bacteria-targeting conjugates based on antimicrobial peptide for bacteria diagnosis and therapy. Mol Pharm 12:2505–2516
Chen W-Y, Chang H-Y, Lu J-K, Huang Y-C, Harroun SG, Tseng Y-T, Li Y-J, Huang C-C, Chang H-T (2015b) Self-assembly of antimicrobial peptides on gold nanodots: against multidrug-resistant bacteria and wound-healing application. Adv Func Mater 25:7189–7199
Chen YS, Wu HC, Kuo CY, Chen YW, Ho S, Yanagida F (2018) Leucocin C-607, a novel bacteriocin from the multiple-bacteriocin-producing Leuconostoc pseudomesenteroides 607 Isolated from Persimmon. Probiotics Antimicrob Proteins 10:148–156
Chu-Kung AF, Nguyen R, Bozzelli KN, Tirrell M (2010) Chain length dependence of antimicrobial peptide-fatty acid conjugate activity. J Colloid Interface Sci 345:160–167
Cleophas RTC, Riool M, Quarles van Ufford HLC, Zaat SAJ, Kruijtzer JAW, Liskamp RMJ (2014) Convenient preparation of bactericidal hydrogels by covalent attachment of stabilized antimicrobial peptides using thiol-ene click chemistry. ACS Macro Lett 3:477–480
Colgrave ML, Craik DJ (2004) Thermal, chemical, and enzymatic stability of the cyclotide kalata B1: the importance of the cyclic cystine knot. Biochemistry 43:5965–5975
Conibear AC, Bochen A, Rosengren KJ, Stupar P, Wang C, Kessler H, Craik DJ (2014) The cyclic cystine ladder of theta-defensins as a stable, bifunctional scaffold: a proof-of-concept study using the integrin-binding RGD motif. ChemBioChem 15:451–459
Conlon JM, Mechkarska M, Abdel-Wahab YH, Flatt PR (2018) Peptides from frog skin with potential for development into agents for Type 2 diabetes therapy. Peptides 100:275–281
Contardi M, Heredia-Guerrero JA, Perotto G, Valentini P, Pompa PP, Spanò R, Goldoni L, Bertorelli R, Athanassiou A, Bayer IS (2017) Transparent ciprofloxacin-povidone antibiotic films and nanofiber mats as potential skin and wound care dressings. Eur J Pharm Sci 104:133–144
Corrêa JAF, Evangelista AG, Nazareth TM, Luciano FB (2019) Fundamentals on the molecular mechanism of action of antimicrobial peptides. Materialia 8:100494
Costa F, Teixeira C, Gomes P, Martins MCL (2019) Clinical application of AMPs. Adv Exp Med Biol 1117:281–298
Cruz GF, de Araujo I, Torres MDT, de la Fuente-Nunez C, Oliveira VX, Ambrosio FN, Lombello CB, Almeida DV, Silva FD, Garcia W (2020) Photochemically-generated silver chloride nanoparticles stabilized by a peptide inhibitor of cell division and its antimicrobial properties. J Inorg Organomet Polym Mater 30:2464–2474
Cruz-Maya I, Guarino V, Almaguer-Flores A, Alvarez-Perez MA, Varesano A, Vineis C (2019) Highly polydisperse keratin rich nanofibers: scaffold design and in vitro characterization. J Biomed Mater Res Part A 107:1803–1813
da Costa JP, Cova M, Ferreira R, Vitorino R (2015) Antimicrobial peptides: an alternative for innovative medicines? Appl Microbiol Biotechnol 99:2023–2040
David AA, Park SE, Parang K, Tiwari RK (2018) Antibiotics-peptide conjugates against multidrug-resistant bacterial pathogens. Curr Top Med Chem 18:1926–1936
de la Fuente-Núñez C, Korolik V, Bains M, Nguyen U, Breidenstein EB, Horsman S, Lewenza S, Burrows L, Hancock RE (2012) Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob Agents Chemother 56:2696–2704
De Lucca AJ, Walsh TJ (1999) Antifungal peptides: novel therapeutic compounds against emerging pathogens. Antimicrob Agents Chemother 43:1–11
Deng L, Taxipalati M, Zhang A, Que F, Wei H, Feng F, Zhang H (2018) Electrospun chitosan/poly(ethylene oxide)/lauric arginate nanofibrous film with enhanced antimicrobial activity. J Agric Food Chem 66:6219–6226
Dhir A, Dhir S, Borowski LS, Jimenez L, Teitell M, Rötig A, Crow YJ, Rice GI, Duffy D, Tamby C, Nojima T, Munnich A, Schiff M, de Almeida CR, Rehwinkel J, Dziembowski A, Szczesny RJ, Proudfoot NJ (2018) Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature 560:238–242
Dhople V, Krukemeyer A, Ramamoorthy A (2006) The human beta-defensin-3, an antibacterial peptide with multiple biological functions. Biochem Biophys Acta 1758:1499–1512
Di Luca M, Maccari G, Nifosì R (2014) Treatment of microbial biofilms in the post-antibiotic era: prophylactic and therapeutic use of antimicrobial peptides and their design by bioinformatics tools. Pathog Dis 70:257–270
Dias Rde O, Franco OL (2015) Cysteine-stabilized αβ defensins: from a common fold to antibacterial activity. Peptides 72:64–72
Duwadi D, Shrestha A, Yilma B, Kozlovski I, Sa-Eed M, Dahal N, Jukosky J (2018) Identification and screening of potent antimicrobial peptides in arthropod genomes. Peptides 103:26–30
Dziuba B, Nalepa B (2012) Identification of lactic acid bacteria and propionic acid bacteria using FTIR spectroscopy and artificial neural networks. Food Technol Biotechnol 50:399
Ebrahimipour GH, Khosravibabadi Z, Sadeghi H, Aliahmadi A (2014) Isolation, partial purification and characterization of an antimicrobial compound, produced by Bacillus atrophaeus. Jundishapur J Microbiol 7:e11802
Edwards IA, Elliott AG, Kavanagh AM, Zuegg J, Blaskovich MA, Cooper MA (2016) Contribution of amphipathicity and hydrophobicity to the antimicrobial activity and cytotoxicity of β-hairpin peptides. ACS Infect Dis 2:442–450
Elias PM, Choi EH (2005) Interactions among stratum corneum defensive functions. Exp Dermatol 14:719–726
Erdem Büyükkiraz M, Kesmen Z (2022) Antimicrobial peptides (AMPs): a promising class of antimicrobial compounds. J Appl Microbiol 132:1573–1596
Essig A, Hofmann D, Münch D, Gayathri S, Künzler M, Kallio PT, Sahl HG, Wider G, Schneider T, Aebi M (2014) Copsin, a novel peptide-based fungal antibiotic interfering with the peptidoglycan synthesis. J Biol Chem 289:34953–34964
Etayash H, Alford M, Akhoundsadegh N, Drayton M, Straus SK, Hancock REW (2021) Multifunctional antibiotic-host defense peptide conjugate kills bacteria, eradicates biofilms, and modulates the innate immune response. J Med Chem 64:16854–16863
Falanga A, Lombardi L, Franci G, Vitiello M, Iovene MR, Morelli G, Galdiero M, Galdiero S (2016) Marine antimicrobial peptides: nature provides templates for the design of novel compounds against pathogenic bacteria. Int J Mol Sci 17:785
Falanga A, Nigro E, De Biasi MG, Daniele A, Morelli G, Galdiero S, Scudiero O (2017) Cyclic peptides as novel therapeutic microbicides: engineering of human defensin mimetics. Molecules (basel, Switzerland) 22:1217
Farouk A, Ahamed NT, Alzahrani OM, Alghamdi AS, Bahobail AA (2017) Inducible antimicrobial compounds (halal) production in honey bee larvae (Apis mellifera) from Rumaida Taif by injecting of various dead microorganisms extracts. J Appl Biol Biotechnol 5:23–29
Florin T, Maracci C, Graf M, Karki P, Klepacki D, Berninghausen O, Beckmann R, Vázquez-Laslop N, Wilson DN, Rodnina MV, Mankin AS (2017) An antimicrobial peptide that inhibits translation by trapping release factors on the ribosome. Nat Struct Mol Biol 24:752–757
Fruitwala S, El-Naccache DW, Chang TL (2019) Multifaceted immune functions of human defensins and underlying mechanisms. Semin Cell Dev Biol 88:163–172
Gajalakshmi P (2017) Selective isolation and characterization of rare actinomycetes adopted in glacier soil of Manali ice point and its activity against Mycobacterium spp. J Microbiol Biotechnol Res 7:1–10
Gan BH, Gaynord J, Rowe SM, Deingruber T, Spring DR (2021) The multifaceted nature of antimicrobial peptides: current synthetic chemistry approaches and future directions. Chem Soc Rev 50:7820–7880
Garcia-Gutierrez E, Mayer MJ, Cotter PD, Narbad A (2019) Gut microbiota as a source of novel antimicrobials. Gut Microbes 10:1–21
Gaspar D, Veiga AS, Castanho MA (2013) From antimicrobial to anticancer peptides. A Review. Front Microbiol 4:294
Ghaffar KA, Hussein WM, Khalil ZG, Capon RJ, Skwarczynski M, Toth I (2015) Levofloxacin and indolicidin for combination antimicrobial therapy. Curr Drug Deliv 12:108–114
Ghag SB, Shekhawat UK, Ganapathi TR (2012) Petunia floral defensins with unique prodomains as novel candidates for development of fusarium wilt resistance in transgenic banana plants. PLoS ONE 7:e39557
Gharsallaoui A, Oulahal N, Joly C, Degraeve P (2016) Nisin as a food preservative: part 1: physicochemical properties, antimicrobial activity, and main uses. Crit Rev Food Sci Nutr 56:1262–1274
Giram PS, Shitole A, Nande SS, Sharma N, Garnaik B (2018) Fast dissolving moxifloxacin hydrochloride antibiotic drug from electrospun Eudragit L-100 nonwoven nanofibrous mats. Mater Sci Eng, C 92:526–539
Gordon YJ, Romanowski EG, McDermott AM (2005) A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res 30:505–515
Gourbal B, Pinaud S, Beckers GJM, Van Der Meer JWM, Conrath U, Netea MG (2018) Innate immune memory: an evolutionary perspective. Immunol Rev 283:21–40
Groves ML, Peterson RF, Kiddy CA (1965) Poliomorphism in the red protein isolated from milk of individual cows. Nature 207:1007–1008
Guzmán-Rodríguez JJ, Ochoa-Zarzosa A, López-Gómez R, López-Meza JE (2015) Plant antimicrobial peptides as potential anticancer agents. Biomed Res Int 2015:735087
Hajipour MJ, Fromm KM, Ashkarran AA, Jimenez de Aberasturi D, de Larramendi IR, Rojo T, Serpooshan V, Parak WJ, Mahmoudi M (2012) Antibacterial properties of nanoparticles. Trends Biotechnol 30:499–511
Hale JD, Hancock RE (2007) Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev Anti Infect Ther 5:951–959
Hamdan N, Yamin A, Hamid SA, Khodir W, Guarino V (2021) Functionalized antimicrobial nanofibers: design criteria and recent advances. J Funct Biomater 12:59
Hammi I, Delalande F, Belkhou R, Marchioni E, Cianferani S, Ennahar S (2016) Maltaricin CPN, a new class IIa bacteriocin produced by Carnobacterium maltaromaticum CPN isolated from mould-ripened cheese. J Appl Microbiol 121:1268–1274
Hancock RE, Patrzykat A (2002) Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr Drug Targets Infect Disord 2:79–83
Hancock RE, Haney EF, Gill EE (2016) The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol 16:321–334
Haney EF, Mansour SC, Hilchie AL, de la Fuente-Núñez C, Hancock RE (2015) High throughput screening methods for assessing antibiofilm and immunomodulatory activities of synthetic peptides. Peptides 71:276–285
Haney EF, Straus SK, Hancock REW (2019) Reassessing the host defense peptide landscape. Front Chem 7:43
Haney EF, Pletzer D, Hancock RE (2021) Impact of host defense peptides on chronic wounds and infections. In: Chronic wounds, wound dressings and wound healing. Springer, Cham, pp 3–19
Hashizume H, Sawa R, Yamashita K, Nishimura Y, Igarashi M (2017) Structure and antibacterial activities of new cyclic peptide antibiotics, pargamicins B, C and D, from Amycolatopsis sp. ML1-hF4. J Antibiot 70:699–704
Hata T, Tanaka R, Ohmomo S (2010) Isolation and characterization of plantaricin ASM1: a new bacteriocin produced by Lactobacillus plantarum A-1. Int J Food Microbiol 137:94–99
Heinrich J, König NF, Sobottka S, Sarkar B, Kulak N (2019) Flexible vs. rigid bis(2-benzimidazolyl) ligands in Cu(II) complexes: impact on redox chemistry and oxidative DNA cleavage activity. J Inorg Biochem 194:223–232
Helmerhorst EJ, Venuleo C, Beri A, Oppenheim FG (2005) Candida glabrata is unusual with respect to its resistance to cationic antifungal proteins. Yeast (chichester, England) 22:705–714
Herrell WE, Heilman D (1941) Experimental and clinical studies on gramicidin. J Clin Investig 20:583–591
Hill CP, Yee J, Selsted ME, Eisenberg D (1991) Crystal structure of defensin HNP-3, an amphiphilic dimer: mechanisms of membrane permeabilization. Science (New York, N.Y.) 251:1481–1485
Hirsch JG (1956) Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J Exp Med 103:589–611
Hollmann A, Martinez M, Maturana P, Semorile LC, Maffia PC (2018) Antimicrobial peptides: interaction with model and biological membranes and synergism with chemical antibiotics. Front Chem 6:204
Holo H, Faye T, Brede DA, Nilsen T, Ødegård I, Langsrud T, Brendehaug J, Nes IF (2002) Bacteriocins of propionic acid bacteria. Lait 82:59–68
Höng K, Austerlitz T, Bohlmann T, Bohlmann H (2021) The thionin family of antimicrobial peptides. PLoS ONE 16:e0254549
Hsu CH, Chen C, Jou ML, Lee AY, Lin YC, Yu YP, Huang WT, Wu SH (2005) Structural and DNA-binding studies on the bovine antimicrobial peptide, indolicidin: evidence for multiple conformations involved in binding to membranes and DNA. Nucleic Acids Res 33:4053–4064
Hu C, Chen X, Huang Y, Chen Y (2018) Co-administration of iRGD with peptide HPRP-A1 to improve anticancer activity and membrane penetrability. Sci Rep 8:2274
Huan Y, Kong Q, Mou H, Yi H (2020) Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front Microbiol 11:582779
Huh AJ, Kwon YJ (2011) “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release 156:128–145
Hwang B, Hwang JS, Lee J, Lee DG (2010a) Antifungal properties and mode of action of psacotheasin, a novel knottin-type peptide derived from Psacothea hilaris. Biochem Biophys Res Commun 400:352–357
Hwang JS, Lee J, Hwang B, Nam SH, Yun EY, Kim SR, Lee DG (2010b) Isolation and characterization of Psacotheasin, a novel Knottin-type antimicrobial peptide, from Psacothea hilaris. J Microbiol Biotechnol 20:708–711
Jäkel CE, Meschenmoser K, Kim Y, Weiher H, Schmidt-Wolf IG (2012) Efficacy of a proapoptotic peptide towards cancer cells. In Vivo (Athens, Greece) 26:419–426
Jia F, Wang J, Peng J, Zhao P, Kong Z, Wang K, Yan W, Wang R (2017) D-amino acid substitution enhances the stability of antimicrobial peptide polybia-CP. Acta Biochim Biophys Sin 49:916–925
Jiang H, Tang X, Zhou Q, Zou J, Li P, Breukink E, Gu Q (2018) Plantaricin NC8 from Lactobacillus plantarum causes cell membrane disruption to Micrococcus luteus without targeting lipid II. Appl Microbiol Biotechnol 102:7465–7473
Jin G, Weinberg A (2019) Human antimicrobial peptides and cancer. Semin Cell Dev Biol 88:156–162
Kalmokoff ML, Banerjee SK, Cyr T, Hefford MA, Gleeson T (2001) Identification of a new plasmid-encoded sec-dependent bacteriocin produced by Listeria innocua 743. Appl Environ Microbiol 67:4041–4047
Kanchanapally R, Viraka Nellore BP, Sinha SS, Pedraza F, Jones SJ, Pramanik A, Chavva SR, Tchounwou C, Shi Y, Vangara A, Sardar D, Ray PC (2015) Antimicrobial peptide-conjugated graphene oxide membrane for efficient removal and effective killing of multiple drug resistant bacteria. RSC Adv 5:18881–18887
Kang SJ, Park SJ, Mishig-Ochir T, Lee BJ (2014) Antimicrobial peptides: therapeutic potentials. Expert Rev Anti Infect Ther 12:1477–1486
Kang HK, Kim C, Seo CH, Park Y (2017) The therapeutic applications of antimicrobial peptides (AMPs): a patent review. J Microbiol (Seoul, Korea) 55:1–12
Kang HK, Seo CH, Luchian T, Park Y (2018) Pse-T2, an antimicrobial peptide with high-level, broad-spectrum antimicrobial potency and skin biocompatibility against multidrug-resistant Pseudomonas aeruginosa infection. Antimicrob Agents Chemother 62:e01493-e1518
Kang X, Dong F, Shi C, Liu S, Sun J, Chen J, Li H, Xu H, Lao X, Zheng H (2019) DRAMP 2.0, an updated data repository of antimicrobial peptides. Sci Data 6:148–149
Kang HK, Lee HH, Seo CH, Park Y (2019b) Antimicrobial and immunomodulatory properties and applications of marine-derived proteins and peptides. Mar Drugs 17:350
Kaunietis A, Buivydas A, Čitavičius DJ, Kuipers OP (2019) Heterologous biosynthesis and characterization of a glycocin from a thermophilic bacterium. Nat Commun 10:1115
Kenawy E-R, Worley SD, Broughton R (2007) The chemistry and applications of antimicrobial polymers: a state-of-the-art review. Biomacromolecules 8:1359–1384
Khurshid Z, Najeeb S, Mali M, Moin SF, Raza SQ, Zohaib S, Sefat F, Zafar MS (2017) Histatin peptides: pharmacological functions and their applications in dentistry. Saudi Pharm J (SPJ) 25:25–31
Kitagawa N, Otani T, Inai T (2019) Nisin, a food preservative produced by Lactococcus lactis, affects the localization pattern of intermediate filament protein in HaCaT cells. Anat Sci Int 94:163–171
Ko SJ, Park E, Asandei A, Choi JY, Lee SC, Seo CH, Luchian T, Park Y (2020) Bee venom-derived antimicrobial peptide melectin has broad-spectrum potency, cell selectivity, and salt-resistant properties. Sci Rep 10:10145
Kościuczuk EM, Lisowski P, Jarczak J, Strzałkowska N, Jóźwik A, Horbańczuk J, Krzyżewski J, Zwierzchowski L, Bagnicka E (2012) Cathelicidins: family of antimicrobial peptides. A review. Mol Biol Rep 39:10957–10970
Kückelhaus SA, Leite JR, Muniz-Junqueira MI, Sampaio RN, Bloch C Jr, Tosta CE (2009) Antiplasmodial and antileishmanial activities of phylloseptin-1, an antimicrobial peptide from the skin secretion of Phyllomedusa azurea (Amphibia). Exp Parasitol 123:11–16
Kurpe SR, Grishin SY, Surin AK, Panfilov AV, Slizen MV, Chowdhury SD, Galzitskaya OV (2020) Antimicrobial and amyloidogenic activity of peptides. Can antimicrobial peptides be used against SARS-CoV-2? Int J Mol Sci 21:9552–9553
Ladokhin AS, White SH (2001) ‘Detergent-like’ permeabilization of anionic lipid vesicles by melittin. Biochem Biophys Acta 1514:253–260
Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C, Chamilos G, Feldmeyer L, Marinari B, Chon S, Vence L, Riccieri V, Guillaume P, Navarini AA, Romero P, Costanzo A, Piccolella E, Gilliet M, Frasca L (2014) The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun 5:5621
Lau QY, Li J, Sani MA, Sinha S, Li Y, Ng FM, Kang C, Bhattacharjya S, Separovic F, Verma C, Chia CSB (1860) Elucidating the bactericidal mechanism of action of the linear antimicrobial tetrapeptide BRBR-NH(2). Biochim Biophys Acta 2018:1517–1527
Lay FT, Anderson MA (2005) Defensins—components of the innate immune system in plants. Curr Protein Pept Sci 6:85–101
Lay FT, Brugliera F, Anderson MA (2003) Isolation and properties of floral defensins from ornamental tobacco and petunia. Plant Physiol 131:1283–1293
Le TN, Do TH, Nguyen TN, Tran NT, Enfors SO, Truong H (2014) Expression and simple purification strategy for the generation of anti-microbial active enterocin P from Enterococcus faecium expressed in Escherichia coli ER2566. Iran J Biotechnol 12:17–25
Le CF, Gudimella R, Razali R, Manikam R, Sekaran SD (2016) Transcriptome analysis of Streptococcus pneumoniae treated with the designed antimicrobial peptides, DM3. Sci Rep 6:26828
Le CF, Fang CM, Sekaran SD (2017) Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob Agents Chemother 61:e02340-e2416
Lee J, Lee DG (2015) Antimicrobial peptides (AMPs) with dual mechanisms: membrane disruption and apoptosis. J Microbiol Biotechnol 25:759–764
Lee SY, Moon HJ, Kawabata S, Kurata S, Natori S, Lee BL (1995) A sapecin homologue of Holotrichia diomphalia: purification, sequencing and determination of disulfide pairs. Biol Pharm Bull 18:457–459
Lee TH, Hall KN, Aguilar MI (2016) Antimicrobial peptide structure and mechanism of action: a focus on the role of membrane structure. Curr Top Med Chem 16:25–39
Lee JH, Seo M, Lee HJ, Baek M, Kim IW, Kim SY, Kim MA, Kim SH, Hwang JS (2019) Anti-inflammatory activity of antimicrobial peptide allomyrinasin derived from the dynastid beetle, Allomyrina dichotoma. J Microbiol Biotechnol 29:687–695
Lei J, Sun L, Huang S, Zhu C, Li P, He J, Mackey V, Coy DH, He Q (2019) The antimicrobial peptides and their potential clinical applications. Am J Transl Res 11:3919–3931
Li H, Anuwongcharoen N, Malik AA, Prachayasittikul V, Wikberg JE, Nantasenamat C (2016) Roles of d-amino acids on the bioactivity of host defense peptides. Int J Mol Sci 17:1023
Li B, Lyu P, Xie S, Qin H, Pu W, Xu H, Chen T, Shaw C, Ge L, Kwok HF (2019a) LFB: a novel antimicrobial brevinin-like peptide from the skin secretion of the Fujian large headed frog, Limnonectes fujianensi. Biomolecules 9:242
Li Y, Liu T, Liu Y, Tan Z, Ju Y, Yang Y, Dong W (2019b) Antimicrobial activity, membrane interaction and stability of the d-amino acid substituted analogs of antimicrobial peptide W3R6. J Photochem Photobiol, B 200:111645
Liang W, Diana J (2020) The dual role of antimicrobial peptides in autoimmunity. Front Immunol 11:2077
Liang X, Zhang X, Lian K, Tian X, Zhang M, Wang S, Chen C, Nie C, Pan Y, Han F, Wei Z, Zhang W (2020) Antiviral effects of Bovine antimicrobial peptide against TGEV in vivo and in vitro. J Vet Sci 21:e80
Lim C, Takahashi E, Hongsuwan M, Wuthiekanun V, Thamlikitkul V, Hinjoy S, Day NP, Peacock SJ, Limmathurotsakul D (2016) epidemiology and burden of multidrug-resistant bacterial infection in a developing country. eLife 5:e18082-85
Lohner K, Prossnigg F (2009) Biological activity and structural aspects of PGLa interaction with membrane mimetic systems. Biochem Biophys Acta 1788:1656–1666
Lu J, Xu H, Xia J, Ma J, Xu J, Li Y, Feng J (2020) D- and unnatural amino acid substituted antimicrobial peptides with improved proteolytic resistance and their proteolytic degradation characteristics. Front Microbiol 11:563030
Luong HX, Thanh TT, Tran TH (2020) Antimicrobial peptides—advances in development of therapeutic applications. Life Sci 260:118407
Lutkenhaus J (1990) Regulation of cell division in E. coli. Trends Genet (TIG) 6:22–25
Luz C, Calpe J, Saladino F, Luciano FB, Fernandez-Franzón M, Mañes J, Meca G (2018) Antimicrobial packaging based on ε-polylysine bioactive film for the control of mycotoxigenic fungi in vitro and in bread. J Food Process Preserv 42:e13370
Mack MR, Kim BS (2016) Superficial Immunity: Antimicrobial responses are more than skin deep. Immunity 45:6–8
Maftoonazad N, Shahamirian M, John D, Ramaswamy H (2019) Development and evaluation of antibacterial electrospun pea protein isolate-polyvinyl alcohol nanocomposite mats incorporated with cinnamaldehyde. Mater Sci Eng C Mater Biol Appl 94:393–402
Mahlapuu M, Håkansson J, Ringstad L, Björn C (2016) Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol 6:194
Maldonado-Barragán A, Caballero-Guerrero B, Martín V, Ruiz-Barba JL, Rodríguez JM (2016) Purification and genetic characterization of gassericin E, a novel co-culture inducible bacteriocin from Lactobacillus gasseri EV1461 isolated from the vagina of a healthy woman. BMC Microbiol 16:37
Malmsten M (2013) Inorganic nanomaterials as delivery systems for proteins, peptides, DNA, and siRNA. Curr Opin Colloid Interface Sci 18:468–480
Manabe T, Kawasaki K (2017) D-form KLKLLLLLKLK-NH(2) peptide exerts higher antimicrobial properties than its L-form counterpart via an association with bacterial cell wall components. Sci Rep 7:43384
Mardirossian M, Pérébaskine N, Benincasa M, Gambato S, Hofmann S, Huter P, Müller C, Hilpert K, Innis CA, Tossi A, Wilson DN (2018) The Dolphin proline-rich antimicrobial peptide Tur1A inhibits protein synthesis by targeting the bacterial ribosome. Cell Chem Biol 25:530-539.e537
Matsuzaki K (1999) Why and how are peptide–lipid interactions utilized for self-defense? Magainins and Tachyplesins as Archetypes. Biochim Biophys Acta 1462:1–10
Mattick AT, Hirsch A (1947) Further observations on an inhibitory substance (nisin) from lactic streptococci. Lancet (London, Engl) 2:5–8
Mills S, Griffin C, O’Connor PM, Serrano LM, Meijer WC, Hill C, Ross RP (2017) A multibacteriocin cheese starter system, comprising nisin and lacticin 3147 in Lactococcus lactis, in combination with plantaricin from Lactobacillus plantarum. Appl Environ Microbiol 83:e00799-e817
Mirzaeei S, Taghe S, Asare-Addo K, Nokhodchi A (2021) Polyvinyl alcohol/chitosan single-layered and polyvinyl alcohol/chitosan/Eudragit RL100 multi-layered electrospun nanofibers as an ocular matrix for the controlled release of ofloxacin: an in vitro and in vivo evaluation. AAPS PharmSciTech 22:170
Mokoena MP (2017) Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini-review. Molecules (Basel, Switzerland) 22:1255
Mookherjee N, Anderson MA, Haagsman HP, Davidson DJ (2020) Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov 19:311–332
Mora C, Tittensor DP, Adl S, Simpson AG, Worm B (2011) How many species are there on Earth and in the ocean? PLoS Biol 9:e1001127
Moravej H, Moravej Z, Yazdanparast M, Heiat M, Mirhosseini A, MoosazadehMoghaddam M, Mirnejad R (2018) Antimicrobial peptides: features, action, and their resistance mechanisms in bacteria. Microb Drug Resist (Larchmont, NY) 24:747–767
Mouro C, Gomes AP, Ahonen M, Fangueiro R, Gouveia IC (2021) Chelidonium majus L. Incorporated emulsion electrospun PCL/PVA_PEC nanofibrous meshes for antibacterial wound dressing applications. Nanomaterials (Basel, Switzerland) 11:1785
Mousavi Maleki MS, Sardari S, Ghandehari Alavijeh A, Madanchi H (2022) Recent patents and FDA-approved drugs based on antiviral peptides and other peptide-related antivirals. Int J Pept Res Ther 29:5
Moyer TB, Heil LR, Kirkpatrick CL, Goldfarb D, Lefever WA, Parsley NC, Wommack AJ, Hicks LM (2019) PepSAVI-MS reveals a proline-rich antimicrobial peptide in Amaranthus tricolor. J Nat Prod 82:2744–2753
Muhammad SA, Ali A, Naz A, Hassan A, Riaz N, Saeed-ul-Hassan S, Andleeb S, Barh D (2016) A new broad-spectrum peptide antibiotic produced by Bacillus brevis strain MH9 isolated from Margalla Hills of Islamabad, Pakistan. Int J Pept Res Ther 22:271–279
Mukhopadhyay J, Sineva E, Knight J, Levy RM, Ebright RH (2004) Antibacterial peptide microcin J25 inhibits transcription by binding within and obstructing the RNA polymerase secondary channel. Mol Cell 14:739–751
Naimah AK, Al-Manhel AJA, Al-Shawi MJ (2017) Isolation, purification and characterization of antimicrobial peptides produced from Saccharomyces boulardii. Int J Pept Res Ther 24:455–461
Nayab S, Aslam MA, Rahman S, Sindhu ZUD, Sajid S, Zafar N, Razaq M, Kanwar R, Amanullah (2022) A review of antimicrobial peptides: its function, mode of action and therapeutic potential. Int J Pept Res Ther 28:46
Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M (2009) Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater 8:543–557
Nguyen LT, Haney EF, Vogel HJ (2011) The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol 29:464–472
Nguyen HLT, Trujillo-Paez JV, Umehara Y, Yue H, Peng G, Kiatsurayanon C, Chieosilapatham P, Song P, Okumura K, Ogawa H, Ikeda S, Niyonsaba F (2020) Role of antimicrobial peptides in skin barrier repair in individuals with atopic dermatitis. Int J Mol Sci 21:7607
Omardien S, Drijfhout JW, Vaz FM, Wenzel M, Hamoen LW, Zaat SAJ, Brul S (1860) Bactericidal activity of amphipathic cationic antimicrobial peptides involves altering the membrane fluidity when interacting with the phospholipid bilayer. Biochim Biophys Acta 2018:2404–2415
Osborn RW, De Samblanx GW, Thevissen K, Goderis I, Torrekens S, Van Leuven F, Attenborough S, Rees SB, Broekaert WF (1995) Isolation and characterisation of plant defensins from seeds of Asteraceae, Fabaceae, Hippocastanaceae and Saxifragaceae. FEBS Lett 368:257–262
Oshiro KGN, Rodrigues G, Monges BED, Cardoso MH, Franco OL (2019) Bioactive peptides against fungal biofilms. Front Microbiol 10:2169
Outlaw VK, Bovier FT, Mears MC, Cajimat MN, Zhu Y, Lin MJ, Addetia A, Lieberman NAP, Peddu V, Xie X, Shi PY, Greninger AL, Gellman SH, Bente DA, Moscona A, Porotto M (2020) Inhibition of coronavirus entry in vitro and ex vivo by a lipid-conjugated peptide derived from the SARS-CoV-2 spike glycoprotein HRC domain. Biology 11:e01935-20
Pan F, Amarjargal A, Altenried S, Liu M, Zuber F, Zeng Z, Rossi RM, Maniura-Weber K, Ren Q (2021) Bioresponsive hybrid nanofibers enable controlled drug delivery through glass transition switching at physiological temperature. ACS Appl Biol Mater 4:4271–4279
Pankajakshan D, Albuquerque MTP, Evans JD, Kamocka MM, Gregory RL, Bottino MC (2016) Triple antibiotic polymer nanofibers for intracanal drug delivery: effects on dual species biofilm and cell function. J Endodontics 42:1490–1495
Panteleev PV, Bolosov IA, Balandin SV, Ovchinnikova TV (2015) Structure and biological functions of β-hairpin antimicrobial peptides. Acta Nat 7:37–47
Panteleev PV, Balandin SV, Ivanov VT, Ovchinnikova TV (2017) A therapeutic potential of animal β-hairpin antimicrobial peptides. Curr Med Chem 24:1724–1746
Parham S, Kharazi AZ, Bakhsheshi-Rad HR, Kharaziha M, Ismail AF, Sharif S, Razzaghi M, RamaKrishna S, Berto F (2022) Antimicrobial synthetic and natural polymeric nanofibers as wound dressing: a review. Adv Eng Mater 24:2101460
Park CB, Kim HS, Kim SC (1998) Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem Biophys Res Commun 244:253–257
Paulsen VS, Blencke HM, Benincasa M, Haug T, Eksteen JJ, Styrvold OB, Scocchi M, Stensvåg K (2013) Structure–activity relationships of the antimicrobial peptide arasin 1 - and mode of action studies of the N-terminal, proline-rich region. PLoS ONE 8:e53326
Pavlukhina S, Lu Y, Patimetha A, Libera M, Sukhishvili S (2010) Polymer multilayers with pH-triggered release of antibacterial agents. Biomacromolecules 11:3448–3456
Perez RH, Ishibashi N, Inoue T, Himeno K, Masuda Y, Sawa N, Zendo T, Wilaipun P, Leelawatcharamas V, Nakayama J, Sonomoto K (2016) Functional analysis of genes involved in the biosynthesis of enterocin NKR-5-3B, a novel circular bacteriocin. J Bacteriol 198:291–300
Pfalzgraff A, Brandenburg K, Weindl G (2018) Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Front Pharmacol 9:281
Pino-Angeles A, Leveritt JM 3rd, Lazaridis T (2016) Pore structure and synergy in antimicrobial peptides of the Magainin family. PLoS Comput Biol 12:e1004570
Pletzer D, Coleman SR, Hancock RE (2016) Anti-biofilm peptides as a new weapon in antimicrobial warfare. Curr Opin Microbiol 33:35–40
Portelinha J, Duay SS, Yu SI, Heilemann K, Libardo MDJ, Juliano SA, Klassen JL, Angeles-Boza AM (2021) Antimicrobial peptides and copper(II) ions: novel therapeutic opportunities. Chem Rev 121:2648–2712
Pouny Y, Rapaport D, Mor A, Nicolas P, Shai Y (1992) Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues with phospholipid membranes. Biochemistry 31:12416–12423
Prasad SV, Fiedoruk K, Daniluk T, Piktel E, Bucki R (2019) Expression and function of host defense peptides at inflammation sites. Int J Mol Sci 21:104
Price DP, Schilkey FD, Ulanov A, Hansen IA (2015) Small mosquitoes, large implications: crowding and starvation affects gene expression and nutrient accumulation in Aedes aegypti. Parasit Vectors 8:252
Pukala TL, Doyle JR, Llewellyn LE, Kuhn-Nentwig L, Apponyi MA, Separovic F, Bowie JH (2007) Cupiennin 1a, an antimicrobial peptide from the venom of the neotropical wandering spider Cupiennius salei, also inhibits the formation of nitric oxide by neuronal nitric oxide synthase. FEBS J 274:1778–1784
Pushpanathan P, Mathew GS, Selvarajan S, Seshadri KG, Srikanth P (2019) Gut microbiota and its mysteries. Indian J Med Microbiol 37:268–277
Radaic A, de Jesus MB, Kapila YL (2020) Bacterial anti-microbial peptides and nano-sized drug delivery systems: the state of the art toward improved bacteriocins. J Control Release 321:100–118
Ragland SA, Criss AK (2017) From bacterial killing to immune modulation: recent insights into the functions of lysozyme. PLoS Pathog 13:e1006512
Rai A, Ferrão R, Palma P, Patricio T, Parreira P, Anes E, Tonda-Turo C, Martins MCL, Alves N, Ferreira L (2022) Antimicrobial peptide-based materials: opportunities and challenges. J Mater Chem B 10:2384–2429
Rajchakit U, Sarojini V (2017) Recent developments in antimicrobial-peptide-conjugated gold nanoparticles. Bioconjug Chem 28:2673–2686
Ramos R, Silva JP, Rodrigues AC, Costa R, Guardão L, Schmitt F, Soares R, Vilanova M, Domingues L, Gama M (2011) Wound healing activity of the human antimicrobial peptide LL37. Peptides 32:1469–1476
Rao M, Wei W, Ge M, Chen D, Sheng X (2013) A new antibacterial lipopeptide found by UPLC-MS from an actinomycete Streptomyces sp. HCCB10043. Nat Prod Res 27:2190–2195
Rathinakumar R, Walkenhorst WF, Wimley WC (2009) Broad-spectrum antimicrobial peptides by rational combinatorial design and high-throughput screening: the importance of interfacial activity. J Am Chem Soc 131:7609–7617
Rathinam VAK, Chan FK (2018) Inflammasome, inflammation, and tissue homeostasis. Trends Mol Med 24:304–318
Reffuveille F, de la Fuente-Núñez C, Mansour S, Hancock RE (2014) A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob Agents Chemother 58:5363–5371
Reinhardt A, Neundorf I (2016) Design and application of antimicrobial peptide conjugates. Int J Mol Sci 17:701
Rodríguez-Rojas A, Baeder DY, Johnston P, Regoes RR, Rolff J (2021) Bacteria primed by antimicrobial peptides develop tolerance and persist. PLoS Pathog 17:e1009443
Rofeal M, Abdelmalek F, Steinbüchel A (2022) Naturally-sourced antibacterial polymeric nanomaterials with special reference to modified polymer variants. Int J Mol Sci 23:4101
Roscetto E, Contursi P, Vollaro A, Fusco S, Notomista E, Catania MR (2018) Antifungal and anti-biofilm activity of the first cryptic antimicrobial peptide from an archaeal protein against Candida spp. clinical isolates. Sci Rep 8:17570
Sader HS, Fedler KA, Rennie RP, Stevens S, Jones RN (2004) Omiganan pentahydrochloride (MBI 226), a topical 12-amino-acid cationic peptide: spectrum of antimicrobial activity and measurements of bactericidal activity. Antimicrob Agents Chemother 48:3112–3118
Sandreschi S, Piras AM, Batoni G, Chiellini F (2016) Perspectives on polymeric nanostructures for the therapeutic application of antimicrobial peptides. Nanomedicine (Lond) 11:1729–1744
Santiago-Morales J, Amariei G, Letón P, Rosal R (2016) Antimicrobial activity of poly(vinyl alcohol)-poly(acrylic acid) electrospun nanofibers. Colloids Surf B 146:144–151
Savelyeva A, Ghavami S, Davoodpour P, Asoodeh A, Los MJ (2014) An overview of Brevinin superfamily: structure, function and clinical perspectives. Adv Exp Med Biol 818:197–212
Schaal JB, Maretzky T, Tran DQ, Tran PA, Tongaonkar P, Blobel CP, Ouellette AJ, Selsted ME (2018) Macrocyclic θ-defensins suppress tumor necrosis factor-α (TNF-α) shedding by inhibition of TNF-α-converting enzyme. J Biol Chem 293:2725–2734
Schafer ME, Browne H, Goldberg JB, Greenberg DE (2021) Peptides and antibiotic therapy: advances in design and delivery. Acc Chem Res 54:2377–2385
Scott MG, Yan H, Hancock RE (1999) Biological properties of structurally related alpha-helical cationic antimicrobial peptides. Infect Immun 67:2005–2009
Sebe I, Ostorhazi E, Fekete A, Kovacs KN, Zelko R, Kovalszky I, Li W, Wade JD, Szabo D, Otvos L Jr (2016) Polyvinyl alcohol nanofiber formulation of the designer antimicrobial peptide APO sterilizes Acinetobacter baumannii-infected skin wounds in mice. Amino Acids 48:203–211
Segura A, Moreno M, Madueño F, Molina A, García-Olmedo F (1999) Snakin-1, a peptide from potato that is active against plant pathogens, Molecular plant-microbe interactions. MPMI 12:16–23
Shahmiri M, Enciso M, Adda CG, Smith BJ, Perugini MA, Mechler A (2016) Membrane core-specific antimicrobial action of cathelicidin LL-37 peptide switches between pore and nanofibre formation. Sci Rep 6:38184
Shai Y (1999) Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochem Biophys Acta 1462:55–70
Sharma S, Sethi S, Prasad R, Samanta P, Rajwanshi A, Malhotra S, Sharma M (2011) Characterization of low molecular weight antimicrobial peptide from human female reproductive tract. Indian J Med Res 134:679–687
Sharma G, Dang S, Gupta S, Gabrani R (2018) Antibacterial activity, cytotoxicity, and the mechanism of action of bacteriocin from Bacillus subtilis GAS101. Med Princ Pract 27:186–192
Sheehan G, Bergsson G, McElvaney NG, Reeves EP, Kavanagh K (2018) The human cathelicidin antimicrobial peptide LL-37 Promotes the growth of the pulmonary pathogen Aspergillus fumigatus. Infect Immun 86:e00097-e118
Shenkarev ZO, Balandin SV, Trunov KI, Paramonov AS, Sukhanov SV, Barsukov LI, Arseniev AS, Ovchinnikova TV (2011) Molecular mechanism of action of β-hairpin antimicrobial peptide arenicin: oligomeric structure in dodecylphosphocholine micelles and pore formation in planar lipid bilayers. Biochemistry 50:6255–6265
Shin JM, Gwak JW, Kamarajan P, Fenno JC, Rickard AH, Kapila YL (2016) Biomedical applications of nisin. J Appl Microbiol 120:1449–1465
Shu G, Chen Y, Liu T, Ren S, Kong Y (2019) Antimicrobial peptide cathelicidin-BF inhibits platelet aggregation by blocking protease-activated receptor 4. Int J Pept Res Ther 25:349–358
Silva JP, Dhall S, Garcia M, Chan A, Costa C, Gama M, Martins-Green M (2015) Improved burn wound healing by the antimicrobial peptide LLKKK18 released from conjugates with dextrin embedded in a carbopol gel. Acta Biomater 26:249–262
Silva ON, de la Fuente-Núñez C, Haney EF, Fensterseifer IC, Ribeiro SM, Porto WF, Brown P, Faria-Junior C, Rezende TM, Moreno SE, Lu TK, Hancock RE, Franco OL (2016) An anti-infective synthetic peptide with dual antimicrobial and immunomodulatory activities. Sci Rep 6:35465
Singh R, Nadhe S, Wadhwani S, Shedbalkar U, Chopade BA (2016) Nanoparticles for control of biofilms of acinetobacter species. Materials (Basel, Switzerland) 9:383
Singh R, Miriyala SS, Giri L, Mitra K, Kareenhalli VV (2017) Identification of unstructured model for subtilin production through Bacillus subtilis using hybrid genetic algorithm. Process Biochem 60:1–12
Som A, Vemparala S, Ivanov I, Tew GN (2008) Synthetic mimics of antimicrobial peptides. Biopolymers 90:83–93
Splith K, Neundorf I (2011) Antimicrobial peptides with cell-penetrating peptide properties and vice versa. Eur Biophys J (EBJ) 40:387–397
Srivastava S, Dashora K, Ameta KL, Singh NP, El-Enshasy HA, Pagano MC, Hesham AE, Sharma GD, Sharma M, Bhargava A (2021) Cysteine-rich antimicrobial peptides from plants: the future of antimicrobial therapy. Phytother Res (PTR) 35:256–277
Starling S (2017) Innate immunity: a new way out for lysozyme. Nat Rev Gastroenterol Hepatol 14:567
Starr CG, Maderdrut JL, He J, Coy DH, Wimley WC (2018) Pituitary adenylate cyclase-activating polypeptide is a potent broad-spectrum antimicrobial peptide: structure-activity relationships. Peptides 104:35–40
Struyfs C, Cammue BPA, Thevissen K (2021) Membrane-interacting antifungal peptides. Front Cell Dev Biol 9:649875
Subbalakshmi C, Sitaram N (1998) Mechanism of antimicrobial action of indolicidin. FEMS Microbiol Lett 160:91–96
Sultana A, Luo H, Ramakrishna S (2021) Antimicrobial peptides and their applications in biomedical sector. Antibiotics (Basel, Switzerland) 10:1094
Sun T, Zhan B, Gao Y (2015) A novel cathelicidin from Bufo bufo gargarizans Cantor showed specific activity to its habitat bacteria. Gene 571:172–177
Tahir HM, Zaheer A, Khan AA, Abbas MST (2017) Antibacterial potential of venom extracted from wolf spider, Lycosa terrestris (Araneae: Lycosiade). Indian J Anim Res 52:286–290
Tang SS, Prodhan ZH, Biswas SK, Le CF, Sekaran SD (2018) Antimicrobial peptides from different plant sources: isolation, characterisation, and purification. Phytochemistry 154:94–105
Tang Z, Ma Q, Chen X, Chen T, Ying Y, Xi X, Wang L, Ma C, Shaw C, Zhou M (2021) Recent advances and challenges in nanodelivery systems for antimicrobial peptides (AMPs). Antibiotics (Basel, Switzerland) 10:990
Taniguchi M, Saito K, Aida R, Ochiai A, Saitoh E, Tanaka T (2019) Wound healing activity and mechanism of action of antimicrobial and lipopolysaccharide-neutralizing peptides from enzymatic hydrolysates of rice bran proteins. J Biosci Bioeng 128:142–148
Tejesvi MV, Segura DR, Schnorr KM, Sandvang D, Mattila S, Olsen PB, Neve S, Kruse T, Kristensen HH, Pirttilä AM (2013) An antimicrobial peptide from endophytic Fusarium tricinctum of Rhododendron tomentosum Harmaja. Fungal Divers 60:153–159
Terras FR, Eggermont K, Kovaleva V, Raikhel NV, Osborn RW, Kester A, Rees SB, Torrekens S, Van Leuven F, Vanderleyden J et al (1995) Small cysteine-rich antifungal proteins from radish: their role in host defense. Plant Cell 7:573–588
Thapa RK, Diep DB, Tønnesen HH (2020) Topical antimicrobial peptide formulations for wound healing: current developments and future prospects. Acta Biomater 103:52–67
Toda H, Williams JA, Gulledge M, Sehgal A (2019) A sleep-inducing gene, nemuri, links sleep and immune function in Drosophila. Science (New York, N.Y.) 363:509–515
Topuz F, Uyar T (2018) Electrospinning of cyclodextrin functional nanofibers for drug delivery applications. Pharmaceutics 11:6
Topuz F, Abdulhamid MA, Holtzl T, Szekely G (2021) Nanofiber engineering of microporous polyimides through electrospinning: influence of electrospinning parameters and salt addition. Mater Des 198:109280
Tossi A, Scocchi M, Skerlavaj B, Gennaro R (1994) Identification and characterization of a primary antibacterial domain in CAP18, a lipopolysaccharide binding protein from rabbit leukocytes. FEBS Lett 339:108–112
Tripathi S, Tecle T, Verma A, Crouch E, White M, Hartshorn KL (2013) The human cathelicidin LL-37 inhibits influenza A viruses through a mechanism distinct from that of surfactant protein D or defensins. J Gen Virol 94:40–49
Tulini FL, Lohans CT, Bordon KC, Zheng J, Arantes EC, Vederas JC, De Martinis EC (2014) Purification and characterization of antimicrobial peptides from fish isolate Carnobacterium maltaromaticum C2: Carnobacteriocin X and carnolysins A1 and A2. Int J Food Microbiol 173:81–88
van der Weerden NL, Bleackley MR, Anderson MA (2013) Properties and mechanisms of action of naturally occurring antifungal peptides. Cell Mol Life Sci (CMLS) 70:3545–3570
van Harten RM, van Woudenbergh E, van Dijk A, Haagsman HP (2018) Cathelicidins: immunomodulatory antimicrobials. Vaccines 6:63
Van Parijs J, Broekaert WF, Goldstein IJ, Peumans WJ (1991) Hevein: an antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta 183:258–264
Vogel H, Badapanda C, Knorr E, Vilcinskas A (2014) RNA-sequencing analysis reveals abundant developmental stage-specific and immunity-related genes in the pollen beetle Meligethes aeneus. Insect Mol Biol 23:98–112
Wang G (2022) Unifying the classification of antimicrobial peptides in the antimicrobial peptide database. Methods Enzymol 663:1–18
Wang KR, Zhang BZ, Zhang W, Yan JX, Li J, Wang R (2008) Antitumor effects, cell selectivity and structure-activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides 29:963–968
Wang DM, Jiao X, Plotnikoff NP, Griffin N, Qi RQ, Gao XH, Shan FP (2017) Killing effect of methionine enkephalin on melanoma in vivo and in vitro. Oncol Rep 38:2132–2140
Wang X, Sun Y, Wang F, You L, Cao Y, Tang R, Wen J, Cui X (2020) A novel endogenous antimicrobial peptide CAMP(211–225) derived from casein in human milk. Food Funct 11:2291–2298
Water JJ, Kim Y, Maltesen MJ, Franzyk H, Foged C, Nielsen HM (2015) Hyaluronic acid-based nanogels produced by microfluidics-facilitated self-assembly improves the safety profile of the cationic host defense peptide novicidin. Pharm Res 32:2727–2735
Wende C, Kulak N (2015) Fluorophore ATCUN complexes: combining agent and probe for oxidative DNA cleavage. Chem Commun (Camb) 51:12395–12398
Wescombe PA, Tagg JR (2003) Purification and characterization of streptin, a type A1 lantibiotic produced by Streptococcus pyogenes. Appl Environ Microbiol 69:2737–2747
Wrońska AK, Boguś MI (2020) Heat shock proteins (HSP 90, 70, 60, and 27) in Galleria mellonella (Lepidoptera) hemolymph are affected by infection with Conidiobolus coronatus (Entomophthorales). PLoS ONE 15:e0228556
Wu C, Wu T, Fang Z, Zheng J, Xu S, Chen S, Hu Y, Ye X (2016) Formation, characterization and release kinetics of chitosan/γ-PGA encapsulated nisin nanoparticles. RSC Adv 6:46686–46695
Wu Q, Patočka J, Kuča K (2018) Insect antimicrobial peptides, a mini review. Toxins 10:461
Wu C, Biswas S, Garcia De Gonzalo CV, van der Donk WA (2019) Investigations into the mechanism of action of sublancin. ACS Infect Dis 5:454–459
Xia S, Liu M, Wang C, Xu W, Lan Q, Feng S, Qi F, Bao L, Du L, Liu S, Qin C, Sun F, Shi Z, Zhu Y, Jiang S, Lu L (2020) Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res 30:343–355
Yadav S, Mahato M, Pathak R, Jha D, Kumar B, Deka SR, Gautam HK, Sharma AK (2014) Multifunctional self-assembled cationic peptide nanostructures efficiently carry plasmid DNA in vitro and exhibit antimicrobial activity with minimal toxicity. J Mater Chem B 2:4848–4861
Yamauchi R, Kawano K, Yamaoka Y, Taniguchi A, Yano Y, Takasu K, Matsuzaki K (2022) Development of antimicrobial peptide-antibiotic conjugates to improve the outer membrane permeability of antibiotics against Gram-negative bacteria. ACS Infect Dis 8:2339–2347
Yan J, Cai J, Zhang B, Wang Y, Wong DF, Siu SWI (2022) Recent progress in the discovery and design of antimicrobial peptides using traditional machine learning and deep learning. Antibiotics (Basel, Switzerland) 11:145
Yang YT, Lee MR, Lee SJ, Kim S, Nai YS, Kim JS (2018) Tenebrio molitor Gram-negative-binding protein 3 (TmGNBP3) is essential for inducing downstream antifungal Tenecin 1 gene expression against infection with Beauveria bassiana JEF-007. Insect Sci 25:969–977
Yang Z, He S, Wu H, Yin T, Wang L, Shan A (2021) Nanostructured antimicrobial peptides: crucial steps of overcoming the bottleneck for clinics. Front Microbiol 12:710199
Yazici A, Ortucu S, Taskin M, Marinelli L (2018) Natural-based antibiofilm and antimicrobial peptides from microorganisms. Curr Top Med Chem 18:2102–2107
Yoshimura T, McLean MH, Dzutsev AK, Yao X, Chen K, Huang J, Gong W, Zhou J, Xiang Y, Badger H, O’Huigin C, Thovarai V, Tessarollo L, Durum SK, Trinchieri G, Bian XW, Wang JM (2018) The antimicrobial peptide CRAMP is essential for colon homeostasis by maintaining microbiota balance. J Immunol (Baltimore, MD 1950) 200:2174–2185
Young-Speirs M, Drouin D, Cavalcante PA, Barkema HW, Cobo ER (2018) Host defense cathelicidins in cattle: types, production, bioactive functions and potential therapeutic and diagnostic applications. Int J Antimicrob Agents 51:813–821
Yu K, Lo JC, Yan M, Yang X, Brooks DE, Hancock RE, Lange D, Kizhakkedathu JN (2017) Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 116:69–81
Zainab A, Ashish N, Ragnath V (2019) Salivary levels of antimicrobial peptides in chronic periodontitis patients with type 2 diabetes. J Int Acad Periodontol 21:36–44
Zanetti M (2004) Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol 75:39–48
Zare M, Dziemidowicz K, Williams GR, Ramakrishna S (2021) Encapsulation of pharmaceutical and nutraceutical active ingredients using electrospinning processes. Nanomaterials (Basel, Switzerland) 11:1968
Zasloff M (2019) Antimicrobial peptides of multicellular organisms: my perspective. Adv Exp Med Biol 1117:3–6
Zeya HI, Spitznagel JK (1963) Antibacterial and Enzymic Basic Proteins from Leukocyte Lysosomes: Separation and Identification. Science 142:1085–1087
Zhang C, Yang M (2022) Antimicrobial peptides: from design to clinical application. Antibiotics (Basel, Switzerland) 11:349
Zhang Z-T, Zhu S-Y (2009) Drosomycin, an essential component of antifungal defence in Drosophila. Insect Mol Biol 18:549–556
Zhang J, Chiang FI, Wu L, Czyryca PG, Li D, Chang CW (2008) Surprising alteration of antibacterial activity of 5″-modified neomycin against resistant bacteria. J Med Chem 51:7563–7573
Zhang F, Cui X, Fu Y, Zhang J, Zhou Y, Sun Y, Wang X, Li Y, Liu Q, Chen T (2017) Antimicrobial activity and mechanism of the human milk-sourced peptide Casein201. Biochem Biophys Res Commun 485:698–704
Zhang C, Zhang J, Liu M, Huang M (2018) Molecular cloning, expression and antibacterial activity of goose-type lysozyme gene in Microptenus salmoides. Fish Shellfish Immunol 82:9–16
Zhang M, Liang W, Gong W, Yoshimura T, Chen K, Wang JM (2019) The critical role of the antimicrobial peptide LL-37/ CRAMP in protection of colon microbiota balance, mucosal homeostasis, anti-inflammatory responses, and resistance to carcinogenesis. Crit Rev Immunol 39:83–92
Zhang R, Jiang X, Qiao J, Wang Z, Tong A, Yang J, Yang S, Yang L (2021a) Antimicrobial peptide DP7 with potential activity against SARS coronavirus infections. Signal Transduct Target Ther 6:140
Zhang QY, Yan ZB, Meng YM, Hong XY, Shao G, Ma JJ, Cheng XR, Liu J, Kang J, Fu CY (2021b) Antimicrobial peptides: mechanism of action, activity and clinical potential. Mil Med Res 8:48
Zhao L, Huang Y, Gao S, Cui Y, He D, Wang L, Chen Y (2013) Comparison on effect of hydrophobicity on the antibacterial and antifungal activities of α-helical antimicrobial peptides. Sci China Chem 56:1307–1314
Zhong C, Zhu N, Zhu Y, Liu T, Gou S, Xie J, Yao J, Ni J (2020) Antimicrobial peptides conjugated with fatty acids on the side chain of D-amino acid promises antimicrobial potency against multidrug-resistant bacteria. Eur J Pharm Sci 141:105123
Zhu M, Liu P, Niu Z-W (2017) A perspective on general direction and challenges facing antimicrobial peptides. Chin Chem Lett 28:703–708
Acknowledgements
This research work was supported by Sri Shanmugha College of Engineering and Technology, Tamil Nadu, India.
Author information
Authors and Affiliations
Contributions
KK: writing draft, review. NRS: Technical correction, editing and validation. GGG: Supervision, conceptualization and validation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kalimuthu, K., Srinivasan, N.R. & Govindarajan, G. Antibiotic-Peptide Conjugation Against Multi-drug Resistant Pathogens: A Comprehensive Review for Therapeutics and Drug Delivery Strategies. Int J Pept Res Ther 29, 91 (2023). https://doi.org/10.1007/s10989-023-10561-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s10989-023-10561-y