Abstract
The 22 kDa of human growth hormone (hGH) is naturally produced and secreted by somatotrophic cells in the anterior part of the pituitary gland. The aim of this study was to clone, express, purify of hGH as fusion to the pelB leader in pET22b (+) plasmid and evaluate it’s biological properties. The hGH polypeptide codon was optimized and subcloned. The recombinant hGH protein was purified by affinity chromatographic system against His-tag and the presence and accuracy of the purified products were evaluated by protein electrophoresis and Western blot. The biological activity of recombinant hGH protein was measured using ELISA assay. The results of sequencing of hGH gene confirmed the presence and proper placement of hGH gene and it’s subcloning in the plasmid pET22b (+). The results of the protein electrophoresis and Western blot assays demonstrated that the expression accuracy of 22 kDa recombinant hGH. The results of Bradford spectroscopy assay showed that the recombinant hGH protein concentration was 1 g/l. The results of classical sandwich ELISA assay, in contrast to the specific antibodies, confirmed the bio-activity of the recombinant hGH protein in its targeting. Consequently, the results of this study showed that pelB leader has the ability to more accurately direct hGH to periplasmic space in Escherichia coli, and the conditions of oxidizing periplasmic space give rise to the correct folding of the protein in this space. Furthermore, the results of current study proved that using bioinformatics tools and combining them with laboratory data, could improve the recombinant hGH expression in E. coli, in addition to preserving bio-activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The human growth hormone (hGH) is an anabolic hormone and a single chain polypeptide with 191 amino acids produced and secreted by the cells called somatotroph in the anterior part of the pituitary. The molecular weight of this hormone is 22 kDa and has two disulfide bonds in its structure (Franklin and Geffner 2011). Growth hormone (GH) stimulates protein synthesis and muscles and connective tissues’ growth. GH also indirectly prolongs bone proliferation by stimulating cell division in the bone cartilage epiphysis epithelium growth plates by insulin-like growth factors (Raven et al. 2013). The hGH is one of the most important human hormones due to its central role in diverse biological functions including metabolism and cell proliferation. Due to the effect of hGH on prolong, growth of body organs, base energy consumption, muscle strength, physical activity, free fat mass loss, bone density, lipid profile and cognitive and physical functions the hormone is used in the treatment of diseases such as defect children’s GH, growth defects due to chronic renal failure until transplantation, adult GH defect, Turner syndrome, Prader-Willi syndrome, SHOX gene defect and Noonan syndrome (Franklin and Geffner 2011; Reh and Geffner 2010).
Natural hGH is a non-glycosylated protein, and therefore the possibility of expressing its recombinant forms is widely available in prokaryotic systems. The host Escherichia coli (E. coli) due to features such as fairly easy and inexpensive culture, ease of growth and manipulation using simple laboratory equipment, rapid access to high density, known genetics and access to the host various plasmids and strains that have been developed to maximize expression, is widely used in expressing recombinant proteins such as GH. Despite all these benefits, the expression and production of recombinant enzymes is not always successful and often results in insoluble and non-functional proteins (Fakruddin et al. 2013). The hGH overexpression in E. coli leads to the accumulation of insoluble protein in the form of inclusion bodies, in which the yield of active protein production is very low biologically, requiring re-folding, as well as costly and time-consuming purification methods. One way to prevent the formation of inclusion bodies is to secrete hGH into the periplasmic bacterial space using a proper signal peptide that reduces costs and facilitates the downstream processes of the given protein (Kim et al. 2013; Thanassi and Hultgren 2000). The advantages of secretion of recombinant proteins can be reduced contamination caused by various cellular components and prevented proteolytic degradation by intracellular proteases and facilitated separation and purification of overexpressed products. The production of recombinant proteins in the periplasmic space, in addition to the above advantages, provides conditions for facilitating the proper folding process in the oxidative medium, which is very similar to the proper folding process of eukaryotic proteins containing disulfide bonds (Forouharmehr et al. 2018; Ghovvati et al. 2018). The periplasmic enzyme disulfide oxidourethactase (Dsb) forms disulfide bonds in proteins (Lilie et al. 1998). E. coli has various systems for transferring proteins from cytosol to the periplasm or extracellular medium (Yoon et al. 2010). In E. coli, proteins are driven by two major sec systems and dual arginine translocation (TAT) through the plasma membrane out of the cell. 96% of exportoms or proteins secreted out of the cell use sec method, which is a very important and unique system among these systems (Saraogi and Shan 2014). Secretory proteins that can be transmitted by this system are identified by signal recognition particles (SRPs) through a signal peptide to be cut. Cutting the signal peptides by signal peptide peptidase (type I for secretory proteins and type II for lipoproteins) releases adult proteins’ domains to the periplasm, which can provide folding or further movement (Chatzi et al. 2014). Another system is TAT, which carries out the removal of multi-domain proteins or binding to the co-factor of pre-folding in the cytoplasm through chaperones. The signal peptide peptidase (SPP) associated with this type transmits substrates to the plasma membrane by detecting the signal peptides having two sequential arginine remains.
The purpose of this study was to evaluate the periplasmic expression of the hGH using pelB leader sequence to accurately transfer this protein to the periplasmic space and perform the correct folding of the recombinant hGH protein in E. coli.
Materials and Methods
Bacterial Strains and Plasmids
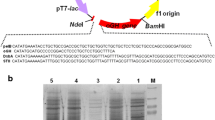
In this study, E. coli strains of DH5α and BL21 (DE3) were used as cloning and expression hosts, respectively. In order to transfer the desired gene during cloning and expression, the pET22b (+) plasmid (Novagen, Madison, USA) was used (Fig. 1a).
Optimization of Codons, Synthesis of hGH Gene and Cloning in the pGH Plasmid
Optimization of hGH polypeptide gene codon based on E. coli host, correction of GC content and investigation of the structural indicators of the desired gene RNA was performed using GeneScript online tool. The optimized sequence of recombinant human growth hormase gene, 573 bp, was synthesized by GeneRay and cloned in the pGH plasmid (Shanghai, China) (Fig. 1b).
Competent Cells Preparation and pGH Plasmid Transformation into DH5α
The bacterial cells DH5α and BL21 (DE3) became competent chemically in accordance with Sambrooke instructions with a few changes as follows (Sambrook and Russell 2001). After inoculation of a bacterial clone in LB liquid culture medium, 16 h culture was considered to achieve sufficient cell density. For re-cultivation of cultured bacterial cells, re-culture was carried out in LB liquid culture medium for about 3 h. After centrifugation at 4 °C, the precipitate was dissolved by cold solution of MgCl2–CaCl2. Competent cells were obtained after centrifugation at 4 °C and solubilized with cold CaCl2. In order to transfer pGH plasmid containing hGH gene, 10 ng of pGH plasmid was added to a volume of 100 μl of competent cells and the gene was transformed by heat shock method according to the standard Sambrooke instructions. An antibiotic-free SOC-enriched culture medium was used to retrieve the competent recombinant cells. Then, the competent cells of the recombinant gene were incubated on a 10 cm plate for approximately 16 h at 37 °C.
Isolation of pGH Plasmid, Double Digestion and Purification of hGH Polypeptide Gene
Evaluation and confirmation of the successful transfer of recombinant gene into DH5α bacteria by PCR, double digestion and sequencing methods were performed based on the standard protocol. The pGH + hGH Plasmid isolation and extraction from DH5α bacteria was performed using QIAprep Spin Miniprep Kit (Qiagen, Germany) according to the manufacturer’s instructions. Plasmid extraction was performed from about 5 ml of bacterial overnight culture and all stages of centrifugation were performed at 13,000 rpm. Separation of the synthesized hGH polypeptide gene from the pGH plasmid using enzymes XhoI and NcoI was performed on the basis of double digestion at 37 °C for 3 h (Thermo Scientific, USA). The product of double digestion was electrophoresed on 1% agarose gel and purification of the synthesized hGH polypeptide gene was performed using Exprep™ Plasmid SV kit (Gene All Biotech. Co., South Korea) according to the manufacturer’s instructions.
Subcloning of hGH Gene into pET22b (+) Plasmid
ligation and subcloning of hGH polypeptide gene was performed in the downstream of T7 promoter, palB leader sequence and the upstream of His-tag gene sequence into pET22b (+) plasmid using Quick Ligation kit (NEB, US), according to the manufacturer’s instructions. The ligation reaction was performed for 5 min at 25 °C and in the presence of T4 DNA ligase enzyme. pET22b + hGH plasmids were transformed into DH5α competent cells using heat shock method and cultured on a plate containing a selective medium containing ampicillin antibiotics. The successful subcloning of pET22b + hGH plasmids to DH5α bacteria was evaluated and confirmed by colony PCR, double digestion and sequencing methods based on the standard protocol.
pET22b + hGH Plasmid Transformation into BL21 (DE3)
The plasmid containing hGH gene (pET22b + hGH) was transformed to competent cells of BL21 (DE3) according to the standard Sambrooke instructions by heat shock method, and transfected competent cells were incubated on a plate containing a selective medium containing ampicillin antibiotics for 16 h at 37 °C. The successful subcloning of pET22b + hGH plasmids into BL21 (DE3) was evaluated and confirmed by colony PCR and double digestion methods according to the standard protocol.
Identification of hGH Polypeptide Gene in pET22 (+) Plasmid
Screening and sequencing of positive clones was performed in order to verify the accuracy of the nucleotide sequence and the accuracy of the subcloned hGH polypeptide reading frame in the expression pET22b (+) plasmid by GeneRay Co. (Shanghai, China) based on Sanger method.
Expression of Recombinant hGH in BL21 (DE3)
A colony of single pET22b + hGH was inoculated into 5 ml of 2 × YT medium containing 2% glucose and ampicillin, and incubated for 16 h at 250 rpm and 37 °C. Dilution and refreshment of overnight cultures was performed in 500 ml of 2 × YT medium containing 0.1% glucose and ampicillin antibiotics. After reaching the optimal OD600 (0.7), induction of gene expression in BL21 (DE3) was performed by adding IPTG to the final concentration of 0.5 mM at 30 °C and 250 rpm. In order to determine the highest expression of hGH in BL21 (DE3), the protein expression was evaluated and compared at 0, 1, 2, 3, 4, 5 and 6 h after induction by SDS-PAGE assay.
Isolation and Extraction of Recombinant hGH from Periplasmic Space
The proteins were expressed and extracted from the periplasmic space of BL21 (DE3) bacteria after optimal time of 5 h induction with IPTG. The incubated culture medium at 30 °C for 20 min was centrifuged at 4 °C and 6000 rpm. The precipitate was dissolved in a cold periplasmic extraction buffer containing protease inhibitors and the resulting solution was incubated for 20 min on ice. The periplasmic protein extract obtained at 13,000 rpm was centrifuged for 30 min at 4 °C and the supernatant containing the recombinant hGH protein was stored at 4 °C.
Isolation and Extraction of Recombinant hGH from Cytoplasmic Space
After separating the supernatant, the obtained bacterial precipitate was dissolved in the osmotic shock buffer (cytoplasmic extraction) containing protease inhibitors and incubated for 20 min on ice. The obtained cytoplasmic protein extract at 13,000 rpm was centrifuged for 30 min at 4 °C and the obtained supernatant was stored at 4 °C.
Dialysis of Recombinant hGH Protein and Purification by Chromatography Assay
The protein extract derived from periplasmic and cytoplasmic space extraction was transferred to separate dialysis bags with cut off of 12 kDa and dialysed for 4 h at 16 °C in PBS. Poly-Prep chromatography column (BIO-RAD, USA) and Ni–NTA agarose matrix (Qiagen, German) were used to purify the recombinant hGH protein using His-tag. The protein extracts of periplasmic and cytoplasmic dialysed hGH were loaded onto the column separately. The column was washed with 20 mM cold imidazole buffer solution, and finally the proteins purified by chromatography column were eluted from the column using 250 mM cold buffer imidazole solution and collected in different tubes.
Evaluation and Confirmation of Recombinant hGH Protein Expression with SDS-PAGE and Western Blot Assays
The presence or absence of recombinant hGH protein in periplasmic and cytoplasmic extracts was evaluated and confirmed using SDS-PAGE assay and proteins were stained using Coomassie Brilliant Blue R250. The expression and presence of recombinant hGH protein in the obtained protein extracts was confirmed specifically using western blot assay after transferring proteins from gel to PVDF paper and anti-poly-histidine-HRP antibodies (Sigma, USA) in the presence of DAB colored substrate to perform the reaction with peroxidase.
Evaluation of Bio-activity of Recombinant hGH Protein Using ELISA Assay
ELISA assay was performed using ELISA kit (Abnova, Taiwan) based on classical sandwich ELISA method according to the manufacturer’s instructions. The optical density was read at the wavelength of 450 nm by ELISA Reader.
Results
Optimization of Codons, Synthesis of hGH Polypeptide Gene and Cloning into pGH Plasmid
The results of the study of the codon optimization index using GeneScript’s online tool showed that the codon adaptation index (CAI) for the studied protein was 0.88, which is an optimal score for expressing the gene in the intended expression system (Fig. 2a). In this index, the number 1 means the best possible case for the expression of the gene in the given expression system and the index higher than 0.8 is considered desired. The process of optimizing hGH gene codons resulted in the human GH content of 42.55% which was in the optimal range (Fig. 2b). Also, evaluation of the sequence of hGH polypeptide after optimization was also evaluated in terms of the presence of negative repetitive elements and negative CIS elements. The obtained results showed that there is no negative element affecting the transcription and translation efficiency in the optimization sequence of hGH polypeptide gene.
Validation and Verification of Subcloning Accuracy of hGH Polypeptide Gene into pET22b (+) Plasmid
The results of electrophoresis of PCR products on agarose gel and presence of 828 bp confirmed the proper insertion of the hGH gene into pGH plasmid (Fig. 3a). Moreover, the results of electrophoresis of colony PCR products, in addition to confirming the subcloning of hGH polypeptide gene, indicate the accuracy of the primer activity for the correct amplification of gene of interest (Fig. 3b). The results of electrophoresis of the enzymatic double digestion after the extraction of pET22b (+) plasmid containing hGH gene demonstrated the presence of 581 bp of the given gene. Also, the linearization or single digestion of pET22b + hGH plasmid by the enzymes XhoI also shows the correct function of the enzymes (Fig. 3c). The accuracy of the nucleotide sequence and the accuracy of the reading frame of hGH gene in the expression pET22b (+) plasmid were confirmed by Sanger sequencing method. The obtained results show the presence of sharp and single peaks, which confirms the accuracy of the given sequence and the reading frame (Fig. 3d).
a Electrophoresis results of colony PCR products derived from transformation of pGH plasmid to DH5α strain. M 100 bp Plus DNA Size Marker (100, 200, 300, 400, 500, 600, 700, 800, 900, 1000, 1500, and 3000 bp), 1–4 colony PCR products of hGH polypeptide gene from the pGH plasmid of the transformer replicated to DH5α strain, 5 the negative control (bacterial extract of DH5α strain is not transformed). b The results of electrophoresis of colony PCR products derived from transformation of the pET22b + hGH plasmid to DH5α and BL21 (D3) strains. M 100 bp Plus DNA Size Marker (100, 200, 300, 400, 500, 600, 700, 800, 900, 1000, 1500 and 3000 bp), 1 the negative control (bacterial extract BL21 (D3) strain that is not transformed), 2 colony PCR products of hGH polypeptide gene amplified from the transformed pET22b + hGH plasmid to DH5α strain, 3 colony PCR products of hGH polypeptide gene amplified from pET22b + hGH plasmid to BL21 (D3) strain. c The results of electrophoresis of double digestion products with enzymes NcoI and XhoI on the pET22b + hGH plasmid in BL21 (D3) strain bacteria. M 1 kbp DNA Size Marker (250, 500, 750, 1000, 1500, 2000, 2500, 300, 4000, 5000, 6000, 8000 and 10,000 bp), 1 pET22b + hGH plasmid extracted and not digested (supercoil), 2 pET22b + hGH plasmid digested with XhoI enzyme (linear plasmid); 3 double digestion of pET22b + hGH plasmid with NcoI and XhoI enzymes; 4 the negative control (double digestion of BL21 (D3) strain bacteria extract not transformed). d Human growth hormone gene sequencing in the pET22b (+) plasmid. The presence of clear and distinct peaks indicates the proper insertion of gene of interest in the correct location and the accuracy of sequencing
Validation and Confirmation of hGH Protein Expression and Purification in BL21 (DE3) Host
The amount of recombinant hGH expression in BL21 (DE3) host was validated and confirmed using SDS-PAGE assay at 1-h intervals. The results of electrophoresis of the products expressed using SDS-PAGE showed no expression of hGH within 0 h. These results confirmed the expression of hGH and a gradual increase in the concentration of the given recombinant protein after induction with IPTG. Also, the study results of the expression of recombinant hGH showed that the highest expression of the given recombinant protein was observed 5 h after induction (Fig. 4a). The desired protein was purified and extracted based on the sequence of polyhystidine in four fractions. The results of SDS-PAGE electrophoresis of purified products by chromatography column against the polyhystidine sequence showed that the recombinant hGH protein was present in the second fraction (Fig. 4b). The quantity and concentration of the purified protein was calculated 1 g/l using Bradford assay.
a The SDS-PAGE results of time point study of hGH polypeptide expression. M Protein size marker (10, 15, 25, 35, 40, 55, 70, 100, 130 and 170 kDa), 0 hour 0 (before induction of hGH gene expression with IPTG), 1 1 h after induction of hGH gene expression with IPTG, 2 2 h after induction of hGH gene expression with IPTG, 3 3 h after induction of hGH gene expression with IPTG, 4 4 h after induction of hGH gene expression with IPTG, 5 5 h after induction of the expression of hGH gene with IPTG, 6 6 h after induction of the expression of hGH gene with IPTG. b The SDS-PAGE results of recombinant hGH protein purified against His-tag using a chromatography column. 1 Fraction 4 after washing with 250 mg Imidazole, 2 fraction 3 after washing with 250 mg imidazole, 3 fraction 2 after washing with 250 mg Imidazole, 4 fraction 1 after rinsing with 250 mg Imidazole, M protein size marker (10, 15, 25, 35, 40, 55, 70, 100, 130 and 170 kDa) based on the expectation of recombinant human growth hormone protein having a molecular weight of about 22 kDa
Validation and Verification of Recombinant hGH Protein Bio-activity by Western Blot and ELISA Assayes
The results of Western blot on a specific antibody binding (Anti-Poly-Histidine conjugated to HRP) confirmed the recombinant hGH expressed at different intervals on PVDF membrane and proved the expression of recombinant hGH in the BL21 (D3) bacterial system (Fig. 5). The results of classical sandwich ELISA confirmed the specific antibody binding to recombinant hGH. These results proved the detection of recombinant hGH protein superficial domain expressed in the strain BL21 (D3) by specific antibodies.
The western blot results of human growth hormone protein. 1—The negative control sample (BL21 non-transformed bacterial extract), 2—hour 0 (before induction of hGH gene expression with IPTG), 3—2 h after induction of hGH gene expression with IPTG, 4—5 h after induction of hGH gene expression with IPTG. As expected recombinant human growth hormone protein has a molecular weight of about 22 kDa
Discussion
Medical knowledge is moving towards a new era in which individuals’ diseases’ management is possible and progressing through genetic and protein information. Thus, recombinant medicine proteins play an increasingly important role in this system. Currently, recombinant human proteins form a significant part of FDA approved biotechnology medicines. Medical proteins have achieved much success due to their features, such as purity, and these are very important in treating diseases that require protein replacement, such as treatment by the hormone. Also, unlimited access to these medical proteins has led to the development of related diseases’ treatment that would require the use of recombinant technology for production on a high-efficiency with no side effect industrial scale (Leader et al. 2008). On the other hand, the success of any bio-pharmaceutical company depends on the ability to achieve large-scale and cost-effective production of the recombinant product (Ghovvati et al. 2018; Vahedi et al. 2018). As a result, the cost of producing a recombinant product can be minimized by increasing production efficiency on a large scale. In order to achieve high efficiency, applying solutions such as: the use of a proper host, optimal plasmid, scale increase process, as well as easy and inexpensive purification process are essential (Gupta and Shukla 2016).
Escherichia coli system is one of the most commonly used systems among the expression hosts, which is widely used for the production of recombinant proteins due to its desired features. However, E. coli has some disadvantages. For example, the correct folding of many proteins requires the formation of a disulfide bond or post-translation changes such as glycosylation, which is not available in the cytoplasm (Waegeman and Soetaert 2011). As a result, the overexpression of recombinant human heterologous proteins in E. coli has produced large amounts of unfolded and incorrectly folded proteins in the cytoplasm, leading to their accumulation and formation of inclusion bodies. The inclusion bodies are insoluble and biologically inactive, and their efficient re-folding would lead to increased production costs (Sorensen and Mortensen 2005). In order to overcome these challenges, signal peptides can be used to direct recombinant proteins to E. coli periplasm, which, due to the oxidizing medium, results in the correct folding of the recombinant protein as well as the proteolytic degradation of proteins is minimized. For this reason, the use of periplasmic expression is used for the commercial production of biopharmaceuticals.
Among different strains of the bacteria E. coli, the strain DH5α has high transformation efficiency, which is due to cases such as mutations in endA1 that inhibits the intracellular endonuclease degrading plasmid DNA. Also due to mutations in recA, they lack the homologous recombination and reduce the plasmid multimerization (Selvarasu et al. 2009). Consequently, in the present study the strain DH5α was used for cloning and replication of plasmids. In this study, E. coli strain BL21 (DE3), commonly used to express recombinant proteins, was used for the expression of the growth hormone gene. BL21 strains have been manipulated in Lon and OmpT proteases, which this defect reduces the degradation of the recombinant protein and leads to higher efficiency, also the strain BL21 (DE3) in comparison with the strain DH5α, has better metabolic and growth features (Jeong et al. 2015; Phue et al. 2008). pET system is one of the most powerful plasmid systems ever developed for the cloning and expression of recombinant proteins. In the meanwhile, the expression pET22b (+) plasmid has the ability to direct the protein expressed to E. coli’s periplasm due to its pelB signal peptide at N terminal at the given gene entering site, in order to produce the correct folding. Also in this study, at C terminal at the given gene entering site, there was polyhistidine gene sequence that facilitated the protein purification process using affinity chromatographic column.
In silico studies and simulations showed the optimum content of GC content between 30% and 70%, and values out of this range will have an adverse effect on the efficiency of transcription and translation (Boycheva et al. 2003). Also, in the optimization process for codons, a score of 0.8 to 1 is desired, suggesting the use of available and more abundant codons of the expression system in the given gene sequence (Gutman and Hatfield 1989).
Basically, eukaryotic cells using translocation process to escape nascent protein from cytoplasm and move it to rough endoplasmic reticulum. This process depends on signal recognition particle complex which can identify signal peptide from nascent protein and translocate that to rough endoplasmic reticulum through transposon (Forouharmehr et al. 2018). Prokaryotic cells have no rough endoplasmic reticulum but they have periplasmic compartment which provides well conditions for facilitating the proper folding process, which is very similar to the proper folding process of eukaryotic cells. Furthermore, the main pathways in prokaryotic cells in which directing nascent protein to periplasmic compartment are Sec, TAT and SPR. In transferring secretory proteins through the bacterial cytoplasmic membrane, in order to not affect the function and structure of the protein, various transfer mechanisms are used such as secretion pathway (Novello et al. 2009) and Dual Arginine Transluctation (TAT). This system plays a critical role in the secretion of unfolded proteins through the cytoplasmic membrane, while TAT system secretes folded proteins and cofactor-containing proteins (Beckwith 2013; Berks 2015). Therefore, we could conclude that the signal peptides have key roles in proper folding of secretory proteins in both eukaryotic and prokaryotic cells and this is not only interesting for it’s potential bio-technological applications but also for large scale production of heterologous proteins in pharmaceutical industries, too.
The periplasmic expression of recombinant proteins is commercially used including Fab components, other cases include some medicines, such as scFvs and antibodies that lack a glycosylated structure (Hsu et al. 2016). In various studies, a variety of peptides have been signaled for expression of recombinant proteins in bacteria, some of which are variety of peptides’ signals have been used including OmpA, LamB, PhoA, DsbA, STII, endoxylanase, and pelB (Choi et al. 2000; Kaderbhai et al. 2001; Kotzsch et al. 2011; Nausch et al. 2013; Schierle et al. 2003; Su et al. 2015; Zhou et al. 2016). In a study LTB signal peptide was used to direct the hGH into E. coli periplasmic space. The results indicated the desired coupling of the growth hormone to the receptor and the correct periplasmic processing (Ghorpade and Garg 1993). The hGH fused to Trx as soluble in cytoplasm was developed by Levarski et al. (2014). The results confirmed the production of 1 g/l of the recombinant protein soluble in the bioreactors and no significant increase was found in the amount obtained compared to the periplasmic expression (Levarski et al. 2014). In another study, Zamani et al. (2016) used L-asparaginase II peptide and pET15b plasmid signals to direct the hGH to the peri-plasmic space, and the obtained results showed that the expression of hGH protein even in the presence of the above signal peptide resulted in the creation of inclusion bodies, as a result, this signal peptide is not effective on the production of efficient hGH periplasmic. Bagherinejad et al. (2016) evaluated the secretory expression of rhGH by the two signals of TorA and SufI peptides that have TAT system, together with the simultaneous expression of DsbA, in the expression plasmid pET22b. TorA signal peptide had the ability to secrete hGH into the culture medium, but when using SufI, a significant amount of hGH remained in the periplasmic space. The production of hGH periplasmic was studied by Sockolosky and Szoka (2013) using pET22b system and pelB and OmpA signal peptides in the strain BL21. The use of pelB resulted in higher yield compared to OmpA and production about 1.4 mg/l. The proteins produced by both signal peptides have a good bio-activity, which shows the effect of the correct selection of the signal peptide on the periplasmic expression of rhGH. The expression induction was performed in TB medium at 25 °C for 16–18 h (Sockolosky and Szoka 2013).
The above research suggest that the periplasmic expression of hGH is very common in E. coli as a host. In current study, the periplasmic expression of hGH was performed using pelB signal peptide and pET22b expression plasmid. The results of current study showed production of about 1 g/l of hGH polypeptide that despite the same signal peptide and even expression plasmid compared with Sockolosky and Szoka (2013) study, the effect of optimizing the codon, type of optimized medium, temperature and induction duration are evident in the expression of rhGH. Consequently, in addition to the main role of the signal peptide in directing the proteins to the desired space, the set of different conditions have a significant effect on the expression of recombinant proteins, which, if properly engineered, can achieve a higher degree of desired expression.
Conclusion
The use of cost-effective expression systems is always a wish of big business companies. Because these systems have a high popularity for mass production due to the simplicity of the culture medium, easy genes’ manipulation, and abundant plasmids. But along with all these desired features, the inability to produce and fold the complex proteins properly is one of their common concerns. In summary, the present study showed that the use of a suitable signal peptide as fusion to the hGH in the case of computerized evaluations and simulations based on valid in silico studies, optimization of codons, and the proper engineering of the conditions for the expression of recombinant proteins in an appropriate expression system can produce desired results on the correct amount of biopharmaceuticals targeted for periplasmic space. The results of this study showed that pelB signal peptide has a high efficiency in expressing the recombinant hGH into the periplasmic space. These results, in addition to the significant amount of expression of recombinant hGH protein in the bacterial system, confirmed its precise expression into the periplasmic space and bio-activity.
References
Bagherinejad M, Sadeghi H, Abedi D, Chou C, Moazen F, Rabbani M (2016) Twin arginine translocation system in secretory expression of recombinant human growth hormone. Res Pharm Sci 11:461–469
Baradaran A, Sieo CC, Foo HL, Illias RM et al (2013) Cloning and in silico characterization of two signal peptides from Pediococcus pentosaceus and their function for the secretion of heterologous protein in Lactococcus lactis. Biotechnol Lett 35:233–238
Beckwith J (2013) The Sec-dependent pathway. Res Microbiol 164:497–504
Berks BC (2015) The twin-Arginine protein translocation pathway. Annu Rev Biochem 84:843–848
Boycheva S, Chkodrov G, Ivanov I (2003) Codon pairs in the genome of Escherichia coli. Bioinformatics 19:987–998
Chatzi KE, Sardis MF, Economou A, Karamanou S (2014) SecA-mediated targeting and translocation of secretory proteins. Biochim Biophys Acta 1843:1466–1474
Choi JH, Jeong KJ, Kim SC, Lee SY (2000) Efficient secretory production of alkaline phosphatase by high cell density culture of recombinant Escherichia coli using the Bacillus sp. endoxylanase signal sequence. Appl Microbiol Biotechnol 53:640–645
Fakruddin M, Mohammad Mazumdar R, Bin Mannan KS, Chowdhury A, Hossain MN (2013) Critical factors affecting the success of cloning, expression, and mass production of enzymes by recombinant E. coli. ISRN Biotechnol 3:1–7
Forouharmehr A, Nassiri M, Ghovvati S, Javadmanesh A (2018) Evaluation of different signal peptides for secretory production of recombinant bovine pancreatic ribonuclease A in gram negative bacterial system: an in silico study. Curr Proteomics 15:24–33
Franklin SL, Geffner ME (2011) Growth hormone: the expansion of available products and indications. Pediatr Clin North Am 58:1141–1165
Ghorpade A, Garg LC (1993) Efficient processing and export of human growth hormone by heat labile enterotoxin chain B signal sequence. FEBS Lett 330:61–65
Ghovvati S, Pezeshkian Z, Mirhoseini SZ (2018) In silico analysis of different signal peptides to discover a panel of appropriate signal peptides for secretory production of Interferon-beta 1b in Escherichia coli. Acta Biochim Pol 65:521–534
Gupta SK, Shukla P (2016) Advanced technologies for improved expression of recombinant proteins in bacteria: perspectives and applications. Crit Rev Biotechnol 36:1089–1098
Gutman GA, Hatfield GW (1989) Nonrandom utilization of codon pairs in Escherichia coli. Proc Natl Acad Sci USA 86:3699–3703
Hsu CC, Thomas ORT, Overton TW (2016) Periplasmic expression in and release of Fab fragments from Escherichia coli using stress minimization. J Chem Technol Biotechnol 91:815–822
Jeong H, Kim HJ, Lee SJ (2015) Complete genome sequence of Escherichia coli strain BL21. Genome Announc 3:e00134-15
Kaderbhai MA, Ugochukwu CC, Kelly SL, Lamb DC (2001) Export of cytochrome P450 105D1 to the periplasmic space of Escherichia coli. Appl Environ Microbiol 67:2136–2138
Kim MJ, Park HS, Seo KH, Yang HJ, Kim SK, Choi JH (2013) Complete solubilization and purification of recombinant human growth hormone produced in Escherichia coli. PLoS ONE 8:e56168
Kotzsch A, Vernet E, Hammarstrom M, Berthelsen J, Weigelt J, Graslund S, Sundstrom M (2011) A secretory system for bacterial production of high-profile protein targets. Protein Sci 20:597–609
Leader B, Baca QJ, Golan DE (2008) Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov 7:21–39
Levarski Z, Soltysova A, Krahulec J, Stuchlik S, Turna J (2014) High-level expression and purification of recombinant human growth hormone produced in soluble form in Escherichia coli. Protein Expr Purif 100:40–47
Lilie H, Schwarz E, Rudolph R (1998) Advances in refolding of proteins produced in E. coli. Curr Opin Biotechnol 9:497–501
Nausch H, Huckauf J, Koslowski R, Meyer U, Broer I, Mikschofsky H (2013) Recombinant production of human interleukin 6 in Escherichia coli. PLoS ONE 8:e54933
Novello D, da Fonseca RA, Pollonio MAR, Franceschini P (2009) Physicochemical evaluation and fatty acids profile of broiler chicken fed broiler diets containing barley brewer (Hordeum vulgare). Ciênc Tecnol Aliment Campinas 29:495–503
Phue JN, Lee SJ, Trinh L, Shiloach J (2008) Modified Escherichia coli B (BL21), a superior producer of plasmid DNA compared with Escherichia coli K (DH5alpha). Biotechnol Bioeng 101:831–836
Raven PH, Johnson GB, Mason LA, Losos J, Singer S (2013) Biology, 10th edn. McGraw-Hill, New York
Reh CS, Geffner ME (2010) Somatotropin in the treatment of growth hormone deficiency and Turner syndrome in pediatric patients: a review. Clin Pharmacol 2:111–122
Sambrook JF, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Saraogi I, Shan SO (2014) Co-translational protein targeting to the bacterial membrane. Biochim Biophys Acta 1843:1433–1441
Schierle CF, Berkmen M, Huber D, Kumamoto C, Boyd D, Beckwith J (2003) The DsbA signal sequence directs efficient, cotranslational export of passenger proteins to the Escherichia coli periplasm via the signal recognition particle pathway. J Bacteriol 185:5706–5713
Selvarasu S, Ow DS, Lee SY, Lee MM, Oh SK, Karimi IA, Lee DY (2009) Characterizing Escherichia coli DH5alpha growth and metabolism in a complex medium using genome-scale flux analysis. Biotechnol Bioeng 102:923–934
Sockolosky JT, Szoka FC (2013) Periplasmic production via the pET expression system of soluble, bioactive human growth hormone. Protein Expr Purif 87:129–135
Sorensen HP, Mortensen KK (2005) Advanced genetic strategies for recombinant protein expression in Escherichia coli. J Biotechnol 115:113–128
Su L, Yu L, Xu C, Wu J (2015) Extracellular expression of Thermobifida fusca cutinase with pelB signal peptide depends on more than type II secretion pathway in Escherichia coli. J Biotechnol 204:47–52
Thanassi DG, Hultgren SJ (2000) Multiple pathways allow protein secretion across the bacterial outer membrane. Curr Opin Cell Biol 12:420–430
Vahedi F, Nassiri M, Ghovvati S, Javadmanesh A (2018) Evaluation of different signal peptides using bioinformatics tools to express recombinant erythropoietin in mammalian cells. Int J Pept Res Ther. https://doi.org/10.1007/s10989-018-9746-1
Waegeman H, Soetaert W (2011) Increasing recombinant protein production in Escherichia coli through metabolic and genetic engineering. J Ind Microbiol Biotechnol 38:1891–1910
Yoon SH, Kim SK, Kim JF (2010) Secretory production of recombinant proteins in Escherichia coli. Recent Pat Biotechnol 4:23–29
Zamani M, Nezafat N, Ghasemi Y (2016) Evaluation of recombinant human growth hormone secretion in E. coli using the L-asparaginase II signal peptide. Avicenna J Med Biotechnol 8:182–187
Zhou Y, Liu P, Gan Y, Sandoval W et al (2016) Enhancing full-length antibody production by signal peptide engineering. Microb Cell Fact. https://doi.org/10.1186/s12934-016-0445-3
Funding
This study was no funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that we have no conflict of interest.
Ethical Approval
No human or animals subjects were involved in this study.
Informed Consent
This study contain no individual participant data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doozandeh-Juibari, A., Ghovvati, S., Vaziri, H.R. et al. Cloning, Expression, Purification and Evaluation of the Biological Properties of the Recombinant Human Growth Hormone (hGH) in Escherichia coli. Int J Pept Res Ther 26, 487–495 (2020). https://doi.org/10.1007/s10989-019-09854-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-019-09854-y