Abstract
Fifty signal peptides of Pediococcus pentosaceus were characterized by in silico analysis and, based on the physicochemical analysis, (two potential signal peptides Spk1 and Spk3 were identified). The coding sequences of SP were amplified and fused to the gene coding for green fluorescent protein (GFP) and cloned into Lactococcus lactis pNZ8048 and pMG36e vectors, respectively. Western blot analysis indicated that the GFP proteins were secreted using both heterologous SPs. ELISA showed that the secretion efficiency of GFP using Spk1 (0.64 μg/ml) was similar to using Usp45 (0.62 μg/ml) and Spk3 (0.58 μg/ml).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactococcus lactis and other lactic acid bacteria (LAB) have been traditionally used for the manufacturing of fermented dairy products. L. lactis is a generally regarded as safe (GRAS) organism and does not produce any endotoxin or lipopolysaccharide. LAB also have a monolayer cell wall that permits direct secretion of the desired protein to the extracellular environment (Sriraman and Jayaraman 2006). Apart from having a single outer membrane, L. lactis possesses only one extracellular housekeeping protease (HtrA) and one extracellular scavenger protease, PrtP (Le Loir et al. 2005).
In L. lactis, achieving protein secretion is important due to low yield of the end-products using available systems. L. lactis secretes up to 210 mg foreign protein/l (e.g. staphylococcal nuclease) with optimized gene constructs, pH and induction conditions (Tremillon et al. 2010). According to Le Loir et al. (2001), the substitution of the native staphylococcal signal peptide (SPNuc) by L. lactis homologous signal peptide (Usp45) led to increased secretion efficiency. Nonetheless, Lindholm et al. (2004) and Morello et al. (2008) showed that the application of Lactobacillus brevis S-layer signal peptide (SIpA) for the secretion of the E. coli FedF adhesion factor and application of SP Exp4 (a synthetic SP with a structure and amino acid composition best fit with the Gram-positive consensus) for the secretion of the Nuc in L. lactis is more efficient than or at least equal with SP Usp45. Thus, the properties of L. lactis have attracted the use of L. lactis as prospective candidate for the production of bioactive proteins in functional food and as oral vaccines. In this study, several heterologous signal peptides of Pediococcus pentosaceus were computationally analyzed and from these, two were used for the construction of secretory vectors for L. lactis.
Materials and methods
Bacterial strains, plasmids and growth condition
Bacterial strains and plasmids used in this work are listed in Supplementary Table 1. L. lactis was grown at 30 °C without shaking in M17 media supplemented with 0.5 % (w/v) glucose (GM17); and GM17 medium with 5 μg erythromycin/ml or 7.5 μg chloramphenicol/ml was used to select plasmid transformants. P. pentosaceus ATCC 25745 was propagated at 30 °C in MRS media.
In silico analysis of physicochemical properties of signal peptide
The signal peptide potential was analyzed using several commonly used prediction algorithms. All Sec-dependent, non-lipoprotein signal peptides of P. pentosaceus were separately analyzed according to size (20–30 amino acids), cleavage site (amino acids A–X-A) and SignalIP3 (D-score > 0.95). The ExPASy tools (http://expasy.org/tools/) were used for the analysis of physicochemical properties of selected signal peptides. The SP and signal peptide cleavage sites were predicted using SignalP 3.0 server (Bendtsen 2004), at http://www.cbs.dtu.dk/services/SignalP/, SOSUIsignal program (Gomi et al. 2004), at http://bp.nuap.nagoyau.ac.jp/sosui/sosuisignal/sosuisignal_submit.html, and the Predisi program (Hiller et al. 2004) at http://www.predisi.de/. Transmembrane predictions of the SP were performed using the TMHMM server v. 2.0, which is accessible at http://www.cbs.dtu.dk/services/TMHMM-2.0/. The hydrophobicity of the SPs was estimated using the ProtScale program and the Kyte & Doolittle scale on the ExPASy server at http://ca.expasy.org/tools/protscale.html, using a sliding window of seven residues.
Construction of recombinant expression vector and transformation
The spk1, spk3 and gfp DNA fragments were amplified, digested with PstI and fused to make SP-GFP fusion cassettes. All primers used are listed in Supplementary Table 2. As a control, the Usp45 and gfp DNA fragments were digested with BamHI and fused to make USP-GFP fusion cassette. The SPs were fused to the GFP reporter protein by a 6 nucleotide linker that created a unique PstI and BamHI restriction sites. All the constructs (Spk1-GFP, Spk3-GFP, and Usp45-GFP) had alanine (A) and aspartic acid (D) in positions +1 and +2 relative to the cleavage site, respectively. The SP-GFP gene cassettes were subsequently ligated to the L. lactis pNZ8048 (inducible) and pMG36e (constitutive) expression vectors after double digestions with the PaeI (SphI)/HindIII and SacI/HindIII, respectively. The mixture was purified using the Wizard PCR Preps DNA Purification Systems (Promega) and the recombinant plasmids were electrotransformed into L. lactis NZ9000 and MG1363, using Bio-Rad Gene Pulser Electroporation System.
Protein expression analysis
Overnight cultures of recombinant clones were diluted 1:50 (v/v) in fresh GM17 broth containing the appropriate antibiotic and incubated at 30 °C until the OD600 value reached 0.5. The inducible transformants were induced with 10 ng nisin/ml for 6 h at 30 °C before proceeding to the subsequent steps. Cell and supernatant fractions were prepared and processed separately from 40 ml cultures. Bacterial cell pellets were collected by centrifugation at 6,000×g for 15 min at 4 °C, then washed and resuspended in 100 μl of TES-lysis buffer [25 % sucrose (w/v), 1 mM EDTA, 50 mM Tris/HCl, pH 8.0, lysozyme (10 mg/ml) supplemented with 1 mM PMSF and 10 mM of dithiothreitol]. The mixture was incubated at 37 °C for 1 h and the cells were disrupted by vortexing with 1 % (w/v) glass beads for 1 min 10 times with an interval of 1 min on ice. The supernatant was collected by centrifugation at 14,000×g for 15 min at 4 °C and then one volume of loading buffer was added (Villatoro-Hernandez et al. 2008).
Extracellular proteins were obtained by collecting the supernatant after the cells were pelleted by centrifugation. The supernatant was then treated with 1 mM of PMSF and 10 mM DTT to avoid proteolysis and oxidation, respectively. Proteins were precipitated with 1:10 (v/v) of 100 % trichloroacetic acid and held on ice for 15 min followed by centrifugation at 14,000×g at 4 °C for 15 min. The precipitated protein was dissolved in 50 μl 50 mM NaOH and one volume of SDS-PAGE loading buffer was added to the protein samples prior to SDS-PAGE analysis on a 12 % gel. Resolved peptides were then subjected to western blotting and analyzed by using primary polyclonal rabbit anti-GFP antibodies (Calbiochem) at 1:2,000 dilution and secondary goat anti-rabbit immunoglobulin G conjugated with alkaline phosphatase (Calbiochem) at 1:5,000, respectively.
GFP quantification by ELISA
The concentrations of GFP in the extracellular cell fractions of the inducible recombinant strains, which secreted into the growth medium were analyzed with an ELISA kit according to manufacturer’s recommendations.
Results
Computational analysis of physicochemical properties of signal peptides Spk1 and Spk3
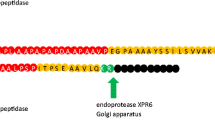
It is critical to predict precisely the location of signal peptide cleavage sites when designing constructs for producing recombinant secreted proteins or receptors. Prediction of the signal peptide probability and cleavage site was performed using SignalP 3.0, SOSUI and Predisi program, consisting of two different predictors based on Neural Network and Hidden Markov Model algorithms. The SignalP 3.0 server was able to predict the probability of a particular amino acid sequence, whether it might act as a signal peptide or not by calculating a discriminating score, termed the D-score. The default cutoff value of D-scores > 0.7 was used for predicting signal peptide potential, and the highest scoring cleavage site was assumed to be the correct prediction (Bendtsen, 2004). Among the 50 performing SPs identified in the screening, two (Spk1, Spk3) showed the high D-score of above 0.95 with the length ranging between 20 and 30 amino acid and the cleavage site pattern of A–X-A. Table 1 presents the cleavage site and score prediction by the SignalP program. In addition the results of physicochemical analysis showed that Spk1 has the highest potential to target the proteins through the Sec compartment pathway compared to Usp45 and Spk3 (Table 2).
Expression of green fluorescent protein
To determine the ability of the signal peptides to direct GFP to the extracellular compartment of L. lactis, SDS-PAGE and western blotting of cytoplasmic and extracellular fractions were carried out. All constructs were electrotransformed into L. lactis NZ9000 and MG1363. The expression of the recombinant GFP in L. lactis NZ9000 was analyzed after 6 h of induction with 10 ng nisin/ml at 30 °C. A band of ~31 kDa representing the expressed GFP was visualized in the intracellular fraction of protein that was extracted from all transformants. Interestingly, a band of ~27 kDa was observed only in the western blot of the extracellular fraction from the secretory construct in pNZ8048 after induction in suitable condition (Supplementary Fig. 1).
Quantification of extracellular GFP using an ELISA kit
GFP expression in the extracellular fraction was quantified. The quantity of extracellular GFP in pNZ8048 inducible construct was determined by comparing its absorbance with that of the known recombinant GFP standard. The concentration of GFP in the supernatant of transformants harbouring Spk1-GFP, Spk3-GFP and Usp45-GFP cloned into the inducible pNZ8048 are given in Fig. 1.
ELISA analysis of GFP in extracellular fraction of inducible constructs. Detection of recombinant proteins from pNZ:GFP (negative control, intracellular constructs), pNZ:Spk1-GFP, pNZ:Spk3-GFP and pNZ:Usp45-GFP. The standard error bar represented in the figure above was calculated by standard deviation of three replicate
Discussion
A high expression level of heterologous proteins can lead to aggregation and misfolding when expressed intracellularly. To circumvent this problem, proteins could be secreted into the extracellular fraction. Secretion of targeted protein is a promising separation step that facilitates downstream purification of the product (Desvaux et al. 2009). Additionally, for proteins that need post-translational modification, secretion is preferable to intracellular production due to the cell wall associated chaperone and corrects folding. However, the yields of the secreted heterologous proteins were often reported to be insufficiently low.
In general, a successful secretion process is a multi-stage effort that requires an optimal balance between all stages of the secretory pathway. One of the most important factors that have a strong influence on the all stages of secretion cascade and consequently, on the yield of a secreted protein are the signal peptides. Therefore, the physicochemical and structural characteristics of a signal peptide play an important role in the functionality of secretion. For this purpose, various computational tools can be applied to predict and characterize the physicochemical properties of signal peptides. These tools were applied to compute the different parameters like number of amino acids and the physicochemical properties of a signal peptide such as molecular weight, isoelectric point, GRAVY, aliphatic index and instability index. Choo and Ranganathan (2008) revealed that the median net charge for SPs of Gram-positive are +3, the aliphatic index for Gram-positive SPs are between 75 and 200, the isoelectric point for SPs of Gram-positive bacteria are predominantly represented within single clusters with median values of 10.3 and from hydropathicity calculations, the GRAVY score of Gram positive bacteria SPs are 93.5 %.
In this study, two signal peptides from the Gram-positive bacteria P. pentosaceus were isolated, characterized and used as a heterologous signal peptide for the secretion of GFP in L. lactis. Commonalities and differences in similarity comparisons between genomic and proteomic analysis of L. lactis and P. pentosaceus show that both microorganisms share many important characteristics such as protein size, amino acid distribution and GC content. The secretory machinery analysis of both organisms also showed close similarity (Bolotin et al. 2001). The computational analyses of the signal peptides showed that the SP Spk1 has higher hydrophobicity, GRAVY index, aliphatic index and stability as compared to Usp45. Although the optimization and characterization of signal peptides using bioinformatics tools can be successfully carried out, theoretically the characterized SPs must be tested experimentally.
The functionality of the P. pentosaceus signal peptides, Spk1 and Spk3 in L. lactis was determined through the production of the heterologous reporter protein GFP, using both lactococcal constitutive and inducible expression vectors. The presence of the expressed recombinant proteins of GFP, Spk1-GFP, Spk3-GFP, and Usp45-GFP in all transformants were analyzed and monitored by SDS-PAGE and immunoblotting. Although the recombinant proteins were not observed in SDS-PAGE (data not shown), the excreted GFP proteins (Spk1-GFP, Spk3-GFP and Usp45-GFP) in the transformed L. lactis cells harbouring inducible promoter were detected by Western blot analysis using anti-GFP antibodies. In the extracellular protein fraction of cells transformed with pMG:Sp-GFP, no band was observed in recombinant using western blotting probably due to very low levels of expression. The low level of secretion and expression in pMG36e construct may, in fact, be due to the weak constitutive promoter (P32) used in this vector (Le Loir et al. 2005). The GFP expressions in the extracellular fraction of inducible constructs were also quantified to determine the amount of extracellular protein expression and verify the result of western blot. ELISA results demonstrated that the amount of extracellular GFP expressed by cells harbouring pNZ:Spk1-GFP (0.64 μg/ml) was almost similar to those expressed by cells carrying the other two constructs pNZ:Usp45-GFP (0.62 μg/ml) and pNZ:Spk3-GFP (0.58 μg/ml).
Interestingly, the level of intracellular GFP expression in cells harboring either the secretory or non-secretory construct were almost similar, indicating that the detection of low extracellular yield was not a result of low precursor production. Furthermore, this shows that the factors hampering the SP efficiency possibly influenced the fraction of secreted precursor rather than creating a fixed limit on the amount of secreted protein. The lower secretion efficiency might be due to several factors such as the structural characteristic of the signal peptides, inefficient cleavage of signal peptide from GFP, incompatibility between the mature protein and the signal peptides, incomplete translocation process across translocation machinery or insufficient balance between the capacities of the export machinery in terms of the rate of protein synthesis. The results of the present study demonstrated that Spk1 and Spk3 from P. pentosaceus could be a potential SP for the secretion of heterologous proteins in L. lactis although they are suboptimal. Hence, there is a need to study the possibility of the modifing SP sequences to enhance the level of heterologous protein secretion in L. lactis.
References
Baradaran A, Foo HL, Sieo CC, Raha AR (2012) Isolation, identification and characterization of lactic acid bacteria from Polygonum minus. Rom Biotechnol Lett 17(2):7245–7252
Bendtsen D (2004) Improved prediction of signal peptides: Signalp 3.0. J Mol Biol 340(4):783–795
Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J, Ehrlich SD, Sorokin A (2001) The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Gen Res 11(5):731–753
Brockmeier U, Caspers M, Freudl R, Jockwer A, Noll T, Eggert T (2006) Systematic screening of all signal peptides from Bacillus subtilis: a powerful strategy in optimizing heterologous protein secretion in Gram-positive bacteria. J Mol Biol 362(3):393–402
Choo K, Ranganathan S (2008) Flanking signal and mature peptide residues influence signal peptide cleavage. BMC Bioinformatics 9:S15. doi:10.1186/1471-2105-9-S12-S15
Desvaux M, Hébraud M, Talon R, Henderson I (2009) Secretion and subcellular localizations of bacterial proteins: a semantic awareness issue. Trends Microbiol 17(4):139–145
Gomi M, Sonoyama M, Mitaku S (2004) High performance system for signal peptide prediction: SOSUIsignal. Chem-Bio Inform J 4(4):142–147
Hiller K, Grote A, Scheer M, Munch R, Jahn D (2004) PrediSi: prediction of signal peptides and their cleavage positions. Nucleic Acid Res 32(Web Server Issue):W375–W379
Le Loir Y, Nouaille S, Commissaire J, Bretigny L, Gruss A, Langella P (2001) Signal peptide and propeptide optimization for heterologous protein secretion in Lactococcus lactis. Appl Environ Microbiol 67(9):4119–4127
Le Loir Y, Azevedo V, Oliveira SC, Freitas DA, Miyoshi A, Bermudez-Humaran LG, Nouaille S, Ribeiro LA, Leclercq S, Gabriel JE, Guimaraes VD, Oliveira MN, Charlier C, Gautier M, Langella P (2005) Protein secretion in Lactococcus lactis: an efficient way to increase the overall heterologous protein production. Microb Cell Fact 4(1):2
Lindholm A, Smeds A, Palva A (2004) Receptor binding domain of Escherichia coli F18 fimbrial adhesin FedF can be both efficiently secreted and surface displayed in a functional form in Lactococcus lactis. Appl Environ Microbiol 70:2061–2071
Morello E, Bermdez-Humarn L, Llull D, Solé V, Miraglio N, Langella P, Poquet I (2008) Lactococcus lactis, an efficient cell factory for recombinant protein production and secretion. J Mol Microbiol Biotechnol 14(1–3):48–58
Sriraman K, Jayaraman G (2006) Enhancement of recombinant streptokinase production in Lactococcus lactis by suppression of acid tolerance response. Appl Microbiol Biotechnol 72(6):1202–1209
Tremillon N, Issaly N, Mozo J, Duvignau T, Ginisty H, Devic E, Poquet E (2010) Production and purification of staphylococcal nuclease in Lactococcus lactis using a new expression-secretion system and a pH-regulated mini-reactor. Microb Cell Fact 9(1):37. doi:10.1186/1475-2859-9-37
Villatoro-Hernandez J, Loera-Arias M, Gamez-Escobedo A, Franco-Molina M et al (2008) Secretion of biologically active interferon-gamma inducible protein-10 (IP-10) by Lactococcus lactis. Microb Cell Fact 7:22–32
Acknowledgments
We would like to thank the Ministry of Science, Technology and Innovation, Malaysia and Malaysian Genome Institute (MGI) for funding this study under the grant number 07-05-MGI-GMB011. We also thank Kees Leenhouts for the kind gift of plasmids pNZ8048 and pMG36e, and L. lactis hosts NZ9000 and MG1363.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baradaran, A., Sieo, C.C., Foo, H.L. et al. Cloning and in silico characterization of two signal peptides from Pediococcus pentosaceus and their function for the secretion of heterologous protein in Lactococcus lactis . Biotechnol Lett 35, 233–238 (2013). https://doi.org/10.1007/s10529-012-1059-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-1059-4