Abstract
Context
Land use change modifies landscapes’ original compositions and configurations, which can have a positive, negative, or neutral effect on species diversity. The direction and magnitude of the effect depends on how each species responds to these conditions and can change depending on the scale in which it is evaluated.
Objectives
We evaluated the effect of landscape composition and configuration on amphibian diversity at multiple scales in two fragmented regions in the Sierra Madre del Sur, Oaxaca, Mexico, in order to identify the determinant landscape characteristics for amphibian species.
Methods
We sampled amphibian populations at 16 sites and measured 15 landscape metrics at five different scales from focal patches. We then modelled the association between these metrics and amphibian abundance and richness for each scale.
Results
We found positive associations between amphibian richness and abundance with Forest Patch Density at the 200 m scale, and negative associations with Urban Total Area and Forest Edge Density at 200 m, 500 m and 1000 m scales. Single-species models revealed different responses to landscape metrics at varying scales, suggesting a differential response to landscape’s transformations that could be due to species life history traits.
Conclusions
Most amphibian species in these regions may be abundant in heterogeneous and fragmented landscapes as long as small forest patches are present. Nevertheless, large scale changes in forest amount and patch size due to fragmentation and urbanization could eventually affect some species negatively. Other variables at finer scales may be important and will depend on species-specific requirements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Land use change is one of the biggest threats to biodiversity worldwide (Bogaert et al. 2011). It is defined as the transformation of natural land covers into some of anthropic origin, such as plantations or crops, as well as the recovery of natural land covers from abandoned artificial land uses (Turner and Gardner 2015). These transformations change the landscapes’ original compositions and configurations, forming a heterogeneous mosaic of different land use types with variable environmental conditions (McGarigal and Cushman 2005). This can have a positive, negative, or neutral effect on species diversity (Bogaert et al. 2011). For most taxonomic groups, this effect tends to be negative, in which land use change causes species in communities become locally extinct, changes in population structure occur, and functional connectivity and gene flow are lost (Bell and Donnelly 2006; Dixo et al. 2009; García-García and Santos-Moreno 2013), leading to a substantial decline in biodiversity (Pardini et al. 2017).
Amphibians are highly affected by land use change because of their specific ecological requirements and their need for different habitats during their life cycle (Cushman 2006). Amphibian responses to landscape transformations have been reported by several authors, including positive effects of habitat amount (i.e., forest cover; Herrmann et al. 2005; Almeida-Gomes et al. 2019), riparian forest cover (Canessa and Parris 2013) and patch size (Cabrera-Guzmán and Reynoso 2012) on species richness and functional diversity, as well as negative effects of road density (Canessa and Parris 2013), ponds’ insolation and shrublands’ degradation (Lescano et al. 2015) on species richness. Some authors have also observed positive effects of crop diversity and negative effects of mean field size on amphibian diversity, which suggest that some species benefit from more heterogeneous landscapes (Collins and Fahrig 2017).
Ecological responses to landscape transformations, however, depend on the scale on which landscape structure is measure, so important species-landscape relationships can be missed if landscape structure is not measured at the appropriate scale (Wiens 1989; Jackson and Fahrig 2015). This is called the scale of effect and it refers to the appropriate scale at which the ecological response is best predicted by landscape structure (Jackson and Fahrig 2012). This particular scale ultimately depends on the life history traits of the species (e.g., dispersal capacity, Jackson and Fahrig 2012), the landscape variables measured, the ecological response evaluated and the regional context (Martin and Fahrig 2012; Jackson and Fahrig 2015; Miguet et al. 2016; Moraga et al. 2019). Since for most species the scale of effect is unknown a priori, landscape ecologists often measure species-landscape relationships at a wide range of scales in order to determine the one where the effect is strongest (McGarigal et al. 2016).
Amphibians also respond to landscape transformations and land use changes at specific scales. For example, some authors have found positive relationships between amphibian abundance and the amount of wetland within 1 km of ponds, suggesting an important effect of landscape context at that scale (Sawatzky et al. 2019). Other authors have found that the most predictive scales for amphibian diversity to landscape transformations relationships are those at 200 m (Benítez-Fernández 2018), 500 m (Canessa and Parris 2013), and between 100 and 1000 m (Herrmann et al. 2005) from focal patches. Some have observed variable associations among anuran species to agricultural intensity at different scales (Koumaris and Fahrig 2016) and different associations to environmental and structural variables depending on the scale evaluated (Duarte-Ballesteros et al. 2021). These results suggest that we cannot make general statements about the impacts and solutions to landscape transformations on amphibian diversity, and that more studies in different landscapes and contexts are necessary to better understand this phenomenon.
The Sierra Madre del Sur (SMS) in Mexico represents a physiographic unit with high amphibian diversity; however, many regions within this unit are seriously affected by land use change (Espinosa et al. 2016). As far as known, no studies have been conducted to analyze the effect of landscape transformations on amphibians on this unit, nor the scale of effect for the species distributed there. Therefore, we evaluated the effect of landscape composition and configuration on amphibian diversity patterns in two regions within SMS. Considering that species-landscape relationships and scale of effect may be different between species (Koumaris and Fahrig 2016), we analyzed these patterns for the whole amphibian community and for each individual species. Scale of effect could also be different depending on the landscape variables measured (Miguet et al. 2016). We measured 15 landscape metrics related to area, edge, shape, aggregation and diversity at five different geographic distances of 100 m, 200 m, 500 m, 1000 m, and 1500 m radii from focal patches, in order to impartially explore which structural and compositional characteristics of the landscape are most important for amphibians and at which scales these relationships are strongest. We expected that metrics related to forest patch area and aggregation will be the most important determinants for the amphibian community and individual species, since habitat amount tends to have a positive effect on amphibian diversity (Almeida-Gomes et al. 2019). Also, we expected that these metrics will have a greater effect on scales between 100 m and 1000 m, since amphibians tend to be small-sized organisms with restricted home ranges (Vitt and Caldwell 2014). Although we didn’t have a prediction on how any individual species will respond, we expected different responses at different scales of effect among species.
Methods
Study site
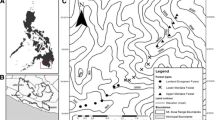
The SMS is a physiographic unit located in southern Mexico, represented by a 1200 km-long and 120 km-wide mountain range arranged in an East-West fashion (Fig. 1). It covers the states of Jalisco, Michoacán, Guerrero, Oaxaca and Puebla. The predominant vegetation types are coniferous and oak forest, tropical and temperate deciduous forest, mesophyllic cloud forest, evergreen and subdeciduous tropical forest, mixed forest, xerophilous scrubland and grassland (Espinosa et al. 2016). We worked in two regions of approximately 812 km2 each (Region A and Region B) located in the central southern limit of the SMS (Fig. 1). These regions cover tropical and temperate deciduous forest, coniferous forest, evergreen forest, and mixed forest. Both regions were chosen as they are severely affected by fragmentation processes and land use changes, with large proportions of agricultural crops, pastures for livestock and urban settlements (Fig. 1). All surveys were performed in tropical deciduous forest, as these were accessible places to survey, as well as the most fragmented land use/cover in these regions (Fig. 1).
Sampling design
Land use/cover classification
In order to measure landscape metrics, classified land use/cover maps were created for both regions from Landsat OLI 8 satellite imagery, obtained from the USGS EarthExplorer platform (https://earthexplorer.usgs.gov/). For this, a supervised classification by maximum likelihood method was performed (Horning et al. 2010), and seven land use/cover classes were defined for Region A: (1) Urban settlement, (2) Water body, (3) Agriculture, (4) Grassland/Scrubland, (5) Temperate deciduous forest, (6) Tropical deciduous forest and (7) Coniferous forest; and nine classes for Region B: (1) Urban settlement, (2) Water body, (3) Agriculture, (4) Grassland/Scrubland, (5) Temperate deciduous forest, (6) Tropical deciduous forest, (7) Coniferous forest, (8) Evergreen forest, and (9) Mixed forest. This classification was based on the land use map from the National Commission for the Knowledge and Use of Biodiversity (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad 2020).
In order to assess accuracy of land use/cover classifications, we used high-resolution images from Google Earth Pro (Hu et al. 2013; Google Earth v.7 2020) and National Commission for the Knowledge and Use of Biodiversity’s land use map (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad 2020) as reference images (Horning et al. 2010). These images were used to calculate the number of pixels classified correctly or incorrectly to a given class in the classification maps; with these, confusion matrices were created. With the confusion matrices, we calculated: total accuracy, producer accuracy, user accuracy and the Kappa index (Cohen 1960). Total accuracy represents a percentage of the overall correctly classified pixels. Producer accuracy is the probability that a pixel in a given class was classified correctly, and user accuracy is the probability that a pixel predicted to be in a certain class is really in that class (Horning et al. 2010). The kappa index measures the agreement between classification and truth-values and ranges between 0 (with no agreement) and 1 (perfect agreement) (Cohen 1960). Image processing, classification and accuracy assessment were performed with software ENVI 5.3 (Exelis Visual Information Solutions v. 5.3 2020).
Amphibian surveys
Amphibian richness and abundance data were obtained from May to September 2019. Three 11-days field trips were performed and, in each field trip, 16 sites were visited across both regions (8 sites per region; Fig. 1). In each region, four sites were chosen in conserved forested areas and four sites in degraded areas (i.e., grassland/scrubland or agriculture classes) (Fig. 1). All surveys in conserved forested areas were performed in tropical deciduous forest class. We avoided sampling in other forest types as these aren’t proportionally present in both regions evaluated and because these weren’t accessible for us. All sites were more than 500 m apart, and at least one water body (e.g., pond or stream) was present at each site. Night surveys were carried out by visual encounter surveys (VES) using two different sampling units: (1) 50 m x 2 m rectangular transects, and (2) 500 m x 4 m rectangular transects, as these are standardized methods used in herpetology that allowed us to compare amphibian diversity on different land uses/covers (Aguirre-León 2014). We sampled 1.50–2 h in each 50 m x 2 m transect, and 0.75–1 h in each 500 m x 4 transect. Two 50 m x 2 m transects and one 500 m x 4 m transect were performed at each site. Total sampling effort for conserved and degrades sites is presented in Supplementary Information 1, Appendix 1, Table S1.
Each amphibian captured was identified to the species level, and relative abundance for each species was calculated as the number of individuals observed in each transect relative to the total of amphibians recorded. Amphibian abundance data obtained by the two sampling units were summed to be used in further analysis. A specimen’s reference collection was obtained, which was deposited in the Herpetology Collection of the Museo de Zoología “Alfonso L. Herrera” at the Facultad de Ciencias of the Universidad Nacional Autónoma de México. The collection permit used was FAUT-0317 issued to the corresponding author of this paper. A total of 208 amphibian specimens were collected, with no more than two specimens per species per site collected to prevent over-collecting. All species observed are not cited in any CITES appendix, and all are classified as least concerned in the IUCN Red List (IUCN 2021).
We calculated Jackknife indices and species accumulation curves for conserved and degraded areas, as well as for the two regions evaluated in order to determine the completeness of the amphibian survey (Magurran and McGill 2011). We also calculated the proportion of species found as Sobs/(a/b), where a is the rate of increase of new species, b is a parameter related to the shape of the curve, and S is the number of species observed (Jiménez-Valverde and Hortal 2003). These analyzes were performed with the software EstimateS 9.1.0 (Colwell 2013) and STATISTICA 8.0 (Weiß 2007).
Landscape metrics
For each site, 15 landscape metrics were measured at five different scales, defined by the area of five concentric circles with radii of 100 m, 200 m, 500 m, 1000 m, and 1500 m from sampled sites (Fig. 1). Landscape metrics were measured with the software FragStats (McGarigal and Marks 1995; Supplementary Information 1, Appendix 1, Table S2). Although no studies have evaluated amphibian dispersal movements for the species observed in these regions, some studies elsewhere suggest that dispersal movements for some similar species range from 37 up to ~ 1000 m (Semlitsch and Bodie 2003; Tozetti and Toledo 2005; Heemeyer 2011; Horan 2011; Heemeyer and Lannoo 2012; Peterson et al. 2013; Henrique and Grant 2019; Arreortúa-Martínez 2020; DeVore et al. 2021; Covarrubias et al. 2022), therefore we chose a maximum radius of 1500 m. Since there could be greater measurement error at smaller scales when resolution is low (Miguet et al. 2016) a minimum radius of 100 m was chosen due to the spatial resolution of Landsat 8 OLI images (i.e., 30 m).
Metrics were calculated separately for tropical deciduous forest, agriculture, grassland/scrubland, urban settlement, and water body classes. Metrics for temperate deciduous forest and coniferous forest classes obtained zero values for all sites at all scales, so they were discarded from further analyses. Metrics for evergreen forest and mixed forest classes obtained zero values for all sites at the 100 m, 200 m and 500 m scales and for all but three sites at the 1000 m and 1500 m scales. For the three sites where these forest types were actually present, we combined their area with the one of the tropical deciduous forest class and calculated the landscape metrics for this combined forest class, since these two forest types could potentially provide habitat amount for some amphibian species (Urbina-Cardona et al. 2006; Wells 2007; Crump 2015; Suazo-Ortuño et al. 2015; Schneider-Maunourya et al. 2016; Ramírez-González 2016; Luna-Gómez et al. 2017; IUCN 2021; Mata-Silva et al. 2021; Naturalista 2021; See Supplementary Information 1, Appendix 1, Table S3). Contagion index and diversity metrics were calculated using data from all land use/cover classes. All metrics calculated are presented in Supplementary Information 2, Table S1.
Because model results can be affected by collinearity among predictor variables, we performed Spearman correlation analyses between landscape metrics measured at each scale, using the correlation_finder function in the R package ntbox (Osorio-Olvera et al. 2020) to choose the metrics in each scale that were least correlated under a threshold of 0.80. Only those metrics that were not correlated across all scales were used to make the models comparable. Water body Total Area was not correlated with other metrics, however, it obtained zero values for all sites at three scales (i.e., 100, 200 and 500 m), so it was discarded from further analyzes. This left a set of seven predictor variables that were used in the models: Urban Total Area, Forest Total Area, Agriculture Total Area, Contagion index, Forest Edge Density, Forest Patch Density and Patch Richness. Here, “Forest” refers to “Tropical deciduous forest” as all metrics were calculated for this forest type class or for the combined forest class as explained above. These metrics were standardized to obtain values between 0 and 1, since they had different units (Martin et al. 2016).
Generalized additive models
Generalized additive models were performed to analyze possible associations between landscape metrics and amphibian richness, amphibian abundance and individual species abundance, as these allow greater flexibility in the modeling process by including linear and non-linear terms (Wood 2017). Because we had no hypothesis about the linearity of these relationships, we adjusted each of the seven metrics with thin plate regression splines with a maximum of eight basis dimensions, to avoid high complexity (Wood 2017). Because our dependent variables consist of species and individual counts, our data primarily fit a Poisson distribution; however, because there was overdispersion in the data, we decided to fit a negative binomial distribution with a log link function (Quinn and Keough 2002). All models were made with the gam function of the mgcv package version 1.8–36 in R (Wood 2021). For the response variables, we fit amphibian richness, amphibian abundance, and abundance of each species separately.
For the modeling process, we first fit full models with all independent variables at each scale. With these, we used the dredge function of the MuMin package in R (Barton 2012), which constructs models with all possible combinations of predictor variables and then compares them using the second-order Akaike information criterion (AICc), which is more informative when sample size is small (Akaike 1973; Burnham and Anderson 2002). Those with a ΔAICc < 2 were considered the best models (Burnham and Anderson 2002). Then, values of AIC weights were calculated for each model to determine the relative importance of each variable at each scale (Wagenmakers and Farrell 2004). Relative importance for a particular variable was defined as the sum of the AIC weight of all models where that variable was included (Rusch et al. 2011; Martin et al. 2016). The AIC weight can be interpreted as the probability that a variable will be included in the best models (Rusch et al. 2011).
In order to determine at which scales the selected landscape variables are more predictive for amphibian richness and abundance, full models (one for amphibian richness and one for total amphibian abundance) were fit using the two most supported predictors for each scale (for a total of 10 predictors for each full model), then the dredge function was used to obtain models with all possible combinations of predictor variables. Similarly, the resulting models were ordered by ΔAICc, and AIC weight was calculated for each. The relative importance of each scale was calculated by summing the AIC weight of all models where the variables of a particular scale were included. All models were performed with the software R 3.5.0 (R Core Team 2018).
Results
Land use/cover map classification accuracy
A total accuracy of 93.43% was obtained for Region A and 95.31% for Region B. In addition, the Kappa index had a value of 0.91 for Region A and 0.94 for Region B, which represents excellent accuracy according to Monserud and Leemans (1992). However, some classes had lower producer and user accuracy values, which translates as omission and commission errors in the classification. For Region A, the largest classification errors were for “Agriculture” and “Grassland/Scrubland” classes, while, for Region B, the largest classification errors were for the “Agriculture” class (Supplementary Information 1, Appendix 1, Tables S4 and S5, Regions A and B, respectively).
Amphibian richness and abundance
We observed a total of 1922 individuals belonging to eight families, 13 genera and 18 species, considering the two sampling units evaluated. We found significant differences for richness (χ2 = 167.14, df = 47, P < 0.0001) and abundance (χ2 = 3356.70, df = 47, P < 0.0001) between sampled points by Chi-Square tests. Seventeen species were observed in conserved forested areas and 16 species in degraded areas. Also, for Region A, a total of 16 species were observed, while for Region B, 17 species were observed. The species accumulation curves indicate a reasonably comprehensive sampling effort, as the curves reached a shallow slope but did not quite asymptote (Supplementary Information 1, Appendix 2, Figure S1). Richness estimators for both conserved forested areas and degraded areas, as well as the whole Region A and B, indicate that more species could be observed as less than 90% of species have been found (Supplementary Information 1, Appendix 1, Table S6).
Landscape metrics effects on amphibian richness and abundance
We found a positive linear relationship between amphibian richness and abundance and Forest Patch Density, and a negative linear relationship with both Urban Total Area and Forest Edge Density, for the 200 m scale (Fig. 2). These metrics were chosen in the best model (Table 1 and Supplementary Information 1, Appendix 1, Table S7), however, only Urban Total Area and Forest Patch Density had a relative importance value greater than 0.70 (Supplementary Information 1, Appendix 2, Figure S2). At other scales, no metric showed a statistically supported positive or negative relationship with amphibian richness and abundance, as they were not chosen in the best model (Table 1 and Supplementary Information 1, Appendix 1, Table S7) and all variables had a relative importance value less than 0.5 (Supplementary Information 1, Appendix 2, Figure S2). The most predictive scale for both amphibian richness and abundance was the 200 m scale with an AIC weight value of 0.91 and 0.89, respectively; other scales were not considered good predictors since they had relative importance values of ~ 0.5 or less (Supplementary Information 1, Appendix 2, Figure S3). At the 200 m scale, the most important metric for both dependent variables was Urban Total Area.
Generalized additive model (GAM) plots showing partial effects of selected landscape metrics on amphibian richness and abundance from studied regions in SMS, Oaxaca, Mexico. Only metrics chosen in the best model at the 200 m scale are plotted. Tick marks on the y- and x-axis are observed data points. Grey points represent partial residuals. The y-axis represents the partial effect of each variable. Shaded areas indicate 95% confidence intervals
Single-species abundance models showed that only five species had strong relationships with some of the landscape metrics at the 200 m scale. These were Agalychnis dacnicolor with a positive linear relationship with Forest Patch Density and a negative linear relationship with Urban Total Area; Eleutherodactylus pipilans with a positive linear relationship with Forest Patch Density and Patch Richness, and a negative linear relationship with Urban Total Area; and Hypopachus ustus, Leptodactylus melanonotus and Rhinella horribilis with non-linear negative relationships with Urban Total Area (Fig. 3). Interestingly, some species showed relationships with some landscape metrics at the 500 m and 1000 m scales, despite no associations found with any of the landscape metrics at these or other scales when considering amphibian richness and abundance as a whole. At the 500 m scale, these species were Lithobates forreri with a negative linear relationship with Forest Edge Density, and Rhinella horribilis with a non-linear negative relationship with Urban Total Area and a linear negative relationship with Forest Edge Density (Supplementary Information 1, Appendix 2, Figure S4). At the 1000 m scale, these species were Eleutherodactylus pipilans with a positive linear relationship with Patch Richness, and Rhinella horribilis with a non-linear negative relationship with Forest Edge Density (Supplementary Information 1, Appendix 2, Figure S5). Species did not show relationships with landscape metrics at other scales, and while some presented apparent associations, confidence intervals were too large to be considered robust models.
Generalized additive model (GAM) plots showing partial effects of selected landscape metrics on Agalychnis dacnicolor, Eleutherodactylus pipilans, Hypopachus ustus, Leptodactylus melanonotus and Rhinella horribilis abundance at the 200 m scale from studied regions in SMS, Oaxaca, México. Only plots with metrics that were chosen in the best model at 200 m scale and with an importance value greater than 0.70 are presented. Tick marks on the y- and x-axis are observed data points. Grey points represent partial residuals. The y-axis represents the partial effect of each variable. Shaded areas indicate 95% confidence intervals
Discussion
The results of this study showed that a positive linear relationship exists between amphibian richness and abundance with Forest Patch Density, partially supporting our prediction that metrics related to forest area and aggregation are the most important determinants for the amphibian community and individual species. This metric measures the aggregation of forest patches (i.e., habitat available) in a landscape, although higher values can also be interpreted as a more fragmented landscape (i.e., more smaller patches; McGarigal and Marks 1995). Since few amphibian species presented a negative relationship with Forest Edge Density, which suggest a negative effect of fragmentation, the relationship observed could mean that most amphibians species in these regions may be abundant in a heterogeneous and fragmented landscape as long as small forest patches are present. Some authors have found that amphibians can be abundant in agricultural landscapes with high crop diversity and small sized forest elements interspersed through these landscapes, which could potentially provide habitat for many amphibian species (Mendenhall et al. 2014; Collins and Fahrig 2017).
Although a negative linear relationship between amphibian richness and abundance with Forest Edge Density was observed in the most supported models at the 200 m scale, this variable presented a low relative importance value, which suggest that most amphibian species in these regions could be tolerating or benefiting from a mix of land uses. This could probably explain why one species (Eleutherodactylus pipilans) had a positive relationship with Patch Richness. Although, forest patch edges can be uninhabitable for many specialist species (Urbina-Cardona et al. 2006), some generalists may benefit if edges between two land cover types provide greater structural complexity and number of microhabitats (Knutson et al. 1999), which could be the case in our study. Two species (Rhinella horribilis and Lithobates forreri), however, had a strong negative relationship with Forest Edge Density, but only at the 500 m and 1000 m scales. This could probably mean that large scale changes in forest amount and patch size due to an increase in fragmentation, could negatively affect the abundance for some species, as habitat amount is crucial for most amphibian species (Almeida-Gomes et al. 2019). Long-term monitoring is needed to clarify this relationship in order to explore changes in abundance as well as landscape changes.
Amphibian richness and abundance had a strong negative relationship with Urban Total Area at several scales, a variable that we did not consider in our predictions. We were able to observe a linear decrease in amphibian diversity as human area, either towns, cities, or roads, increased. Other studies have shown that when urban settlements are established, there is a loss of natural covers, changes in the physical and chemical properties of water bodies, and production of air pollution, making some amphibian species go extinct at these sites as they fail to withstand such degraded conditions (Knutson et al. 1999; Canessa and Parris 2013; Treglia et al. 2018). Roads, in turn, increase mortality of adult individuals by vehicle collision (Pinto et al. 2020) and cause excessive noise, which prevents females of some species from hearing the males’ calls (Simmons and Narins 2018). Urban Total Area was the landscape metric with the highest relative importance value in the best models, so it appears to be one of the greatest determinants of amphibian richness and abundance in these regions.
Single-species models revealed different responses of some species to landscape metrics at varying scales, which could be due to differences in their life history traits (García-Llamas et al. 2019; See Supplementary Information 1, Appendix 1, Table S3). For example, Agalychnis dacnicolor positive response to Forest Patch Density could be due to the species need for water bodies and stand vegetation for reproduction (Wells 2007; Suazo-Ortuño et al. 2015). Eleutherodactylus pipilans positive response to Patch Richness could be due to the species resistance to land degradation and its reproductive mode, that allow it to reproduce without the need of water bodies (Wells 2007; IUCN 2021). All species responded negatively to Urban Total Area, despite most species being able to survive in degraded lands (See Supplementary Information 1, Appendix 1, Table S3). Since urbanized areas produce changes in the physical and chemical properties of water bodies (Canessa and Parris 2013), most species can be affected as they may need these water bodies for reproduction (See Supplementary Information 1, Appendix 1, Table S3). Considering these results, we suggest that species-specific studies including life history traits must be considered when analyzing landscape transformations effects on biodiversity.
Most associations between amphibian species and landscape metrics were observed at the 200 m scale, and some were observed at the 500 m and 1000 m scales, which support our prediction that metrics will have a greater effect on scales between 100 m and 1000 m. It is likely that no relationship was observed at the largest scale (i.e., 1500 m) because most amphibian species possess small home ranges (e.g., 0.0003–0.03 ha, Vitt and Caldwell 2014; 1.92 ha, Incilius spiculatus, Arreortúa-Martínez 2020) and perform short daily movements (e.g., 37 m, Incilius spiculatus, Arreortúa-Martínez 2020), so species are probably responding to landscape elements within their home ranges (Jackson and Fahrig 2012). For the 100 m scale, there was large measurement error due to the Landsat resolution images used (i.e., 30 m; Miguet et al. 2016) that didn’t allow models to reflect possible relationships between amphibians and landscape elements. It is important to mention that the different scales evaluated here, are actually different extents for the same grain (i.e., resolution; Turner and Gardner 2015), so we were only analyzing at which extents species-landscape relationships are strongest. Changing grain could potentially help us analyze if other landscape elements too small for our satellite images to identify, like small ponds (Ribeiro et al. 2019) or linear strips of vegetation (Biaggini and Corti 2015; Hansen et al. 2019), are also important for amphibian diversity in these regions.
Some other local variables could also be important for amphibian species in these regions. Some authors have found that relative humidity, canopy cover, understory density, leaf litter depth, percentage of bare soil, among other variables, explained part of the amphibian taxonomic and functional diversity patterns in some fragmented landscapes (Urbina-Cardona et al. 2006; Ribeiro et al. 2017). Mendenhall et al. (2014) found that countryside forest elements that are often too small for most remote sensing techniques to identify, contribute to approximately 95% of available habitat for forest-dependent amphibians. This could probably explain why the other 12 species in the community did not show associations with selected landscape metrics at any scale as some may be responding more to environmental conditions at finer scales than the ones considered here. For this reason, we recommend using high-resolution satellite imagery and measuring explanatory variables at finer scales to better discern the specific conditions that are necessary for each species (Schindler et al. 2013; Treglia et al. 2018).
Most amphibian species observed in our regions are distributed in various forest types in addition to tropical deciduous forests (see Supplementary Information 1, Appendix 1, Table S3). Since we didn’t performed surveys in other forest types and metrics couldn’t be calculated, our models are probably showing just one part of the species true response to landscape transformations (i.e., their response for one part of their distribution), as they may respond differently depending on the regional context (Miguet et al. 2016). However, analyzing species-landscape relationships along several forest types, should incorporate the measurement of other important local climatic and structural variables, such as temperature, relative humidity, canopy cover or leaf litter depth, as these can change drastically between forest types and could explain part of the species abundance variation (Urbina-Cardona et al. 2006; Ribeiro et al. 2017). An empirical study that compares species-landscape relationships between forest types could be a nice contribution that may help us determine if the species responses to landscape transformations and its scale of effect could change along their distribution, which may help us propose specific conservation measures.
Implications for conservation.
The results of our study showed that most amphibians species in these regions may be abundant in heterogeneous and fragmented landscapes as long as small forest patches are present. Nevertheless, large scale changes in forest amount and patch size due to an increase in fragmentation and urbanization, could eventually affect some species abundance in these regions, as habitat amount is crucial for most amphibian species (Almeida-Gomes et al. 2019). Since species responded differently to landscape transformations at different scales, conservation and management measures should be species-specific, although some generalization could be made. For example, a high density of relatively small forest patches (e.g., 1–5 ha) may help in the protection and conservation of most amphibian species in these regions, especially if these are interspersed along other land uses. Conserving small, interconnected forest patches may be a realistic way to maintain suitable habitats for many species, as has been observed and proposed by other authors (Ribeiro et al. 2017; Lindenmayer 2019; Wintle et al. 2019), as it simultaneously permits the development of economic activities for people living in these regions. Since landscape transformation effects on amphibian richness and abundance typically occur at small spatial scales, management measures should also contemplate other important environmental and structural variables at finer scales, such as high-quality water bodies. Future multi-scale studies should be conducted to further understand amphibian-environmental interactions in these complex Oaxacan regions of southern Mexico.
Data availability
Upon publication, requests can be made to the corresponding Author for data.
Code availability
Upon publication, requests can be made to the corresponding author for R scripts.
References
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Proceedings of the 2nd International symposium on information theory, Budapest, Hungary
Aguirre-León G (2014) Métodos de estimación, captura y contención de anfibios y reptiles. In: Gallina-Tessaro S, López-González C (eds) Manual de técnicas para el estudio de la fauna. Instituto de Ecología A.C., Veracruz, pp 63–84
Almeida-Gomes M, Vieira MV, Rocha CFD, Melo AS (2019) Habitat amount drives the functional diversity and nestedness of anuran communities in an Atlantic Forest fragmented landscape. Biotropica 51:874–884
Arreortúa-Martínez M (2020) Patrones de movimiento de Incilius spiculatus (Anura: Bufonidae) en bosque mesófilo de montaña con distinto grado de perturbación. Dissertation, Instituto Politécnico Nacional, México
Bell KE, Donnelly MA (2006) Influence of Forest Fragmentation on Community Structure of Frogs and Lizards in Northeastern Costa Rica. Conserv Biol 20:1750–1760
Barton K (2012) MuMIn: multi-model inference. R package version 1.7.11. https://cran.r-project.org/package=MuMIn
Biaggini M, Corti C (2015) Reptile assemblages across agricultural landscapes: where does biodiversity hide? Anim Biodiv Conserv 38:163–174
Benítez-Fernández FR (2018) La Importancia De Áreas De Bosque En Paisajes Urbanos Para La Estructuración De Metacomunidades De Anfibios. Dissertation, Universidad Federal de Integración Latino-Americana, Brasil
Bogaert J, Barima YSS, Waya-Mongo LI, Bamba I, Mama A, Toyi M, Lafortezza R (2011) Forest Fragmentation: Causes, Ecological Impacts and Implications for Landscape Management. In: Li C, Lafortezza R, Chen J (eds) Landscape Ecology in Forest Management and Conservation. Springer, Berlin, pp 273–296
Burnham KP, Anderson DR (2002) Model selection and multimodel inference. Springer-Verlag, New York
Cabrera-Guzmán E, Reynoso VH (2012) Amphibian and reptile communities of rainforest fragments: minimum patch size to support high richness and abundance. Biodivers Conserv 21:3243–3265
Colwell RK (2013) EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples, Software and User’s Guide, Version 9.1.0. Freeware for Windows and Mac OS. Available from http://purl.oclc.org/estimates
Canessa S, Parris KM (2013) Multi-Scale, Direct and Indirect Effects of the Urban Stream Syndrome on Amphibian Communities in Streams. PLoS ONE 8:e70262
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Meas 20:37–46
Collins SJ, Fahrig L (2017) Responses of anurans to composition and configuration of agricultural landscapes. Agric Ecosyst Environ 239:399–409
Covarrubiasa S, Gutiérrez-Rodríguez C, Rojas-Soto O, Hernández-Guzmán R, González C (2022) Functional connectivity of an endemic tree frog in a highly threatened tropical dry forest in Mexico. Ecoscience 29:69–85
Crump ML (2015) Anuran Reproductive Modes: Evolving Perspectives. J Herpetol 49:1–16
Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (2020) Cobertura del Suelo de México a 30 m. http://geoportal.conabio.gob.mx/metadatos/doc/html/nalcmsmx15gw.html. Accessed 4 March 2021
Cushman SA (2006) Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biol Conserv 128:231–240
DeVore JL, Shine R, Ducatez S (2021) Spatial ecology of cane toads (Rhinella marina) in their native range: a radiotelemetric study from French Guiana. Sci Rep-UK 11:11817
Dixo M, Metzger JP, Morgante JS, Zamudio KR (2009) Habitat fragmentation reduces genetic diversity and connectivity among toad populations in the Brazilian Atlantic Coastal Forest. Biol Conserv 142:1560–1569
Duarte-Ballesteros L, Urbina-Cardona JN, Saboyá-Acosta LP (2021) Ensamblajes de anuros y heterogeneidad espacial en un ecosistema de páramo de Colombia. Caldasia 43:126–137
Exelis Visual Information Solutions v. 5.3 (2020) ENVI software. https://www.l3harrisgeospatial.com/Software-Technology/ENVI. Accessed 5 November 2020
Espinosa D, Ocegueda-Cruz S, Luna-Vega I (2016) Introducción al estudio de la biodiversidad de la Sierra Madre del Sur: Una visión general. In: Luna-Vega I, Espinosa D, Contreras-Medina R (eds) Biodiversidad de la Sierra Madre del Sur. Universidad Nacional Autónoma de México, Ciudad de México, pp 23–36
García-García JL, Santos-Moreno A (2013) Efectos de la estructura del paisaje y de la vegetación en la diversidad de murciélagos filostómidos (Chiroptera: Phyllostomidae) de Oaxaca, México. Rev Biol Trop 62:217–239
García-Llamas P, Rangel TF, Calvo L, Suárez-Seoane S (2019) Linking species functional traits of terrestrial vertebrates and environmental filters: A case study in temperate mountain systems. PLoS ONE 14:e0211760
Google Earth v.7 (2020) High resolution images. http://earth.google.com. Accessed 4 March 2021
Hansen NA, Scheele BC, Driscoll DA, Lindenmayer DB (2019) Amphibians in agricultural landscapes: the habitat value of crop areas, linear plantings and remnant woodland patches. Anim Conserv 22:72–82
Heemeyer JL(2011) Breeding migrations, survivorship, and obligate Crayfish Burrow use by adult Crawfish frogs (Lithobates areolatus). Dissertation, Indiana State University, United States
Horan RV(2011) Evaluation and application of novel telemetry methods for the study of movements and ecology of tropical hylids. Dissertation, University of Georgia, United States
Heemeyer JL, Lannoo MJ (2012) Breeding Migrations in Crawfish Frogs (Lithobates areolatus): Long Distance Movements, Burrow Philopatry, and Mortality in a Near-Threatened Species. Copeia 3:440–450
Henrique RS, Grant T (2019) Influence of Environmental Factors on Short-Term Movements of Butter Frogs (Leptodactylus latrans). Herpetologica 75:38–46
Herrmann HL, Babbitt KJ, Baber MJ, Congalton RG (2005) Effects of landscape characteristics on amphibian distribution in a forest-dominated landscape. Biol Conserv 123:139–149
Horning N, Robinson JA, Sterling EJ, Turner W, Spector S (2010) Remote sensing for ecology and conservation: A handbook of techniques. Oxford University Press, New York
Hu Q, Wu W, Xia T, Yu Q, Yang P, Li Z, Song Q (2013) Exploring the use of Google Earth Imagery and Object-Based methods in land use/cover mapping. Remote sens 5:6026–6042
IUCN (2021) The IUCN Red List of Threatened Species. Version 2021-2. https://www.iucnredlist.org. Accessed 10 April 2022
Jackson HB, Fahrig L (2012) What size is a biologically relevant landscape? Landsc Ecol 27:929–941
Jackson HB, Fahrig L (2015) Are ecologist conducting research at the optimal scale? Global Ecol. Biogeogr 24:52–63
Jiménez-Valverde A, Hortal J (2003) Las curvas de acumulación de especies y la necesidad de evaluar la calidad de los inventarios biológicos. Rev Iber Aracnol 8:51–61
Knutson MG, Sauer JR, Olsen DA, Mossman MJ, Hemesath LM, Lannoo MJ Effects of landscape composition and wetland fragmentation on frog and toad abundance and species richness in Iowa and Wisconsin(1999) U.S.A. Conserv Biol 13:1437–1446
Koumaris A, Fahrig L (2016) Different Anuran Species Show Different Relationships to Agricultural Intensity. Wetlands 36:731–744
Lescano JN, Bellis LM, Hoyos LE, Leynaud GC (2015) Amphibian assemblages in dry forests: Multi-scale variables explain variations in species richness. Acta Oecol 65–66:41–50
Lindenmayer D (2019) Small patches make critical contributions to biodiversity conservation. P Natl Acad Sci USA 116:717–719
Luna-Gómez MI, García A, Santos-Barrera G (2017) Spatial and temporal distribution and microhabitat use of aquatic breeding amphibians (Anura) in a seasonally dry tropical forest in Chamela. Mexico Rev Biol Trop 65:1082–1094
McGarigal K, Marks B(1995) FRAGSTATS: spatial pattern analysis program for quantifying landscape structure. USDA Forest Service General Technical Report PNW-351. Corvallis, Oregon, U.S.A
Magurran AE, McGill BJ (2011) Biological Diversity. Oxford University Press, New York
Martin AE, Fahrig L (2012) Measuring and selecting scales of effect for landscape predictors in species–habitat models. Ecol Appl 22:2277–2292
Martin EA, Seo B, Park CR, Reineking B, Steffan-Dewenter I (2016) Scale-dependent effects of landscape composition and configuration on natural enemy diversity, crop herbivory, and yields. Ecol Appl 26:448–462
Mata-Silva V, García-Padilla E, Rocha A, Desantis DL, Johnson JD, Ramírez-Bautista A, Wilson LD (2021) A reexamination of the Herpetofauna of Oaxaca, Mexico: Composition Update, Physiographic Distribution, and conservation Commentary. Zootaxa 4996:201–252
McGarigal K, Cushman SA (2005) The gradient concept of landscape structure. In: Wiens JA, Moss MR (eds) Issues and Perspectives in Landscape Ecology. Cambridge University Press, Cambridge, pp 112–119
McGarigal K, Wan HY, Zeller KA, Timm BC, Cushman SA (2016) Multi-scale habitat selection modeling: a review and outlook. Landsc Ecol 31:1161–1175
Mendenhall CD, Frishkoff LO, Santos-Barrera G, Pachecho J, Mesfun E, Mendoza-Quijano F, Ehrlich PR, Ceballos G, Daily GC, Pringle RM (2014) Countryside biogeography of neotropical reptiles and amphibians. Ecology 95:856–870
Miguet P, Jackson HB, Jackson ND, Martin AE, Fahrig L (2016) What determines the spatial extent of landscape effects on species? Landsc Ecol 31:1177–1194
Monserud RA, Leemans R (1992) Comparing global vegetation maps with the Kappa statistic. Ecol Model 62:275–293
Moraga AD, Martin AE, Fahrig L (2019) The scale of effect of landscape context varies with the species’ response variable measured. Landsc Ecol 34:703–715
Naturalista(2021) Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. http://www.naturalista.mx. Accessed 11 April 2022
Osorio-Olvera L, Lira-Noriega A, Soberón J, Peterson AT, Falconi M, Contrera-Díaz RG, Martínez-Meyer E, Barve V, Barve N (2020) ntbox: An r package with graphical user interface for modelling and evaluating multidimensional ecological niches. Methods Ecol Evol 11:1199–1206
Pardini R, Nichols E, Püttker T (2017) Biodiversity response to habitat loss and fragmentation. Encyclopedia of the Anthropocene 3:229–239
Peterson AC, Richgels KLD, Johnson PTJ, McKenzie VJ (2013) Investigating the dispersal routes used by an invasive amphibian, Lithobates catesbeianus, in human-dominated landscapes. Biol Invasions 15:2179–2191
Pinto FAS, Clevenger AP, Grilo C (2020) Effects of roads on terrestrial vertebrate species in Latin America. Environ Impact Assess 81:106337
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologist. Cambridge University Press, Cambridge
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Ramírez-González CG(2016) Anfibios de Oaxaca: Riqueza y distribución. Dissertation, Instituto Politécnico Nacional, México
Ribeiro J, Colli GR, Batista R, Soares A (2017) Landscape and local correlates with anuran taxonomic, functional and phylogenetic diversity in rice crops. Landsc Ecol 32:1599–1612
Ribeiro J, Colli GR, Soares A (2019) Landscape correlates of anuran functional connectivity in rice crops: A graph-theoretic approach. J Trop Ecol 35:118–131
Rusch A, Valantin-Morison M, Sarthou JP, Roger-Estrade J (2011) Multi-scale effects of landscape complexity and crop management on pollen beetle parasitism rate. Landsc Ecol 26:473–486
Sawatzky ME, Martin AE, Fahrig L (2019) Landscape context is more important than wetland buffers for farmland amphibians. Agric Ecosyst Environ 269:97–106
Schindler S, von Wehrden H, Poirazidis K, Wrbka T, Kati V (2013) Multiscale performance of landscape metrics as indicators of species richness of plants, insects and vertebrates. Ecol Indic 31:41–48
Schneider-Maunourya L, Lefebvre V, Ewers RM, Medina-Rangel GF, Peres CA, Somarriba E, Urbina-Cardona N, Pfeifer M (2016) Abundance signals of amphibians and reptiles indicate strong edge effects in Neotropical fragmented forest landscapes. Biol Conserv 200:207–215
Semlitsch RD, Bodie JR (2003) Biological Criteria for Buffer Zones around Wetlands and Riparian Habitats for Amphibians and Reptiles. Conserv Biol 17:1219–1228
Simmons AM, Narins PM (2018) Effects of anthropogenic noise on amphibians and reptiles. In: Slabbekoorn H, Dooling R, Popper A, Fay R (eds) Effects of Anthropogenic Noise on Animals. Springer Handbook of Auditory Research, New York, pp 179–208
Suazo-Ortuño I, Alvarado-Díaz J, Mendoza E, López-Toledo L, Lara-Uribe N, Márquez-Camargo C, Paz-Gutiérrez JG, Rangel-Orozco JD (2015) High resilience of herpetofaunal communities in a human-modified tropical dry forest landscape in western Mexico. Trop Conserv Sci 8:396–423
Tozetti AM, Toledo LF (2005) Short-Term Movement and Retreat Sites of Leptodactylus labyrinthicus (Anura: Leptodactylidae) during the Breeding Season: A Spool-and-Line Tracking Study. J Herpetol 39:640–644
Treglia ML, Landon AC, Fisher RN, Kyle G, Fitzgerald LA (2018) Multi-scale effects of land cover and urbanization on the habitat suitability of an endangered toad. Biol Conserv 228:310–318
Turner MG, Gardner RH (2015) Landscape Ecology in Theory and Practice: Pattern and Process. Springer, New York
Urbina-Cardona JN, Olivares-Pérez M, Reynoso VH (2006) Herpetofauna diversity and microenvironment correlates across a pasture–edge–interior ecotone in tropical rainforest fragments in the Los Tuxtlas Biosphere Reserve of Veracruz, Mexico. Biol Conserv 132:61–75
Vitt LJ, Caldwell JP (2014) Herpetology: An Introductory Biology of Amphibians and Reptiles. Academic Press, London
Wagenmakers EJ, Farrell S (2004) AIC model selection using Akaike weights. Psychon B Rev 11:192–196
Wells KD (2007) The ecology and behavior of amphibians. The University of Chicago Press, Chicago
Wiens JA (1989) Spatial Scaling in Ecology. Func Ecol 3:385–397
Wintle BA, Kujala H, Whitehead A, Cameron A, Veloz S, Kukkala A, Moilanen A, Gordon A, Lentini PE, Cadenhead NCR, Bekessy SA (2019) Global synthesis of conservation studies reveals the importance of small habitat patches for biodiversity. P Natl Acad Sci USA 116:909–914
Wood SN (2017) Generalized additive models: An Introduction with R. CRC Press, Florida
Weiß CH StatSoft Inc, Tulsa OK(2007) : STATISTICA, Version 8. AStA. Adv Stat Anal 91:339–341
Wood SN(2021) Package mgcv. R package version 1.8–36. https://cran.r-project.org/web/packages/mgcv/mgcv.pdf
Acknowledgements
We thank the Posgrado de Ciencias Biológicas from the Universidad Nacional Autónoma de México for providing permissions and facilities to DGRA for the realization of the doctoral thesis on which this manuscript is based. We thank the Consejo Nacional de Ciencia y Tecnología (CONACYT) for financial support to perform this research, grant PN2271 to LMOO, and PhD grant to DGRA. We also thank the Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT) for providing the collection permit FAUT-0317, used for the amphibian specimens in this study, granted to LMOO. We are thankful to Gerardo Soria Ortiz, Rafael Peralta Hernández and Rigomar López Gómez for their support during fieldwork. We thank Alejandra Selene Membrillo Abad and Lizbeth González Gómez for their assistance and suggestions regarding image classification. We also thank Carlos Martorell Delgado for his assistance and suggestions in performing generalized additive models and statistical analysis. Finally, we express our special thanks to Brett O. Butler for English proofing this manuscript.
Funding
PhD Grant and Grant PN2271 came from Consejo Nacional de Ciencia y Tecnología (CONACYT), México, Granted to DGRA and LMOO, respectively.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design; DGRA and LMOO defined the sampling design and collected the data; DGRA and LMOO contribute to the analysis of the data; DGRA led the writing of the manuscript; and all authors contributed to the draft and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest nor competing interest.
Ethical approval
Specimens’ reference collections were obtained with collection permit FAUT-0317 issued to the corresponding author of this paper. A maximum of two voucher specimens per species per site were obtained to prevent over-collecting. The project collection protocols used in this work were evaluated and found to comply with the standards approved by the Ethics and Scientific Responsibility Commission (CEARC, Comisión de Ética y Responsabilidad Científica), of the Facultad de Ciencias, UNAM.
Consent to participate
All authors agree in participating in this manuscript.
Consent for publication
All authors agree with the publication of this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramírez-Arce, D.G., Ochoa-Ochoa, L.M. & Lira-Noriega, A. Effect of landscape composition and configuration on biodiversity at multiple scales: a case study with amphibians from Sierra Madre del Sur, Oaxaca, Mexico. Landsc Ecol 37, 1973–1986 (2022). https://doi.org/10.1007/s10980-022-01479-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-022-01479-9