Abstract
Context
To detect an effect of landscape context on a species’ response, the landscape variables need to be measured within the appropriate distance from the species’ response, i.e. at the scale of effect. However, it is not clear what factors determine the scale of effect.
Objective
Our objective was to test the prediction that the scale of effect should be smallest when the species’ response is fecundity, larger for abundance, and larger still for occurrence.
Methods
We compared the scale of effect of two landscape variables (road density, forest proportion) on the three responses (fecundity, abundance, occurrence) for the wood frog (Lithobates sylvaticus) in eastern Ontario, Canada. We used egg mass surveys of 34 ponds to estimate fecundity (mean eggs/mass), abundance (number of masses), and occurrence (presence/absence of egg masses). We then empirically estimated the scale of effect of each landscape variable on each response.
Results
The scale of effect differed among responses, from 0.2 to 3.0 km radii; however, it did not vary in the predicted order. Furthermore, the order was not consistent between the two landscape variables.
Conclusions
Our results show that the scale of effect of a landscape variable on a given species can differ for different response variables. However, they also suggest that these differences in the scale of effect are not predictable. Thus, the most reliable way to ensure a landscape context study is conducted at the correct spatial extent is to estimate the scale of effect empirically, rather than ‘guesstimating’ the extent a priori.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Landscape context, i.e. the amount and spatial pattern of land cover types surrounding a given site, can have important effects on a species’ response (e.g. abundance, occurrence) at that site (Thornton et al. 2011). For example, the abundance and occurrence of anurans (frogs and toads) in ponds is influenced by the amount of forest, the density of roads, the mean size of crop fields, and the proportion of different crop types in the landscapes surrounding the ponds (Eigenbrod et al. 2008; Koumaris and Fahrig 2016; Collins and Fahrig 2017). The abundances and occurrences of birds and bats are influenced by the amount and fragmentation of forest in landscapes surrounding forest sites (Smith et al. 2011; Ethier and Fahrig 2011). In urban environments, the abundances and occurrences of birds, lichens, and bats are influenced by the amount of green space in the landscapes surrounding sample sites (Coffey and Fahrig 2012; Smith et al. 2014; Moretto 2018).
Our ability to detect and estimate these landscape context effects depends on measuring the landscape variables (e.g. forest amount, road density) at the ‘right’ spatial extent around the sample sites. The spatial extent, or range of extents, within which a landscape variable has its strongest effect(s) on a particular species’ response has been called the scale of effect of that variable on that species (Jackson and Fahrig 2012). The scale of effect varies widely across species, from 0.01 to 100 km (Jackson and Fahrig 2015). Estimates of the scale of effect can also vary widely for a given species. For example, Moretto (2018) found a very small scale of effect (about 0.2 km radius) of tree cover on bat abundance in an urban setting, while Ethier and Fahrig (2011) found the scale of effect was much larger, between 1 and 5 km radius around sample sites, for the same bat species in a rural setting. The scale of effect of the landscape on a given species can also vary with the landscape variable measured. For example, striped skunk occurrence at a site is related most strongly to forest cover within 0.45 km, but to wetland cover, crop cover, and edge density within ≥ 3.45 km (Martin and Fahrig 2012).
Measuring landscape context effects within the appropriate spatial extent, i.e. at the scale of effect, is important not only for correctly identifying landscape context effects on particular species but also for testing ecological hypotheses. For example, Holland et al. (2005a) found strong support for a predicted negative relationship between beetle species’ reproductive rates and extinction thresholds (minimum habitat amount needed for species occurrence) when the extinction threshold for each species was estimated at its scale of effect. Scale of effect varied from 0.02 to 1.6 km (radius) across species. In contrast, when a common landscape size (1 km radius) was used to estimate the extinction thresholds for all species, Holland et al. (2005a) found no relationship between species’ reproductive rates and extinction thresholds. Thus, estimating landscape context effects at the appropriate spatial extent can be critical for making reliable inferences about ecological relationships.
Despite the importance of measuring landscape variables at the scale of effect, most studies have used a single spatial extent for studying landscape context effects, where the spatial extent chosen is either arbitrary or loosely based on information about the home range size or the dispersal distance of the organism. Even in studies that estimate landscape context effects at multiple spatial extents, only 29% used a biological rationale (home range size or dispersal distance) to select the range of spatial extents evaluated (Jackson and Fahrig 2015). It therefore seems likely that landscape context effects are often under-estimated or mis-identified due to inappropriate selection of the landscape extent within which a landscape variable is measured.
This leads to the question: can we do a better job selecting the landscape extent, or the range of landscape extents, in studies of landscape context effects? As already mentioned, studies that provide a biologically-based rationale for the selected landscape extent generally use a rationale based on the movement ecology of the species (Jackson and Fahrig 2015). This choice has some support in the literature. A simulation study (Jackson and Fahrig 2012) predicted that the scale of effect of habitat amount on abundance is ~ 4–9 times the median dispersal distance or ~ 0.3–0.5 times the maximum dispersal distance of a species. In addition, empirical studies have found that the scale of effect was larger for larger wood-boring beetle species (Holland et al. 2005b) and for larger bird species (Thornton and Fletcher 2014) relative to smaller species, possibly because larger species have larger movement ranges. On the other hand, in a review of multi-scale empirical studies, Jackson and Fahrig (2015) did not find a relationship between the scale of effect and species movement range.
This lack of strong support for a predictive relationship between a species’ movement range and its scale of effect could be due to a variety of other factors that might influence the scale of effect, thus muddying the relationship between movement range and the scale of effect (Miguet et al. 2016). As mentioned above, the scale of effect appears to depend on the particular context of the study (e.g. urban vs. rural) and the particular landscape variable measured (e.g. wetland vs. forest cover). Miguet et al. (2016) also hypothesized that the scale of effect could be influenced by species interactions. For example, the scale of effect of the landscape context on a prey species may be determined or affected by the scale of effect of the landscape context on its predators. And, they hypothesized that the scale of effect might be larger for species with lower reproductive rates. If all of these factors are simultaneously in play, this might explain the generally weak relationship between the scale of effect and movement range found by Jackson and Fahrig (2015).
In addition to the factors mentioned above, Miguet et al. (2016) also hypothesized that the scale of effect of a landscape variable on a species should depend on the temporal scale over which the landscape context effect occurs, and that this should depend on the particular species’ response variable measured. This idea was supported in a simulation study in which, for a given species, the scale of effect of habitat amount on population abundance was much smaller than the scale of effect of habitat amount on occurrence, which was in turn much smaller than the scale of effect of habitat amount on genetic diversity (Jackson and Fahrig 2014). Following the logic from the simulations, Miguet et al. (2016) hypothesized that the scale of effect on fecundity should be even smaller than the scale of effect on abundance. The argument is that fecundity is mainly affected by within-territory resource acquisition during a single season; thus, fecundity should be most strongly affected by the landscape context within the species’ foraging range. Abundance is affected not only by conditions within the foraging range that influence reproduction and mortality, but also by immigration and emigration. Thus, abundance should be affected by the landscape context at a larger spatial extent than fecundity, i.e. an extent that encompasses the dispersal distance of the species. Occurrence, which depends on extinction-colonization dynamics over a number of generations, should be influenced by the landscape over an even larger spatial extent that encompasses the landscape context experienced by multiple generations of dispersers. Finally, genetic diversity plays out over even more generations and therefore over an even larger distance. Martin (2018) reviewed support for the prediction that the scale of effect for a given species (or species group) increases in the order: fecundity < abundance < occurrence < genetic diversity. In most cases (70%) the scale of effect was different for different response variables, but overall the difference was not consistently in the predicted direction. Martin (2018) suggested that the lack of support for the prediction may be due to inaccurate estimation of the scale of effect of the landscape variable on a species’ response, because the range of scales included in most studies was too small to encompass the true scale of effect for each response.
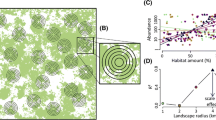
In this study we used estimates of wood frog (Lithobates sylvaticus) fecundity, abundance, and occurrence in ponds to test the prediction that the scale of effect of the landscape context on fecundity should be smaller than the scale of effect on abundance, which in turn should be smaller than the scale of effect on occurrence. We included two landscape variables—road density (km/km2) and the proportion of the landscape in forest (‘forest proportion’)—to evaluate whether the predicted order of the scale of effect (fecundity < abundance < occurrence) is consistent across different landscape variables. The wood frog is an ideal test species for this prediction because it is relatively abundant in eastern Ontario, its egg masses are relatively easy to identify and count, and its populations are known to respond negatively to road density and positively to forest proportion in the surrounding landscape (Findlay et al. 2001; Eigenbrod et al. 2009). For each of the three response variables (fecundity, abundance, and occurrence) and each of the two predictor variables (road density and forest proportion) we estimated the scale of effect using a multi-scale focal sample site study design (Brennan et al. 2002). This involves: (1) measuring the species’ responses (here, fecundity, abundance, and occurrence) at a set of sample sites (here, ponds) that vary in the landscape variables of interest (here, road density and forest proportion) in the surrounding landscapes (Fig. 1a); (2) measuring the landscape variables within multiple spatial extents centered on each sample site (Fig. 1a); (3) estimating the strength of relationship between the species’ response and landscape variable within each spatial extent (Fig. 1b); and (4) comparing the strength of relationship across spatial extents and selecting the spatial extent within which the relationship is strongest (Fig. 1c).
Example showing how to empirically estimate the scale of effect using a multi-scale focal sample site study design. a Measure a species’ response (e.g. abundance) at a set of sample sites that vary in the landscape variable of interest (e.g. forest proportion) in the surrounding landscapes. Measure the landscape variable within multiple spatial extents centered on each sample site. b Estimate the strength of relationship (e.g. Akaike Information Criterion; AIC) between the response and the landscape variable for each spatial extent. c Compare the strength of relationship across spatial extents and select the spatial extent, or range of extents, within which the relationship is strongest (e.g. the extent with the smallest AIC)
Methods
Site selection and landscape variables
We selected 34 ponds to represent the range of variability in road density within eastern Ontario (0.13–6.54 km/km2; Fig. 2). For pond selection, road density was estimated within 1 km of the center of each pond. We limited our initial selection of ponds to those having a road within 0.5 km and forest near the pond, to allow access to the pond for sampling and to maximize the probability of wood frog occurrence, respectively. We note that, although landscapes were not explicitly selected to minimize the correlation between our two landscape variables, the |Pearson correlation| was ≤ 0.26 within all spatial extents. The pond data and forest data were provided by the Ontario Ministry of Natural Resources and Forestry (https://www.ontario.ca/page/land-information-ontario; contains information licensed under the Open Government Licence—Ontario). The road data were from Statistics Canada (2011 Census; https://www12.statcan.gc.ca/census-recensement/2011/geo/RNF-FRR/index-eng.cfm).
To test our prediction, we needed to measure each landscape variable—road density and forest proportion—within multiple extents, over a wide enough range to encompass the scale of effect for all responses, and with short enough distances between tested spatial extents to pinpoint the scale of effect. Thus, we chose to measure road density and forest proportion within each of 30 nested extents from 0.1 to 3.0 km radii (areas of 0.03–28.3 km2), in 0.1 km increments. We chose a minimum spatial extent that was within the range of home range sizes identified by Blomquist and Hunter (2010) and Groff et al. (2017). We chose a maximum extent to ensure that we included extents well beyond the dispersal range of the wood frog. Estimates of mean dispersal distances for the wood frog range from approximately 0.5 km (Groff et al. 2017) to 1 km (Berven and Grudzien 1990), which suggests that our maximum extent is likely 3–6 times the mean wood frog dispersal distance.
Wood frog fecundity, abundance, and occurrence
We surveyed for egg masses two times at each pond, once between April 12 and April 29, 2016 and again between April 29 and May 17, 2016. The wood frog begins breeding during a period of warmth after a heavy rain, sometime between late winter (in February or March) and early spring (in April or May; Berven 1982, 1990; Browne et al. 2009). We surveyed three ponds per day, for 20 min to 2 h per survey depending on pond size and the number of egg masses present. All surveys were conducted between 0900 and 1800 h. We searched the ponds for egg masses by looking through the water’s surface using polarized sunglasses. We started from the southern corner of the pond and walked towards the northern corner of the pond moving back and forth across the pond until the entire pond had been surveyed. We used a double observer method to allow for estimates of detectability across observers (Grant et al. 2005). The pond was divided in half and Observer 1 surveyed the first half for egg masses while Observer 2 recorded the masses found by Observer 1 and noted any masses missed by Observer 1. The observers then switched roles for the second half of the pond. Detectability was estimated for each observer in each survey as
where nunobserved = the number of egg masses not discovered by the primary observer (i.e. Observer 1 in the first half or Observer 2 in the second half of the pond), and nobserved = the number of egg masses discovered by the primary observer (Grant et al. 2005). We then estimated the detectability for each observer across all the pond surveys in which the observer participated. Detectability was highly consistent across observers (see Results).
When an egg mass was found by the primary observer, the egg mass was gently lifted from the water and placed in an Ovagram (Karraker 2007; Fig. 3), which consisted of a flat-bottom plastic basin that contained the mass, and a flat-bottom glass basin which was used to gently compress the egg mass from above until individual eggs were distinguishable. A photograph was then taken of the compressed egg mass, using an Olympus Stylus TG-4 camera. The number of eggs per mass was estimated from the photo in ImageJ (Schindelin et al. 2015; Moraga and Pervin 2018), using the “Multi-point” feature to manually place a point on each egg in the photo. The software then tabulated the number of points present in the photo.
Wood frog fecundity, abundance, and occurrence were estimated from the egg mass surveys for each pond. To avoid non-independence of observations, we used the egg mass survey data from the survey with the most masses to estimate fecundity and abundance. This is because we could not know whether or not an egg mass present during the second survey was counted during the first survey. Fecundity was the mean number of eggs per egg mass from the survey. Abundance was indexed as the number of egg masses (Crouch and Paton 2000; Grant et al. 2005; Raithel et al. 2011). Occurrence was the presence/absence of egg masses, where the wood frog was considered ‘present’ if we found at least one egg mass in at least one of the surveys of the pond.
Potentially confounding variables
We measured nine additional variables that may influence wood frog fecundity, abundance, or occurrence at each sample pond. We intended to include variables that were correlated with our species’ response variables in the statistical models used to select the scale of effect (see Statistical analysis, below). This was to avoid erroneously selecting a scale of effect where the apparent landscape context effect is not actually due to the landscape variable but rather to the effect of another important variable that happens to be strongly correlated with the landscape variable within that particular extent.
The first potentially confounding variable we measured was the Julian date of sampling. The remaining eight variables indexed different aspects of the local habitat quality at the sampled pond: dissolved oxygen, electrical conductivity, temperature, pH, vegetative cover (emergent plus submerged), pond depth, pond perimeter, and pond area. Each of the local habitat variables was measured once per survey. Water quality can influence the survival of developing embryonic and larval wood frog (Babbitt et al. 2006). Attachment sites for egg masses are found in submerged and emergent vegetation, with egg masses unlikely to be found in ponds with no such vegetation (Grant et al. 2005). Pond size, perimeter, and depth can influence wood frog survival (Rowe and Dunson 1995), for example, because larger ponds are more likely to support predatory fish than smaller ponds (Raithel et al. 2011). We measured dissolved oxygen (mg/L) using a LAQUA D-75 probe at approximately 15 cm below the water surface and 1–2 m from the pond edge. Electrical conductivity (µS), temperature (°C), and pH were measured with a Hannah Instruments probe at approximately 5 cm below the water surface and 1–2 m from the pond edge. We visually classified the vegetative cover (emergent plus submerged) in each pond on a five-point scale—0 = 0%, 1 = 1–10%, 2 = 11–25%, 3 = 26–50%, 4 = 51–75%, or 5 = ≥ 76%—as per Grant et al. (2005). We measured pond depth (cm) using a meter stick placed in the deepest area of the pond. To estimate pond area and perimeter we walked the entire perimeter of each pond and saved the walked route on a Garmin GPSMAP 64st, and later calculated area and perimeter in ArcMap (ESRI). Note that we did not include observer identity as a potential confounding variable because our detectability analysis indicated very high consistency in detectability across observers (see Results), and any variation due to observer identity was further minimized by the fact that each pond was simultaneously surveyed by two observers.
Statistical analysis
The number of sites included in our analyses varied based on the response. Occurrence (presence-absence of egg masses) was measured at all 34 ponds. Abundance (number of egg masses) was measured at the 21 ponds where egg masses were found. For fecundity, we could not measure the number of eggs per mass if individuals had begun emerging by the time the mass was found, because the number of missing individuals was unknown. Thus, fecundity was only estimated at the 17 ponds where at least one intact egg mass was found.
We estimated the scale of effect six times, once for each combination of the species’ response (fecundity, abundance, occurrence) and landscape variable (road density, forest proportion). To estimate the scale of effect for each combination, we modeled the relationship between the response and one landscape variable within each of the 30 spatial extents (i.e. road density within 0.1 km, road density within 0.2 km, etc.). Thus, the scale of effect of road density on a species’ response was estimated independently of the scale of effect of forest proportion. We used general linear models to estimate the effect of each of the two landscape variables on fecundity, generalized linear models with a negative binomial distribution and log link to estimate the effects of the landscape variables on abundance, and generalized linear models with a binomial distribution and logit link to estimate the effects of the landscape variables on occurrence. We then estimated the scale of effect for each predictor-response combination as the spatial extent with the smallest AIC. We note that we did not model non-linear relationships between the response and landscape variable when selecting the scale of effect because visual examination of the raw data did not suggest the relationships were non-linear.
We tested for positive spatial autocorrelation in the residuals for each response-predictor combination at each spatial extent, i.e. whether similarity in residual values declined with distance between the sampling sites. To do so we used a one-tailed Global Moran’s I, with a permutation approach (1000 permutations) to calculate the significance level. Residuals were considered spatially autocorrelated at p < 0.05.
To determine if the potential confounding variables affected our selection of the scale of effect, we repeated the above analysis, with the exception that here we modeled the relationship between the response and landscape variable within each spatial extent while controlling for the potentially confounding variable that was most strongly related to that response. We included only the single most important potentially confounding variable in each case, rather than multiple variables, because of our limited sample size (17–34 landscapes per analysis). For fecundity and abundance, we identified the most important potentially confounding variable based on Pearson correlations between the response and each of the potentially confounding variables. For occurrence, we used Nagelkerke’s R2 from a logistic regression of the relationship between egg mass presence-absence and each potentially confounding variable.
We used bootstrapping to estimate the uncertainty around the selected scale of effect. For each predictor-response combination we randomly re-sampled the data from n ponds, with replacement, from the set of n surveyed ponds, 1000 times, where n = 17, 21, and 34 for analyses of fecundity, abundance, and occurrence, respectively (see above). We then estimated the scale of effect for each resampled data set, as described above. Finally, we summed the total number of times (out of 1000) that each spatial extent was selected as the scale of effect.
All analyses were conducted in R (R Core Team 2018), using the ‘MASS’ package for generalized linear models with a negative binomial distribution (Venables and Ripley 2002), the ‘ape’ package for Global Moran’s I tests (Paradis and Schliep 2019), and the ‘fmsb’ package to calculate Nagelkerke’s R2 (Nakazawa 2018).
Results
We observed the wood frog at 21 of the 34 sampled ponds. We found between 1 and 401 wood frog egg masses per occupied pond. Mean fecundity ranged from 266 to 1220 eggs per mass. Detectability of egg masses ranged from 95 to 100% across observers.
The estimated scale of effect did not vary in the order we predicted (i.e. fecundity < abundance < occurrence). The scale of effect of road density was smallest for abundance (0.4 km), intermediate for occurrence (0.7 km), and largest for fecundity (2.1 km; Fig. 4). The scale of effect of forest proportion was smallest for fecundity (0.2 km), intermediate for occurrence (0.4 km), and largest for abundance (3.0 km; Fig. 4). All Global Moran’s I tests for positive spatial autocorrelation of model residuals were non-significant (Online Resource 1).
Comparison of the model support for the effect of each landscape variable (road density, forest proportion) on each species’ response (fecundity, abundance, occurrence), at each of 30 spatial extents. We used general linear models to estimate the effect of each of the two landscape variables on fecundity, generalized linear models with a negative binomial distribution and log link to estimate the effects of the landscape variables on abundance, and generalized linear models with a binomial distribution and logit link to estimate the effects of the landscape variables on occurrence. The estimated scale of effect (arrow) is the spatial extent with the smallest AIC. Grey shading is used to identify all spatial extents with substantial support, i.e. ∆AIC ≤ 2
Our conclusions did not depend on whether we controlled for potentially confounding variables. The scale of effect was the same when we did and did not control for the most influential potentially confounding variable for five of the six predictor-response combinations (Online Resource 2). The only exception was that the estimated scale of effect of forest proportion on abundance was much smaller (0.1 vs. 3.0 km) when we included the most influential potentially confounding variable (vegetative cover; Online Resource 2). However, the order of the scale of effect also did not vary as predicted in this analysis. The scale of effect of forest proportion was smallest for abundance, intermediate for fecundity, and largest for occurrence (Online Resource 2).
Our bootstrapping analysis suggested substantial uncertainty in the selected scale of effect for most of our predictor-response combinations (Fig. 5). For example, although the best road density-fecundity model occurred within the 2.1 km extent, the selected scale of effect ranged from 0.1 to 3.0 km for the 1000 bootstrapped analyses for this predictor-response combination (Fig. 5a). Moreover, in one case, the most frequently selected scale of effect based on the bootstrapped analysis was different from the scale of effect that was selected based on the lowest AIC (Fig. 5e).
Uncertainty around the estimated scale of effect, estimated by bootstrapping. For each predictor-response combination we randomly re-sampled the data from n ponds, with replacement, from the set of n surveyed ponds, 1000 times, where n = 17, 21, and 34 for analyses of fecundity, abundance, and occurrence, respectively. We then estimated the scale of effect for each resampled data set. Plotted is the total number of times (out of 1000) that each spatial extent was selected as the scale of effect. The estimated scale of effect (arrow) is the spatial extent with the smallest AIC for the complete data set (see Fig. 4)
While our results did not support our prediction, and the estimated scale of effect was often very uncertain, the predictor-response relationships at their estimated scale of effect did conform to our expectations for abundance and occurrence. Specifically, we found significantly lower abundance and probability of occurrence in landscapes with greater road density than in landscapes with lower road density (abundance: Nagelkerke’s R2 = 0.32, p = 0.002; occurrence: Nagelkerke’s R2 = 0.22, p = 0.04). We also found (significantly) greater abundance and (non-significantly) higher probability of occurrence in landscapes with more forest than in landscapes with less forest (abundance: Nagelkerke’s R2 = 0.47, p = 0.002; occurrence: Nagelkerke’s R2 = 0.12, p = 0.10). However, we found the opposite relationships between fecundity and our landscape variables, i.e. significantly higher fecundity in landscapes with greater road density (R2 = 0.28, p = 0.03) and lower fecundity in landscapes with more forest (R2 = 0.53, p = 0.001).
Discussion
Our results do not support the predicted order of the scale of effect—fecundity < abundance < occurrence—for either landscape variable. Instead, for road density the order of the scale of effect was abundance < occurrence < fecundity, and for forest proportion the order was fecundity < occurrence < abundance. This result is consistent with results from a review of multi-scale studies (Martin 2018) which found that, while the scale of effect does vary with the response variable, it does not vary in a predictable order.
Martin (2018) speculated that failure to support the prediction might be due to inadequacies in study design in the majority of studies reviewed; studies with stronger designs were more likely to be consistent with the prediction (i.e. the scale of effect increases in the order: fecundity < abundance < occurrence). However, study design issues cannot explain why we found no support for the prediction here. In particular, Martin (2018) suggested that in most studies the range of extents analyzed—smallest to largest—was too narrow, and the distance between tested extents was too wide to accurately estimate the scale of effect. Our study design explicitly avoided these problems. We measured spatial extents from 0.1 to 3.0 km and used a fine resolution of extents in 0.1 km increments to accurately estimate the scale of effect. Moreover, only one of the six predictor-response relationships we tested was strongest at the smallest or largest tested extent. Thus it is likely that the true scale of effect was between the smallest and largest extents measured for at least five of the predictor-response relationships.
Our predicted order of the scale of effect (Miguet et al. 2016) was based on simulations (Jackson and Fahrig 2014), and on the well-established linkage between temporal and spatial scaling in ecology (Levin 1992). That we did not find support for our prediction might suggest that we incorrectly inferred the temporal scale (and therefore the spatial scale) of landscape context effects on the responses. This could occur if feedbacks between responses influence the manifested scale of effect. For example, the fact that fecundity was positively related to road density and negatively related to forest proportion—in contrast to the negative effect of road density on abundance and the positive effect of forest proportion on abundance—could indicate a negative density-dependent effect of abundance on fecundity. This would obscure the expected difference between the two in their temporal scales of regulation. A negative density-dependent effect of abundance on fecundity is supported by an experimental study examining wood frog populations, which found that higher densities lead to delayed onset of sexual maturity and smaller individual egg masses (Harper and Semlitsch 2007). However, this explanation seems unlikely. If the effects of the landscape variables on fecundity are indirect effects through their effects on population density, then we should have seen the same scale of effect of these landscape variables for both abundance and fecundity. We did not see this.
A second possible reason for finding results that do not support the predicted order of scale of effect relates to low variation in the strength of some predictor-response relationships across spatial extents. For example, there was only ∆AIC = 2.36 between the most and least supported models relating occurrence to forest proportion (Fig. 4f). This suggests a similar level of statistical support across extents, either because the landscape variable is a poor predictor of the species’ response or the response is not scale-dependent (Martin and Fahrig 2012). This would insert a strong element of uncertainty in the estimated scale of effect. However, we argue this explanation is unlikely. The fact that there is a range of similarly-supported spatial extents (i.e. extents with ∆AIC ≤ 2) does not affect our conclusion. For road density, it is clear that our prediction is also not supported if we compare the ranges of similarly-supported spatial extents across the species’ responses; i.e. all supported extents for fecundity are much larger than for occurrence (Fig. 4a, c). Bootstrap estimates of uncertainty also suggest this explanation is unlikely. In particular, bootstrapping revealed that the estimated scale of effect of forest proportion on abundance is highly uncertain, showing a bimodal distribution (Fig. 5e). If we assume the smaller scale is accurate, this would place the scale of effect in our predicted order (fecundity < abundance < occurrence). However, the differences in estimated scales would then be very small (0.2, 0.3, and 0.4 km respectively), each with high local uncertainty (Fig. 5d–f). Thus, low variation in the strength of the predictor-response relationship across scales does not appear to explain our lack of support for the predicted order of the scale of effect.
Although the order of the scale of effect was not consistent with our predicted order, the scale of effect did vary substantially depending on the response variable measured, and the order differed between the two landscape variables. Previous studies have also found differences in scale of effect for amphibian species depending on the response variable measured. For example, a study of urbanization effects on the spotted salamander (Ambystoma maculatum) found that species occurrence was best predicted with a model that contained road length measured within 1 km, whereas population abundance was best predicted with a model that contained road length measured within 0.3 km (Clark et al. 2008). A study of forest effects on the European common frog (Rana temporaria), found species occurrence was most strongly affected by forest proportion within 0.4 km, while population abundance was most strongly affected by forest proportion within 1 km (Boissinot et al. 2015). Our finding of a different scale of effect for different responses in the wood frog is further supported by comparing different wood frog studies that used different response variables. These studies found the scale of effect of road density (Homan et al. 2004; Veysey et al. 2011) and forest proportion (Porej et al. 2004; Herrmann et al. 2005; Clark et al. 2008) on either wood frog abundance or occurrence were within extents ranging from 0.2 to 2 km, highlighting the response-specific nature of landscape context effects.
It remains possible that the order of the scale of effect is consistent between species, for a given landscape variable, particularly for organisms that share similar dispersal patterns and life histories. In fact, two other multi-scale studies have estimated the same order of the scale of effect of road density and forest proportion on amphibian abundance and occurrence as we did. Clark et al. (2008) found that the scale of effect of road density was greater on occurrence than abundance (for A. maculatum) and Boissinot et al. (2015) found that the scale of effect of forest proportion was greater on abundance than occurrence (for R. temporaria).
Implications
Our results indicate three findings that together have important implications for research. First, as in previous meta-analyses of the scale of effect (Jackson and Fahrig 2015; Martin 2018), relationships between a species’ response and landscape variable are typically strongest at a specific spatial extent (or range of spatial extents), i.e. they exhibit a scale of effect. Second, for a single species, the scale of effect of a given landscape variable varies with the response variable. This is consistent with the findings of Martin (2018) who found that the scale of effect of a given landscape variable differed between response variables 70% of the time. Third, the order of the scale of effect is not consistent with a priori predictions and is not consistent across landscape variables. In other words, although the scale of measurement of a landscape variable strongly influences its estimated relationship with a species’ response, predicting the appropriate scale of measurement a priori is likely impossible in the absence of previous multi-scale studies of the particular species’ response-landscape variable relationship of interest. In the absence of such pre-existing evidence, we recommend that a single scale should not be chosen for estimating landscape context effects on species but rather one should empirically estimate the scale of effect using a multi-scale study design.
References
Babbitt KJ, Baber MJ, Brandt LA (2006) The effect of woodland proximity and wetland characteristics on larval anuran assemblages in an agricultural landscape. Can J Zool 84:510–519
Berven KA (1982) The genetic basis of altitudinal variation in the wood frog Rana sylvatica. I. An experimental analysis of life history traits. Evolution 36:962–983
Berven KA (1990) Factors affecting population fluctuations in larval and adult stages of the wood frog (Rana sylvatica). Ecology 71:1599–1608
Berven KA, Grudzien TA (1990) Dispersal in the wood frog (Rana sylvatica): implications for genetic population structure. Evolution 44:2047–2056
Blomquist SM, Hunter ML Jr (2010) A multi-scale assessment of amphibian habitat selection: wood frog response to timber harvesting. Écoscience 17:251–264
Boissinot A, Grillet P, Besnard A, Lourdais O (2015) Small woods positively influence the occurrence and abundance of the common frog (Rana temporaria) in a traditional farming landscape. Amphibia-Reptilia 36:417–424
Brennan JM, Bender DJ, Contreras TA, Fahrig L (2002) Focal patch landscape studies for wildlife management: optimizing sampling effort across scales. In: Liu J, Taylor WW (eds) Integrating landscape ecology into natural resource management. Cambridge University Press, Cambridge, pp 68–91. https://doi.org/10.1017/CBO9780511613654.006
Browne CL, Paszkowski CA, Foote AL, Moenting A, Boss SM (2009) The relationship of amphibian abundance to habitat features across spatial scales in the Boreal Plains. Écoscience 16:209–223
Clark PJ, Reed JM, Tavernia BG, Windmiller BS, Regosin JV (2008) Urbanization effects on spotted salamander and wood frog presence and abundance. Herpetol Conserv 3:67–75
Coffey HMP, Fahrig L (2012) Relative effects of vehicle pollution, moisture and colonization sources on urban lichens. J Appl Ecol 49:1467–1474
Collins SJ, Fahrig L (2017) Responses of anurans to composition and configuration of agricultural landscapes. Agric Ecosyst Environ 239:399–409
Crouch WB, Paton PWC (2000) Using egg-mass counts to monitor wood frog populations. Wildl Soc Bull 28:895–901
Eigenbrod F, Hecnar SJ, Fahrig L (2008) The relative effects of road traffic and forest cover on anuran populations. Biol Conserv 141:35–46
Eigenbrod F, Hecnar SJ, Fahrig L (2009) Quantifying the road-effect zone: threshold effects of a motorway on anuran populations in Ontario. Canada. Ecol Soc 14:24
Ethier K, Fahrig L (2011) Positive effects of forest fragmentation, independent of forest amount, on bat abundance in eastern Ontario, Canada. Landsc Ecol 26:865–876
Findlay CS, Lenton J, Zheng L (2001) Land-use correlates of anuran community richness and composition in southeastern Ontario wetlands. Écoscience 8:336–343
Grant EHC, Jung RE, Nichols JD, Hines JE (2005) Double-observer approach to estimating egg mass abundance of pool-breeding amphibians. Wetl Ecol Manag 13:305–320
Groff LA, Calhoun AJK, Loftin CS (2017) Amphibian terrestrial habitat selection and movement patterns vary with annual life-history period. Can J Zool 95:433–442
Harper EB, Semlitsch RD (2007) Density dependence in the terrestrial life history stage of two anurans. Oecologia 153:879–889
Herrmann HL, Babbitt KJ, Baber MJ, Congalton RG (2005) Effects of landscape characteristics on amphibian distribution in a forest-dominated landscape. Biol Conserv 123:139–149
Holland JD, Fahrig L, Cappuccino N (2005a) Fecundity determines the extinction threshold in a Canadian assemblage of longhorned beetles (Coleoptera: Cerambycidae). J Insect Conserv 9:109–119
Holland JD, Fahrig L, Cappuccino N (2005b) Body size affects the spatial scale of habitat–beetle interactions. Oikos 110:101–108
Homan RN, Windmiller BS, Reed JM (2004) Critical thresholds associated with habitat loss for two vernal pool-breeding amphibians. Ecol Appl 14:1547–1553
Jackson HB, Fahrig L (2012) What size is a biologically relevant landscape? Landsc Ecol 27:929–941
Jackson HB, Fahrig L (2015) Are ecologists conducting research at the optimal scale? Glob Ecol Biogeogr 24:52–63
Jackson ND, Fahrig L (2014) Landscape context affects genetic diversity at a much larger spatial extent than population abundance. Ecology 95:871–881
Karraker NE (2007) A new method for estimating clutch sizes of ambystomatid salamanders and ranid frogs: introducing the ovagram. Herpetol Rev 38:46–48
Koumaris A, Fahrig L (2016) Different anuran species show different relationships to agricultural intensity. Wetlands 36:731–744
Levin SA (1992) The problem of pattern and scale in ecology: the Robert H. MacArthur Award lecture. Ecology 73:1943–1967
Martin AE (2018) The spatial scale of a species’ response to the landscape context depends on which biological response you measure. Curr Landsc Ecol Rep 3:23–33
Martin AE, Fahrig L (2012) Measuring and selecting scales of effect for landscape predictors in species-habitat models. Ecol Appl 22:2277–2292
Miguet P, Jackson HB, Jackson ND, Martin AE, Fahrig L (2016) What determines the spatial extent of landscape effects on species? Landsc Ecol 31:1177–1194
Moraga AD, Pervin E (2018) Efficient estimation of amphibian clutch using image analysis of compressed globular egg masses. Herpetol Conserv Biol 13:341–346
Moretto L (2018) A small-scale response of urban bat activity to tree cover. Dissertation, Carleton University
Nakazawa M (2018) fmsb: functions for medical statistics book with some demographic data. R package version 0.6.3. https://CRAN.R-project.org/package=fmsb
Paradis E, Schliep K (2019) ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528
Porej D, Micacchion M, Hetherington TE (2004) Core terrestrial habitat for conservation of local populations of salamanders and wood frogs in agricultural landscapes. Biol Conserv 120:399–409
Raithel CJ, Paton PWC, Pooler PS, Golet FC (2011) Assessing long-term population trends of wood frogs using egg-mass counts. J Herpetol 45:23–27
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rowe CL, Dunson WA (1995) Impacts of hydroperiod on growth and survival of larval amphibians in temporary ponds of Central Pennsylvania, USA. Oecologia 102:397–403
Schindelin J, Rueden CT, Hiner MC, Eliceiri KW (2015) The ImageJ ecosystem: an open platform for biomedical image analysis. Mol Reprod Dev 82:518–529
Smith AC, Fahrig L, Francis CM (2011) Landscape size affects the relative importance of habitat amount, habitat fragmentation, and matrix quality on forest birds. Ecography 34:103–113
Smith AC, Francis CM, Fahrig L (2014) Similar effects of residential and non-residential vegetation on bird diversity in suburban neighbourhoods. Urban Ecosyst 17:27–44
Thornton DH, Branch LC, Sunquist ME (2011) The influence of landscape, patch, and within-patch factors on species presence and abundance: a review of focal patch studies. Landsc Ecol 26:7–18
Thornton DH, Fletcher RJ Jr (2014) Body size and spatial scales in avian response to landscapes: a meta-analysis. Ecography 37:454–463
Venables B, Ripley B (2002) MASS: support functions and datasets for Venable’s and Ripley’s MASS. Modern Applied Statistics with S. Fourth Edition, Springer
Veysey JS, Mattfeldt SD, Babbitt KJ (2011) Comparative influence of isolation, landscape, and wetland characteristics on egg-mass abundance of two pool-breeding amphibian species. Landsc Ecol 26:661–672
Acknowledgements
We thank the private landowners who let us sample their ponds. Erik Pervin and Caitlin Brunton were indispensable field assistants. We thank Joseph Bennett and Jeremy Kerr for helpful comments and suggestions. We also thank the associate editor and two anonymous reviewers for their comments on an earlier version of this paper. This work was supported by a Natural Sciences and Engineering Research Council of Canada Grant to Lenore Fahrig.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All work complied with the Canadian Council on Animal Care requirements for the use of eggs in research (Category of Invasiveness A).
Data availability
The data sets generated during the current study are available in the Mendeley Data repository, https://doi.org/10.17632/ccw64rpj22.1.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moraga, A.D., Martin, A.E. & Fahrig, L. The scale of effect of landscape context varies with the species’ response variable measured. Landscape Ecol 34, 703–715 (2019). https://doi.org/10.1007/s10980-019-00808-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-019-00808-9