Abstract
Information on animal communities inhabiting Neotropical fragmented landscapes is important for developing conservation strategies. The structure of amphibian and reptile communities in six tropical rainforest fragments (<20 ha) and two reference areas in continuous forest at Los Tuxtlas, Mexico was studied. A total of 3,481 individuals of 51 species of amphibians and reptiles were recorded across 12 bimonthly surveys during 2 years. Taxonomic composition was different between the smallest fragments and the reference areas. Six species were exclusive to large undisturbed forest and richness was significantly lower in the five smallest fragments (1.4–6.6 ha) compared with the largest patch, one or both of the reference areas. Amphibian abundance tended to be higher in large areas, while reptiles were more abundant in the five smallest fragments. Craugastor loki and Anolis uniformis were the dominant species in all sites, and particularly in the smaller fragments. Amphibian and reptile richness was positively related to larger patch sizes, deeper leaf litter, closed canopy cover, and higher relative humidity and negatively related to linear patch shape and high temperatures. Abundance of reptiles was positively associated to high temperatures, high density of woody debris, and closed canopy cover; it was negatively affected by linear patch shape, low humidity levels, and steeper slopes. While amphibian and reptile communities were strongly affected in vegetation fragments, these patches retained a considerable number of rainforest species. Fragments up to at least 17 ha have the potential for preserving communities with similar structure to those occurring in large tropical rainforests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat fragmentation, the breaking apart and isolation of continuous native vegetation by different vegetation types or land use (the matrix), has important consequences to biodiversity (Wilcove et al. 1986; Saunders et al. 1991). Tropical rainforest is considered the most diverse and most threatened terrestrial ecosystem in the world, and has been subject to extensive fragmentation. From 1990 to 1997 5.8 million hectares of humid tropical forest were lost globally each year, and the deforestation rate in Latin America alone was about 2.5 million hectares per year in the same period (Achard et al. 2002). In Mexico tropical rainforests have been drastically cleared during recent decades by human activities, resulting in a 95 % reduction in the original forested area (Dirzo and García 1992).
In fragmented landscapes, patch isolation and habitat loss produce different abiotic and biotic changes to the original communities, and the intensity varies with size, shape, position in the landscape, time since isolation, edge recovery, matrix context, distance, and degree of connectivity between remnants (Wilcove et al. 1986; Saunders et al. 1991; Laurance et al. 2002).
Area effects and edge effects are mechanisms modifying animal communities inhabiting fragments. Area effects are ecological changes that occur as a result of isolation, and their magnitude is generally proportional to the fragment size. On the other hand, edge effects are caused by strong environmental variations and thus affect biotic factors occurring along forest edges (Laurance and Yensen 1991; Turner 1996; Carvalho and Vasconcelos 1999). The structure of the remnants, as determined by vegetation, topographical features, and fragment shape, also influences animal communities. These effects are rarely investigated.
Island Biogeography theory predicts that large areas maintain higher species richness than small ones because of lower recolonization and higher extinction rates in small areas (MacArthur and Wilson 1967). This pattern has been applied to continental habitat fragments, where it is expected that animal community structure in large patches is less affected by the fragmentation process. However, the effects of habitat fragmentation and patch size on communities can be negative or positive depending on the particular taxonomical group considered. The methods and the scale (patch or landscape) of the studies can also result in different interpretations (Fahrig 2003).
Amphibians and reptiles depend on specific microhabitats and conditions, which make them particularly sensitive to perturbation (Zug et al. 2001); thus, the effects of fragmentation on these groups across Neotropical rainforests need to be evaluated. Studies in central Amazonia have shown that frog richness is positively related to fragment size. However, richness can also be increased in small fragments because the extinction of few rare species after isolation or may be compensated for by invasion of species from the surrounding matrix, which may result in changes to the original community composition (Tocher et al. 1997; Gascon et al. 1999). In other Neotropical rainforests, direct and indirect negative effects of fragmentation on amphibian and reptile communities have been documented (e.g. Vallan 2000; Bell and Donnelly 2006; Lehtinen and Ramanamanjato 2006). Some negative effects of small patch size, high level of isolation, simple structure, and edges are a decrease on amphibian and reptile richness and diversity, and a change in abundances and in species composition. But fragmentation and associated changes can benefit some species or taxonomic groups (Schlaepfer and Gavin 2001; Urbina-Cardona et al. 2006).
The main goal of this paper was to analyze the structure of amphibian and reptile communities in different-sized patches of tropical rainforest using a fragment-scale analysis. The effect of size and structure of rainforest fragments on the species composition, richness and abundance was assessed.
Methods
Study site
The Los Tuxtlas region, located on the coast in the southern portion of the state of Veracruz, was one of the largest tropical rainforests in Mexico, and is situated at the northernmost distributional limit of tropical rainforest on the American continent (Dirzo and Miranda 1991). This area has been strongly affected by deforestation, having lost 84 % of the original vegetation. The regional landscape has been converted into more than 1,005 forest fragments of different shapes, sizes and degrees of isolation. Most fragments are very small, with only 7 % larger than 10 ha (Dirzo and García 1992; Mendoza et al. 2005).
Los Tuxtlas is characterized by its complex topography and high precipitation. Mean annual temperatures range from 22 to 26 °C in the lowest areas (elev. 200–600 m), and annual rainfall is around 2,500 mm in the dry season (January to May) and over 4,000 mm in wet season (June to November). Rainfall increases with tropical storms (September and October) and the lowest temperatures are registered when cold northern winds blow into the area from December to February (Soto and Gama 1997).
Selection of fragments and reference areas
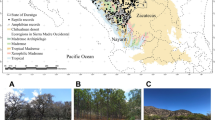
Six isolated tropical rainforest fragments (<20 ha) completely surrounded by cattle pastures were chosen for this study. Each of these fragments has been isolated for approximately 35 years. The size range of these remnants is similar to that of the remaining forest fragments in the Los Tuxtlas region (mean: 13.6 ha; Mendoza et al. 2005). These patches were named Fragment 1 to Fragment 6 (referred as F1–F6), and numbered sequentially according to their size (Fig. 1). All are in the Colonia Agrícola Ganadera Adolfo Ruíz Cortines, San Andrés Tuxtla, 5–8 km northwest of Los Tuxtlas Tropical Biology Station (LTS), Universidad Nacional Autónoma de México. The isolation between patches is similar, and the fragments are occasionally disturbed by human activity (livestock grazing and logging).
For comparison, two reference areas in continuous tropical rainforest were selected: Laguna Escondida (LE) and Lote 67 from within LTS, which represents 700 ha of continuous forest and a Biological Reserve (Fig. 1). Although both reference areas are connected, these were studied and reported separately because of their different environmental contexts: LE is influenced by large permanent water bodies (two lagoons and one river) and some human disturbance (e.g. selective extraction of wood; introduction of exotic plants, and hunting); and, LTS is a protected area with no perturbation and only small temporal streams. In each reference area we surveyed a 20 ha portion of forest.
Characterization of fragments and reference areas
The eight sites were characterized with qualitative and quantitative descriptors (Table 1). Sizes were calculated using ArcView 3.3 software (Environmental Systems Research Institute Inc. [ESRI] 2003), based on the polygon of each fragment outlined on an aerial photography of the area (1:20,000). Fragment shape was calculated using the shape index suggested by Patton (1975), estimating the degree to which a shape differs from a circle. The formula is Sh = P/2√πA, where P is the patch perimeter of the site and A is the area; the minimum value is 1 when the site is a perfect circle, and it increases as the site gets more irregular. Remnant mean elevation was calculated by averaging the highest and the lowest elevations on each site. Mean slope was estimated by averaging the inclination measured with a Brunton compass on 20 plots randomly located in each site. Mean environmental relative humidity and temperature were calculated by averaging data across four time points (1,000, 1,500, 1,800 and 2,300 h) taken every survey day in 10 defined points at the interior and edges of the fragments. Measurements were taken with a digital thermohygrometer after a 30 s exposure.
Vegetation structure was characterized using Gentry’s (1982) method by establishing seven independent, randomly located and oriented 50 × 2 m transects within the interior of each site. Occasionally the steepness of the terrain or the presence of areas with very thick and complex vegetation made us move transects a few meters to more workable locations. Along these transects density of trees and bamboos (>10 cm perimeter at breast height), mean perimeter of trees and bamboos at breast height, density of plants and seedlings (>10 cm high), and density of woody debris (fallen branches, logs and other wood remains >20 cm diameter) were quantified. Leaf litter depth was measured in five points along each transect (10 m between points) by inserting a ruler into the soil litter. The canopy percent cover was estimated in 20 randomly located points, separated by 25 m each. In these points, hemispheric canopy photos were taken with a Nikon E-990 and fisheye lens pointed upward. Pictures were broken down into binary bitmaps (black, canopy; white, sky) and were analyzed using Gap Light Analyzer 2.0 software (Frazer et al. 1999).
Amphibian and reptile sampling
From April 2003 to March 2004, and from July 2006 to June 2007, the eight sites were surveyed every 2 months completing 12 surveys per site. Visual encounter surveys were time-constrained (Crump and Scott 1994), calibrated to 10 h per site per day, and divided into 5 h in the morning (1,000–1,500 h) and 5 h at night (1,800–2,300 h). Surveys were done by two persons on randomly placed transects located within the fragments. The sampling effort was 240 person-hours per site, with a total of 1,920 person-hours, collectively.
Amphibians and reptiles were searched for intensively in all available microhabitats across the understory (leaf litter, plants, tree trunks, buttresses, bark, fallen logs, decomposed wood, rocks, etc.), from ground level to 2 m high. All sightings were recorded. Individuals were captured by hand or by using a snake hook, and then marked and released at the site of capture. Based on the extremely low number of recaptures the marking was stopped after the fourth survey and mark-recapture data were not considered in the analyses.
When the amphibians and reptiles were not identified to the species level in the field, they were collected for identification in the lab. These individuals were sacrificed, preserved and housed in the Colección Nacional de Anfibios y Reptiles, Instituto de Biología, Universidad Nacional Autónoma de México.
Data analysis
After testing for normality and homogeneity of variances, the environmental and vegetation data between sites were compared using nonparametric Kruskal–Wallis one-way analysis of variance (ANOVA) with the Dunn post hoc test. The tests were performed in SigmaPlot 11.0 (Systat Software Inc. 2008). The association between patch size and environmental and vegetation variables was summarized with a principal components analysis (PCA), using PRIMER (Clarke and Gorley 2006). The significance of the relationships between pairs of variables was obtained performing nonparametric Spearman’s rank correlations in STATISTICA 6.0 (StatSoft 2001).
Complementarity of amphibian and reptile species composition between sites was measured using Colwell and Coddington’s (1994) formula: C = U jk/S jk; where S j is the local richness of site 1, S k is the local richness of site 2, V jk is the number of common species between the two sites, S jk = S j + S k − V jk is the total richness for both sites combined, U jk = S j + S k − 2V jk is the number of species unique to either list. Zero indicates two identical communities and 1 indicates that species compositions are completely distinct (Colwell and Coddington 1994).
The potential number of species occurring in each site was evaluated using the nonparametric estimator Chao 2 with the formula S 2 = S obs + L2/2M; where S obs is the observed number of species in a sample, L is the number of species that occur in only one sample, and M is the number of species that occur in exactly two samples (Colwell and Coddington 1994). This estimator was calculated with 500 randomizations in EstimateS 7.0 (Colwell 2004). Inventory completeness was calculated for each site as the difference of the percentage of species observed from the total number of species predicted by Chao 2. To compare richness of fragments with the same number of registered individuals, sample-based rarefaction Mao Tau curves (rescaled by individuals) were calculated with their 95 % confidence intervals (Gotelli and Colwell 2001; Colwell et al. 2004) using EstimateS 7.0 (Colwell 2004).
Rank–abundance curves were used to compare species abundance patterns, species dominance, and evenness among sites. The relative abundance of each species (ni/N) on a logarithmic scale (Log10) was plotted against the rank order of the species from the most to the least abundant for each fragment and reference area (Magurran 2004). Additionally the amphibian:reptile ratio was compared using a Mann–Whitney U test for each site. Abundance of individuals for amphibian and reptile communities was compared using a one-way ANOVA and post hoc Tukey’s HSD test, this in STATISTICA 6.0 (StatSoft 2001).
The principal component scores obtained in the PCA analysis were used to evaluate the influence of the patch size and environmental and vegetation variables on the structure of the communities, to reduce the measured variables to fewer uncorrelated composite variables. The main principal components (PCs) were selected based on the eigenvalues and the percent of explained variance. Then, the relationship between the scores from the retained PCs and richness and abundance of amphibians and reptiles were examined by using linear regression in JMP 5.0.1 (SAS 2002).
Results
Characterization of fragments and reference areas
Significant differences in elevation, slope, tree and bamboo density, tree and bamboo perimeter, canopy cover, woody debris, and leaf litter depth were recorded between the studied forests (Table 1). PCA showed two groups of sites characterized by similar environmental variables and vegetation. One group was composed by the reference areas LTS, LE and the largest fragment (F6); the other group included F3, F4 and F5. F1 and F2 were not grouped (Fig. 2). Significant positive correlation was found for patch size and relative humidity (r = 0.833, p = 0.005), slope and tree–bamboo density (r = 0.853, p = 0.005), and tree perimeter and woody debris density (r = 0.714, p = 0.037). Negative associated variables were patch size and elevation (r = −0.881, p = 0.0001), elevation and relative humidity (r = −0.714, p = 0.037), slope and tree perimeter (r = −0.738, p = 0.028), and tree–bamboo density with tree perimeter (r = −0.905, p = 0.0001).
PCA showing the vectors of environmental and vegetation variables in six tropical rainforest fragments (F1–F6) and two reference areas (LE and LTS) at Los Tuxtlas, Veracruz. 1 Size, 2 shape, 3 elevation, 4 slope, 5 temperature, 6 relative humidity, 7 tree–bamboo density, 8 tree–bamboo perimeter at breast height, 9 plant density, 10 canopy cover, 11 woody debris density, 12 leaf litter depth. PC 1 principal component 1, PC 2 principal component 2. The orientation of the lines represents the association between the variables
Amphibian and reptile community structure
Species composition
In the eight studied remnants, 3,481 individuals (1,654 amphibians and 1,827 reptiles) of 51 species were captured; of these, 17 were amphibians and 34 were reptiles (Table 2). Ten amphibian species were found in the fragments and 13 in the reference areas, while 25 reptile species were recorded in the fragments and 22 in the reference areas. Each site had more reptiles than amphibian species and only five species were present in each of the eight sites: the frog Craugastor loki, the salamander Bolitoglossa rufescens, the lizards Anolis uniformis and Lepidophyma tuxtlae, and the snake Imantodes cenchoa. Seven amphibian and 13 reptile species (most of them snakes) were found only in one site. The salamander Pseudoeurycea orchimelas, the frog Agalychnis callidryas, the lizard Sceloporus salvini, and the snakes Dendrophidion vinitor, Leptophis ahaetulla, and Spilotes pullatus were exclusive to large undisturbed forest (LTS) (Table 2).
For both, amphibians and reptiles, there was a significant difference in community composition between F1, F2 and F3, and the reference areas (Table 3). Complementarity for both taxonomic groups tended to be low in F2, F3, F4, F5 and F6, while the smallest site, F1, showed an increasing complementarity with the increase in the size of the sites. Thus its complementarities with LE and LTS were the highest (Table 3).
Richness estimation
Amphibian richness estimated by Chao 2 was similar to the observed richness in most sites. The completeness was above 75 % for seven sites, reaching 100 % in four of them. The only one site with low completeness was LTS with 55.5 % of the estimated species number. For reptiles, most sites had completeness close to, or much higher than 75 %, nevertheless completeness for F5 was low, reaching only 33.6 % of the estimated species number (Table 4).
Richness comparison between sites
Mao Tau rarefaction test with 95 % confidence intervals showed that amphibian richness was significantly lower in F1 than in all sites (with the exception of F5). Richness in F3 was lower than in F4, while richness in F3, F4 and F5 was lower than in LE (Fig. 3a). For reptiles, richness in F6, LE, and LTS was significantly higher than that found in F1, F2 and F4 (Fig. 3b).
Amphibian and reptile abundance comparison between sites
The highest amphibian abundance was recorded from F6 and LTS, and the lowest from F1 and F2. Amphibian abundance was significantly higher in LTS than in F1, F2, F4, F5 and LE (ANOVA: F = 5.257, p = 0.000; Tukey p < 0.05). For reptiles, the lowest abundances were also found in F1 and F2, but the highest abundance was reached in F4. Reptile abundance was significantly higher in F4 than in the two smallest fragments (ANOVA: F = 3.363, p = 0.003; Tukey p < 0.05).
Difference in amphibian:reptile ratio was significant for F4 (Mann–Whitney: Z = −2.136, p = 0.032) and LTS (Mann–Whitney: Z = 2.367, p = 0.017). In F4 lizards were extremely abundant, while in LTS frogs were the most abundant. In the remaining sites the number of reptiles was always numerically higher than amphibians (except for F6) (Table 2).
Relative abundance of the species
Both amphibian and reptile communities were distributed according to a log-series model with a low number of abundant species and a high number of rare species. Rank–abundance curves showed that the frog C. loki and the lizard A. uniformis were the dominant species in all sites. For amphibians, differences in relative abundance between the dominant species and the remaining species were notably distinct between the smallest fragments with high dominances (except F2) and F6, LE, and LTS, which showed greater evenness (Fig. 4a). For reptiles, abundances of the species were most equitable in F6 and LTS (Fig. 4b).
Rank–abundance curves for the relative abundance of the species (Log10 ni/N) of amphibians (a) and reptiles (b) in six tropical rainforest fragments (F1–F6) and two reference areas (LE, LTS) at Los Tuxtlas, Veracruz. Species codes in Table 2
Effect of patch size, environmental and vegetation variables, on amphibian and reptile community structure
PC 1 and PC 2 explained most of the total variance in the environmental and vegetation data set (73.6 %, collectively), with PC 1 accounting for 39.91 % and PC 2 for 33.74 % of this variation. The subsequent PCs were not used for further analysis because each accounted for only a low percentage of the variance.
There was a significant positive relationship between PC 1 values and amphibian and reptile richness (R 2 = 0.911, p = 0.000; R 2 = 0.686, p = 0.011) (Fig. 5a). There was no significant relationship between PC 1 and amphibian or reptile abundance (R 2 = 0.341, p = 0.128; R 2 = 0.087, p = 0.476) (Fig. 5b). Richness of both groups was not associated to PC 2 (R 2 = 0.003, p = 0.891; R 2 = 0.082, p = 0.490), (Fig. 5c), and likewise for the abundance of amphibians (R 2 = 0.107, p = 0.427) (Fig. 5d). On the contrary, the abundance of reptiles was strongly associated with PC 2 values (R 2 = 0.776, p = 0.003) (Fig. 5d).
Relationship between principal components (PC 1 and PC 2) of environmental and vegetation variables with richness (a, c) and abundance (b, d) in amphibian and reptile communities in six tropical rainforest fragments and two reference areas at Los Tuxtlas, Veracruz. White rhomboids amphibian, black squares reptiles
Inspection of the data showed that size of the site was the variable with the highest importance in PC 1, with five other variables showing important scores. High positive values of PC 1 were positively associated with a combination of large fragment size, deep leaf litter, closed canopy cover, and high relative humidity, and were negatively related to linear fragment shape and high temperatures. On the other hand, high temperatures, high density of woody debris and closed canopy cover were the variables positively associated to PC 2 scores, while linear shape of the fragment, low humidity levels and steeper slopes were negatively related to this PC.
Discussion
Richness and composition of amphibian and reptile communities
Historically, there have been 26 amphibian and 64 reptile species reported from the northern slope of the San Martín volcano in the Los Tuxtlas region (Pérez-Higareda Personal Communication), some of these species are naturally rare or secretive. In our work 65 % of the amphibians and 53 % of the reptiles were found. Our surveys were performed in the forest understory and some burrowing and arboreal species were most likely under-represented or were not recorded. In addition to the 34 reptile species found, two other species, the lizard Iguana iguana and the snake Ninia sebae, were observed during our vegetation surveys in LTS (but not recorded during herpetological surveys). However, completeness for the estimated richness as derived from our surveys was high in the studied sites (Table 4), indicating that most of the possible species were recorded with our sampling design and allowing comparisons between sites. Overall, the number of amphibian and reptile species recorded in all six small fragments was the same as that found in the two reference areas.
Amphibian and reptile composition varies naturally across Los Tuxtlas Biosphere Reserve landscape because of the environmental heterogeneity in the region (Vogt et al. 1997; Ramírez-Bautista and Nieto-Montes de Oca 1997). However, the fragments and the reference sites used in our study are all limited within the northeast slope of the San Martín Volcano between 100 and 700 m. These should be affected by the same general climatic variables as this elevation range has been demonstrated to have similar amphibian and reptile composition and abundance parameters within pristine zones (Hernández-Ordóñez and Reynoso Unpublished Data; Luna-Alcántara and Reynoso Unpublished Data). Thus, the variations in structure found in the communities described can be attributed to environmental modifications derived from fragmentation.
Taxonomical composition patterns found between the sites showed the highest similarities to be between more similarly sized patches. It is assumed that most tropical rainforest species which persist in fragments smaller than 10 ha have broad ecophysiological tolerances and are less vulnerable to fragmentation. Alternatively, these species are vagile enough to visit and/or re-colonize from larger fragments (Laurance et al. 2002). Extinction-prone amphibians in El Refugio-Huanchaca Biological Station, Bolivia were rare and did not use the matrix, whereas extinction-prone reptiles were trophically specialized in continuous forest and fragments (Watling and Donnelly 2007). The species composition recorded in our study seems to be mainly determined by the sensitivity of species to environmental changes and habitat loss, by the influence of the surrounding matrix, and by the differential distributions of the species owing to their unique requirements and life histories. Most of the species inhabiting fragments were found in the reference areas or have been previously reported within preserved areas in Los Tuxtlas Biosphere Reserve (Vogt et al. 1997; Ramírez-Bautista and Nieto-Montes de Oca 1997), while other species distributed in continuous forest were never found in small fragments, and thus appear to be sensitive to fragmentation. Some of the species exclusive to the reference areas LTS and/or LE are the salamander P. orchimelas, the frogs A. callidryas and Gastrophryne elegans, and the lizard Anolis barkeri. Species found in F1 (the salamander B. rufescens, the frog C. loki, the lizards Anolis rodriguezi, A. uniformis, Ameiva undulata, and L. tuxtlae, and the snake I. cenchoa) seem to be tropical rainforest species that are not sensitive to fragment size and the associated abiotic and biotic variables. The lizards A. rodriguezi and A. undulata, are in fact, species characteristic to open sites and may occur in the forest edge and the pasture matrix in Los Tuxtlas (Vogt et al. 1997; Urbina-Cardona et al. 2006).
Differences in species richness and composition between fragments along the studied patch size gradient compared with reference sites may suggest a tendency towards nested extinction. This one defined as an orderly sequence of extinctions with species inhabiting small patches being a poorer subset of most resistant species occurring in large ones (Patterson 1987; Hager 1998). This pattern has been found for amphibian and reptile communities in other tropical fragmented landscapes (Vallan 2000; Lehtinen and Ramanamanjato 2006). In our study, species like the frog Craugastor alfredi and the snakes Clelia scytalina, Coniophanes fissidens, Leptodeira septentrionalis, Scaphiodontophis annulatus, Micrurus limbatus, and Bothrops asper seem to occur only in fragments greater than 6.6 ha as they were not detected in F1–F4 (Table 2). Absence or extreme rarity of large snakes (e.g. Boa constrictor and B. asper) in the smallest fragments (F1 and F2) may be related to their body sizes. This is an important feature found to restrict reptile distribution in small vegetation fragments, where available food resources can be limited (Luiselli and Capizzi 1997; Watling and Donnelly 2007).
Species of anurans that are common in pastures, edges, disturbed or unforested areas such as the frogs Dendropsophus microcephalus, Lithobates brownorum, L. vaillanti, and the toad Rhinella marina (Lee 1996; Vogt et al. 1997; Urbina-Cardona et al. 2006), were found on the edges of the unprotected reference area (LE) reflecting some degree of disturbance, the matrix influence, and even the effect of the lagoon, as anurans with indirect development are common in this area.
Species abundance and dominance
Analysis at the class level showed that amphibians are more common in large areas of vegetation than in fragments, while reptiles appear to be more abundant in fragments. The low amphibian abundance may be an effect of their sensitivity to desiccation caused by an increment of wind and sun exposition in small fragments and edges; also the eggs of the terrestrial species are less protected and more affected by low humidity (Marsh and Pearman 1997; Primack 2004). In addition, respiration of amphibian through their skin represents a physiological constraint, which can increase their vulnerability to desiccation in drier environments (Lehtinen et al. 2003). In Madagascar, frog species were edge-avoiders while reptiles had more varied responses to edges in six fragments (10–457 ha) of littoral rainforest (Lehtinen et al. 2003). Also, a decline in frog abundances reported in 10 ha size Amazonian rainforest fragments appears to be related to amphibian constraints (Funk and Mills 2003).
Craugastor loki and A. uniformis were the most common species in all sites. Leaf-litter frogs with direct terrestrial development like Craugastor spp. and anole lizards are commonly dominant in large primary tropical rainforest in different Neotropical areas (Reagan 1992; Schlaepfer and Gavin 2001). A higher number of frogs of the genera Craugastor, Pristimantis and Diasporus was found in forest interiors of nine premontane wet forest fragments (<1–100 ha) in Fila Cruces, Costa Rica, while individuals of two Anolis lizard species were more abundant in edges (Schlaepfer and Gavin 2001). Additionally, in the tropical rainforest of La Selva, Costa Rica Craugastor noblei and Pristimantis ridens showed a significant increase in density related to fragment size; overall anuran density was lower and lizard density was higher in fragments (1–7 ha) than in continuous forest (Bell and Donnelly 2006). Our results support that leaf-litter frog abundance in tropical rainforests increases with patch size.
Amphibian abundance showed greater evenness in the four sites with water bodies (F2 and F6, and reference areas LE and LTS), while reptiles showed more evenness in the reference area LTS. This difference reflected the important role of water for amphibian populations.
Craugastor loki and A. uniformis were also the most common species recorded in large tropical rainforest patches (26–472 ha) at Los Tuxtlas (Urbina-Cardona et al. 2006). Both species are forest dwellers (edge and interior), but C. loki can be occasionally found in pastures (Campbell 1998; Urbina-Cardona et al. 2006; Cabrera-Guzmán Personal Observation). Nevertheless, Craugastor clutches are generally deposited on leaf litter and under wet fallen logs, and require high humidity levels (Townsend and Stewart 1994; Campbell 1998; Donnelly 1999) expected in large forested areas. The over-dominance of both species in the five smallest fragments in our study can be related to the rarity or absence of competitor and/or predator species that are sensitive to fragmentation. The increase in densities (and crowding) of surviving fauna in forest remnants, at least at the short term level, can also have been induced by reduction in the available habitat (Wilcove et al. 1986; Saunders et al. 1991; Turner 1996; Laurance et al. 2002). However, the studied communities have been isolated for approximately 30–35 years, such that either C. loki and A. uniformis are likely to be structured in populations relatively stable, showing an increase of abundance not by the effect of crowding, but because of the habitat reduction.
Additionally A. uniformis reproduction is continual throughout the year (Campbell et al. 1989), it has an unspecific diet (Cabrera-Guzmán and Reynoso 2008) and a tolerance to wide environmental variation (Urbina-Cardona et al. 2006). The notorious high abundance of this lizard in F4 could also be related to food availability as population density and home range overlap can increase for Anolis humilis when insects are abundant (Guyer 1988). Invertebrate surveys are necessary to evaluate prey abundance and variation in prey availability during the year.
The high abundance of some other species in only one or two fragments, such as the salamander B. rufescens in F2, the lizard L. tuxtlae in F3 and F4, the toad Incilius cavifrons and the skink Scincella silvicola in F6, most likely indicates that these patches offer a particular kind of microhabitat favorable for these species. On the contrary, some species still present in the fragments have critically low abundances (e.g. Corytophanes hernandesii, L. tuxtlae and I. cenchoa) that could be close to falling below viability levels and may become extinct locally in the short term (Table 2). This seems especially true for species that avoid crossing the matrix, preventing recolonization or maintenance of the genetic variability through migration.
Effect of patch size on amphibian and reptile community structure
Most of the recent studies on effects of habitat fragmentation on amphibians and reptiles in tropical rainforests include very small fragments, the smallest being between 0.6 and 1.5 ha (e.g. Bell and Donnelly 2006; Hillers et al. 2008; Watling and Donnelly 2008). The smallest patches in our study (1.4 and 2.8 ha) showed severe declines in richness and abundance of individuals. Fragment size was a good predictor for amphibian and reptile richness, since in the PCA analysis the species numbers were positively related to the PC 1, mainly described by the size of the habitat area. Richness in F6 (17.2 ha) was not significantly lower than richness in the reference sites LE and LTS. The notable change in species number from F5 to F6 suggests the occurrence of a critical size where abiotic and biotic variables change enough to prevent the survival of many species. Because of the short inter-patch distances between all sites, it could be inferred that the species present in F6 but not in the five smaller fragments have suffered local extinctions or show a low recolonization frequency. Our results suggest that the severe defaunation of the largest mammals in small forest fragments (<200 ha) in the Los Tuxtlas region (Dirzo and Miranda 1991; Estrada et al. 1994) can occur for amphibians and reptiles within fragments smaller than 7 ha.
The positive relation between patch size and species richness found in our study is similar to that reported for frogs and other amphibians in tropical rainforest fragments in Manaus, Brazil (1–100 ha), and Ambohitantely, Madagascar (0.16–136 ha), where species number was positively related to fragment size (Tocher et al. 1997; Vallan 2000). Consistently, in rainforest patches (5–122 ha) on Negros Island, the Philippines the main factor influencing the herpetofaunal species number was the area (Alcala et al. 2004). Also in La Selva, Costa Rica, fragment size was the best richness predictor for frogs and lizards in patches of forest (1–7 ha) (Bell and Donnelly 2006), as well as in El Refugio-Huanchaca, Bolivia the richness of generalist frogs increased with fragment area (Watling and Donnelly 2008). Contrary to our results, rainforest fragments (1.5–48, >48 ha) in the Taï National Park, Côte d’Ivoire had low species richness of leaf-litter anurans that was not related to fragment size (Hillers et al. 2008); also richness of leaf-litter lizards in rainforest fragments at the Ibiúna Plateau, Brazil was not influenced by forest area (Dixo and Metzger 2009).
Our study shows that amphibian and reptile abundance does not depend on the fragment size, suggesting that some other patch features may have a more important role in determining the numbers of individuals. Amphibian density in rainforest fragments has been found to be negatively correlated to patch size due to the high density of streams and brooks in small patches (Vallan 2000). Other studies have described no relation between total abundance or density of amphibians and/or reptiles with patch size, even when some particular species density may be associated to the size of the area (Bell and Donnelly 2006; Dixo and Metzger 2009). It seems that in Los Tuxtlas landscape as in other Neotropical regions, small fragments can support large populations of some amphibian and reptile species.
Effect of environmental and vegetation variables on amphibian and reptile community structure
In small rainforest fragments increases in temperature and decreases in humidity, and changes in the vegetal community structure are usually caused by area reduction, isolation, canopy removal, and exposure (Saunders et al. 1991; Murcia 1995; Ferreira and Laurance 1997; Arroyo-Rodríguez et al. 2007). Additionally, some variation in the forest fragments attributes may be related to the spatial distribution of the remnants and some particular characteristics of the sites. The tolerance range of species to environmental conditions can have a strong influence on their distribution pattern (Malanson and Cramer 1999), and amphibian and reptile community structure in tropical forests is not only determined by habitat size, but also by environmental variation, microclimate, vegetation structure, disturbance, microhabitat loss, and isolation (Akani et al. 1999; Hillers et al. 2008; Gillespie et al. 2005).
In the Los Tuxtlas region microclimatic changes on forest edges delimited within the first 20 m of rainforest have an important influence over amphibian and reptile communities (Urbina-Cardona et al. 2006). We have found significant differences in environmental variables and vegetation in the study sites. F1 has experienced major local alteration of microclimatic conditions, contrary to the largest patch (F6), which is only affected by strong environmental changes on the edges and shows high humidity levels, closed canopy cover and a complex vegetation structure in most of its core area. Thus F6 was characterized by relatively similar environmental variables to those in the continuous forest LE and LTS. Though differences were not significant, the smallest fragments studied recorded lower humidity levels than the reference areas (except when a water body was present), such that relative humidity was related to patch size. Both, amphibian and reptile richness were higher in sites with high humidity levels concurring with Urbina-Cardona et al. (2006).
Linear patch shape has been associated with low reptile richness when compared with square or circular fragments (e.g. Driscoll 2004). This effect was reflected in our study as negative relationships occurred for amphibian and reptile richness with elongated patch shapes. F1, with a linear shape, is located in the highest altitude, so that elevation was negatively associated to fragment size. This last association is not general in the Los Tuxtlas region, as many of the largest forest extensions are located at high elevations across the altitudinal gradient (Mendoza et al. 2005). The elongated shape of F1 and its elevation make it totally vulnerable to environmental alterations produced by edge effects (Murcia 1995). Elevation is a determining factor affecting richness of amphibians and reptiles, as it can limit the presence of species in different forests (Fauth et al. 1989). However, we did not find any effects throughout the elevation range of our study sites (119–683 m).
Canopy cover showed significant positive effects on amphibian and reptile richness and reptile abundance in our study, being consistent with former studies in rainforest areas (Urbina-Cardona et al. 2006; Hillers et al. 2008). The two sites with the densest canopy (F4 and LE) contained more leaf litter than forests which were more open, notably F1. In our study sites leaf litter depth was an important determinant for amphibian and reptile richness. A positive relation between accumulated leaf litter depth and cover with the abundance of amphibians and reptiles or any subordinated taxa, such as salamanders or litter frogs occurs in different forest sites. This relation can be explained by the retention of humidity, the availability of invertebrates and the cover provided by leaf litter (Inger 1980; Lieberman 1986; Fauth et al. 1989; Allmon 1991; Heinen 1992; Hillers et al. 2008).
F2 (and to a lesser extent F1) was characterized by a different vegetation structure, with an important proportion of bamboo thickets. A negative relationship between slope and tree perimeter was found for these study sites since the two smallest fragments are located on steeper inclinations. All other sites are composed of woody elements and the largest and more buttressed trees occurred in F3 and F4. These trees offer an important structural component for many amphibians and reptiles. Abundance and species richness of the leaf-litter herpetofauna was much higher in plots containing buttressed trees in the tropical wet forest at La Selva, Costa Rica (Whitfield and Pierce 2005). These trees are absent from F1 and F2, hindering the presence of species specialized in this microhabitat. Additionally, broken bamboo stalks or cut internodes filled with rain water can host particular invertebrate communities different to those inhabiting trees (Louton et al. 1996). Thus, differences in vegetation structure can also affect food availability and composition for the herpetofauna.
Fragments smaller than 10 ha have higher tree mortality and vegetation disturbance by wind than larger fragments (Saunders et al. 1991; Laurance et al. 1998). In our study a high proportion of large trees resulted in a higher density of woody debris. The availability of decaying logs and fallen branches, as well as blocks of wood and planks produced by selective logging was significantly favorable for reptile abundance. Debris provides refuge that protect amphibians and reptiles from predators and desiccation, increasing their abundance and survival (Rothermel and Luhring 2005). In Los Tuxtlas species such as night lizards (Lepidophyma spp.), snakes, and leaf-litter frogs are common in these refuges (Castillo-Cerón and López-González 1990; Cabrera-Guzmán Personal Observation).
Conservation of fragmented landscapes
Our study provides evidence that fragment size has a strong impact on amphibian and reptile communities. However in Los Tuxtlas tropical rainforest the composition and structure of these communities are not totally size-dependent; instead, there are complex interactions between size, environmental variables, vegetation structure, habitat heterogeneity and anthropogenic perturbation. Thus, general conservation strategies and local management must consider not only the fragment size, but also a combination of environment and vegetation features.
The importance of large and continuous forested areas to preserve biodiversity is clear in Los Tuxtlas, as it is in any other Neotropical landscape. However, composition, species richness and abundance recorded in our study shows that a great diversity of amphibians and reptiles is still found throughout a fragmented landscape where a large portion of rainforest remains as patches that average 13.6 ha (Mendoza et al. 2005). The data obtained by our study on amphibians and reptiles attests to the fact that the presence of different-sized vegetation fragments may permit the survival of many populations of birds, mammals and other vertebrates characteristic of large forested areas in the Los Tuxtlas region (Estrada et al. 1994; Graham and Blake 2001).
A fragment-scale analysis instead of a comparison between fragmented versus continuous landscapes can provide important information on a finer scale, to determine patch sizes limits and environmental features for which communities are still structured similar to those in continuous forests. In our study, F6 (17.4 ha) is an important remnant for conservation. Its area has enough environmental variability to shelter amphibian and reptile communities as richness, abundance, and thus, diversity is similar to that in large and continuous tropical rainforest. The conservation value of smaller fragments such as F2, F3, F4 and F5 (3.6–6.6 ha) was also outstanding in this study. Despite wind disturbance, livestock presence and logging, these fragments maintain populations of several rainforest species and are still used by individuals of some large vagile species. Although the Los Tuxtlas region is protected as a Biosphere Reserve, very few efforts have been carried out to preserve rainforest remnants, which are mostly located in private or communal lands. These fragments have persisted due to inaccessibility, unsuitability for farming, or due to the owners’ will to preserve a piece of land that may provide lumber or other minor supplies to cover home necessities. Persistence of these small isolated populations is critical for the preservation of the highly diverse herpetofauna in Los Tuxtlas increasing connectivity within the area. Additionally, an integral conservation program should be proposed to include the restoration of pastureland surrounding small fragments, and the implementation of biological corridors designed to facilitate movement of ground dwelling organisms in order to restore the fauna to the existing fragments. Long term monitoring is necessary to evaluate the responses of animal communities to fragmentation over time in this and other Neotropical landscapes.
Abbreviations
- F1:
-
Fragment 1
- F2:
-
Fragment 2
- F3:
-
Fragment 3
- F4:
-
Fragment 4
- F5:
-
Fragment 5
- F6:
-
Fragment 6
- LE:
-
Laguna Escondida
- LTS:
-
Los Tuxtlas Biological Station Reserve
References
Achard F, Eva HD, Stibig HJ, Mayaux P, Gallego J, Richards T, Malingreau JP (2002) Determination of deforestation rates of the world’s humid tropical forests. Science 297:999–1001
Akani GC, Barieenee IF, Capizzi D, Luiselli L (1999) Snake communities of moist rainforest and derived savanna sites of Nigeria: biodiversity patterns and conservation priorities. Biodivers Conserv 8:629–642
Alcala EL, Alcala AC, Dolino CN (2004) Amphibians and reptiles in tropical rainforest fragments on Negros Island, the Philippines. Environ Conserv 31:254–261
Allmon WD (1991) A plot study of forest floor litter frogs, Central Amazon, Brazil. J Trop Ecol 7:503–522
Arroyo-Rodríguez V, Aguirre A, Benítez-Malvido J, Mandujano S (2007) Impact of rain forest fragmentation on the population size of a structurally important palm species: Astrocaryum mexicanum at Los Tuxtlas, Mexico. Biol Conserv 138:198–206
Bell KE, Donnelly MA (2006) Influence of forest fragmentation on community structure of frogs and lizards in Northeastern Costa Rica. Conserv Biol 20:1750–1760
Cabrera-Guzmán E, Reynoso VH (2008) Anolis uniformis: diet. Herpetol Rev 39:220
Campbell JA (1998) Amphibians and reptiles of Northern Guatemala, the Yucatán, and Belize. Animal natural history series. University of Oklahoma Press, Norman
Campbell JA, Formanowicz DR Jr, Medley PB (1989) The reproductive cycle of Norops uniformis (Sauria: Iguanidae) in Veracruz, Mexico. Biotropica 21:237–243
Carvalho KS, Vasconcelos HL (1999) Forest fragmentation in central Amazonia and its effects on litter-dwelling ants. Biol Conserv 91:151–157
Castillo-Cerón JM, López-González CA (1990) Notes on the biology and status of a population of Lepidophyma tuxtlae (Sauria: Xantusidae) in the Sierra de Santa Marta, Veracruz, México. Bull Md Herpetol Soc 26:153–158
Clarke KR, Gorley RN (2006) PRIMER 6.0 software. User manual/tutorial. PRIMER-E. Plymouth
Colwell RK (2004) EstimateS 7.0: statistical estimation of species richness and shared species from samples. Software and user’s guide
Colwell RK, Coddington JA (1994) Estimating terrestrial biodiversity through extrapolation. Philos Trans R Soc Lond B 345:101–118
Colwell RK, Mao CX, Chang J (2004) Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85:2717–2727
Crump ML, Scott NJ Jr (1994) Visual encounter surveys. In: Heyer WR, Donnelly MA, McDiarmid RW, Hayek LC, Foster MS (eds) Measuring and monitoring biological diversity: standard methods for amphibians. Smithsonian Institution Press, Washington, DC, pp 84–92
Dirzo R, García MC (1992) Rates of deforestation in Los Tuxtlas, a Neotropical area in Southeast Mexico. Conserv Biol 6:84–90
Dirzo R, Miranda A (1991) El límite boreal de la selva tropical húmeda en el continente americano: contracción de la vegetación y solución de una controversia. Interciencia 16:240–247
Dixo M, Metzger JP (2009) Are corridors, fragment size and forest structure important for the conservation of leaf-litter lizards in a fragmented landscape? Oryx 43:435–442
Donnelly MA (1999) Reproductive phenology of Eleutherodactylus bransfordii in Northeastern Costa Rica. J Herpetol 33:624–631
Driscoll DA (2004) Extinction and outbreaks accompany fragmentation of a reptile community. Ecol Appl 14:220–240
Environmental Systems Research Institute Inc. ESRI (2003) ArcView 3.3 software. Redlands
Estrada A, Coates-Estrada R, Meritt D (1994) Non flying mammals and landscape changes in tropical rain forest region of Los Tuxtlas, Mexico. Ecography 17:229–241
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fauth JE, Crother BI, Slowinski JB (1989) Elevational patterns of species richness, evenness, and abundance of the Costa Rican leaf-litter herpetofauna. Biotropica 21:178–185
Ferreira LV, Laurance WF (1997) Effects of forest fragmentation on mortality and damage of selected trees in central Amazonia. Conserv Biol 11:797–801
Frazer GW, Canham CD, Lertzman KP (1999) Gap Light Analyzer (GLA) 2.0. Imaging software to extract canopy structure and gap light transmission indices from true-color fisheye photographs. Users manual and program documentation. Simon Fraser University, Burnaby
Funk WC, Mills LS (2003) Potential causes of population declines in forest fragments in an Amazonian frog. Biol Conserv 111:205–214
Gascon C, Lovejoy TE, Bierregaard RO Jr, Malcom JR, Stouffer PC, Vasconcelos HL, Laurance WF, Zimmerman B, Tocher M, Borges S (1999) Matrix habitat and species richness in tropical forest remnants. Biol Conserv 91:223–229
Gentry AH (1982) Patterns of Neotropical plant species diversity. Evol Biol 15:1–84
Gillespie GR, Howard S, Lockie S, Scroggie D, Boeadi MP (2005) Herpetofaunal richness and community structure of offshore islands of Sulawesi, Indonesia. Biotropica 37:279–290
Gotelli JN, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Graham CH, Blake JG (2001) Influence of patch and landscape-level factors on bird assemblages in a fragmented tropical landscape. Ecol Appl 11:1709–1721
Guyer C (1988) Food supplementation in a tropical mainland anole, Norops humilis: effects on individuals. Ecology 69:362–369
Hager HA (1998) Area-sensitivity of reptiles and amphibians: Are there indicator species for habitat fragmentation? Ecoscience 5:139–147
Heinen JT (1992) Comparisons of the leaf litter herpetofauna in abandoned cacao plantations and primary rain forest in Costa Rica: some implications for faunal restoration. Biotropica 24:431–439
Hillers A, Veith M, Rödel MO (2008) Effects of forest fragmentation and habitat degradation on West African leaf-litter frogs. Conserv Biol 22:762–772
Inger RF (1980) Densities of floor-dwelling frogs and lizards in lowland forests of Southeast Asia and Central America. Am Nat 115:761–770
Laurance WF, Yensen E (1991) Predicting the impacts of edge effects in fragmented habitats. Biol Conserv 55:77–92
Laurance WF, Ferreira LV, Rankin-de Merona JM, Laurance SG (1998) Rain forest fragmentation and the dynamics of Amazonian tree communities. Ecology 79:2032–2040
Laurance WF, Lovejoy TE, Vasconcelos HL, Bruna EM, Didham RK, Stouffer PC, Gascon C, Bierregaard RO, Laurance SG, Sampaio E (2002) Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conserv Biol 16:605–618
Lee JC (1996) The amphibians and reptiles of the Yucatan Peninsula. Cornell University Press, Ithaca
Lehtinen RM, Ramanamanjato JB (2006) Effects of rainforest fragmentation and correlates of local extinction in a herpetofauna from Madagascar. Appl Herpetol 3:95–110
Lehtinen RM, Ramanamanjato JB, Raveloarison JG (2003) Edge effects and extinction proneness in a herpetofauna from Madagascar. Biodivers Conserv 12:1357–1370
Lieberman SS (1986) Ecology of the leaf litter herpetofauna of a Neotropical rain forest: La Selva, Costa Rica. Acta Zool Mex (ns) 15:1–72
Louton J, Gelhaus J, Bouchard R (1996) The aquatic macrofauna of water-filled bamboo (Poaceae: Bambusoideae: Guadua) internodes in a Peruvian lowland tropical forest. Biotropica 28:228–242
Luiselli L, Capizzi D (1997) Influences of area, isolation and habitat features on distribution of snakes in Mediterranean fragmented woodlands. Biodivers Conserv 6:1339–1351
MacArthur RH, Wilson EO (1967) The theory of island biogeography, 1st edn. Princeton University Press, Princeton
Magurran AE (2004) Measuring biological diversity. Blackwell Publishing, Oxford
Malanson GP, Cramer BE (1999) Landscape heterogeneity, connectivity, and critical landscapes for conservation. Divers Distrib 5:27–39
Marsh DM, Pearman PB (1997) Effects of habitat fragmentation on the abundance of two species of Leptodactylid frogs in an Andean montane forest. Conserv Biol 11:1323–1328
Mendoza E, Fay J, Dirzo R (2005) A quantitative analysis of forest fragmentation in Los Tuxtlas, southeast Mexico: patterns and implications for conservation. Rev Chil Hist Nat 78:451–467
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10:58–62
Patterson BD (1987) The principle of nested subsets and its implications for biological conservation. Conserv Biol 1:323–334
Patton DR (1975) A diversity index for quantifying habitat edge. Wildl Soc Bull 3:171–173
Primack RB (2004) A primer of conservation biology, 3rd edn. Sinauer Associates, Sunderland
Ramírez-Bautista A, Nieto-Montes de Oca A (1997) Ecogeografía de anfibios y reptiles. In: González SE, Dirzo R, Vogt RC (eds) Historia Natural de los Tuxtlas. Instituto de Biología, Instituto de Ecología, CONABIO. Universidad Nacional Autónoma de México, Distrito Federal, Mexico City, pp 523–532
Reagan DP (1992) Congeneric species distribution and abundance in three-dimensional habitat: the rain forest anoles of Puerto Rico. Copeia 1992:392–403
Rothermel BB, Luhring TM (2005) Burrow availability and desiccation risk of Mole Salamanders (Ambystoma talpoideum) in harvested versus unharvested forest stands. J Herpetol 39:619–626
SAS (2002) JMP 5.0.1 software. SAS Institute, Cary
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32
Schlaepfer MA, Gavin TA (2001) Edge effects on lizards and frogs in tropical forest fragments. Conserv Biol 15:1079–1090
Soto M, Gama L (1997) Climas. In: González SE, Dirzo R, Vogt RC (eds) Historia Natural de los Tuxtlas. Instituto de Biología, Instituto de Ecología, CONABIO. Universidad Nacional Autónoma de México, Mexico City, pp 7–23
StatSoft (2001) STATISTICA 6.0 software for Windows
Systat Software Inc. (2008) SigmaPlot 11.0 software for Windows
Tocher MD, Gascon C, Zimmerman BL (1997) Fragmentation effects on a central Amazonian frog community: a ten year study. In: Laurance WF, Bierregard RO Jr (eds) Tropical forest remnants: ecology, management, and conservation of fragmented communities. University of Chicago Press, Chicago, pp 124–137
Townsend DS, Stewart MM (1994) Reproductive ecology of the Puerto Rican frog Eleutherodactylus coqui. J Herpetol 28:34–40
Turner IM (1996) Species loss in fragments of tropical rain forest: a review of the evidence. J Appl Ecol 33:200–209
Urbina-Cardona JN, Olivares-Pérez M, Reynoso VH (2006) Herpetofauna diversity and microenvironment correlates across a pasture-edge-interior ecotone in tropical rainforest fragments in the Los Tuxtlas Biosphere Reserve of Veracruz, Mexico. Biol Conserv 132:61–75
Vallan D (2000) Influence of forest fragmentation on amphibian diversity in the nature reserve of Ambohitantely, highland Madagascar. Biol Conserv 96:31–43
Vogt RC, Villarreal BJL, Pérez-Higareda G (1997) Lista anotada de anfibios y reptiles. In: González SE, Dirzo R, Vogt RC (eds) Historia Natural de los Tuxtlas. Instituto de Biología, Instituto de Ecología, CONABIO. Universidad Nacional Autónoma de México, Mexico City, pp 507–528
Watling JI, Donnelly MA (2007) Multivariate correlates of extinction proneness in a naturally fragmented landscape. Divers Distrib 13:372–378
Watling JI, Donnelly MA (2008) Species richness and composition of amphibians and reptiles in a fragmented forest landscape in northeastern Bolivia. Basic Appl Ecol 9:523–532
Whitfield SM, Pierce MSF (2005) Tree buttress microhabitat use by a Neotropical leaf-litter herpetofauna. J Herpetol 39:192–198
Wilcove DS, McLellan CH, Dobson AP (1986) Habitat fragmentation in the temperate zone. In: Soulé ME (ed) Conservation biology: the science of scarcity and diversity. University of Michigan, Sunderland, pp 237–256
Zug GR, Vitt LJ, Caldwell JP (2001) Herpetology: an introductory biology of amphibians and reptiles, 2nd edn. Academic Press, New York
Acknowledgments
We thank Denise Arroyo, Florencia Bertoni, Alejandro Carbajal, Henrry F. Carmona, Jorge A. Cime, Arturo García, Rafael Lara, Marco A. Márquez, Hugo Reyes, J. Carlos Rosas, Cristian Salvatierra, and Martín Sánchez for invaluable help in amphibian and reptile surveys. Álvaro Campos, C. Omar Becerra, Roberto García, Armando Ponce, and Marcia M. Ramírez participated in the vegetation surveys and study site characterization. The Palacios-Domínguez family provided housing and assistance during the field work and permitted us to conduct our research on their lands, same as other families at Los Tuxtlas. Genaro Gutiérrez helped to estimate fragments size. Greg Brown and Gerardo Rivas assisted in the analysis of environmental and vegetation data. Rosamond Coates, Saúl López, Betsabé Ruiz, and J. Nicolás Urbina provided reviews and suggestions on earlier versions of the manuscript. Jesse Meik and Marion Winkler improved the English language. Funding was provided by PAPIIT, DGAPA, UNAM (Grant No. IN 222506) to V. H. R. Consejo Nacional de Ciencia y Tecnología (CONACyT) and Dirección General de Estudios de Posgrado UNAM (DGEP) awarded Masters’ scholarships to E. C.-G. Specimens were collected under permit Num/SGPA/DGVS/02132, DGVS, SEMARNAT to V. H. R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cabrera-Guzmán, E., Reynoso, V.H. Amphibian and reptile communities of rainforest fragments: minimum patch size to support high richness and abundance. Biodivers Conserv 21, 3243–3265 (2012). https://doi.org/10.1007/s10531-012-0312-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-012-0312-4