Abstract
Context
An understanding of species-habitat relationships is required to assess the impacts of habitat fragmentation and degradation. To date, habitat modeling in fragmented landscapes has relied on landscape composition and configuration metrics and the importance of habitat quality in determining species distributions has not been sufficiently explored.
Objectives
We evaluated how habitat use by herbivores and frugivorous mammals is shaped by a potential interaction of habitat amount and quality in the Brazilian Pantanal wetland. We also assessed if the contribution of habitat quality to species´ habitat use varies according to the species sensitivity to habitat loss.
Methods
We combined mammal detection data obtained from camera traps with thematic maps to estimate the amount of habitat and measured habitat quality using local environment variables and distance to waterbodies. Specifically, we used a single-season occupancy approach to evaluate the relative support of univariate, additive, and interactive relationships between species-specific habitat use and measures of habitat quality and quantity.
Results
Habitat quality was more important than habitat amount in determining species habitat use (occupancy) in a naturally fragmented landscape. Habitat quality alone was the best predictor of habitat use for two of the six species (white lipped peccary and collared peccary), but no species’ habitat use was explained solely by habitat amount. Habitat amount was influential only when considered in conjunction with habitat quality covariates and only for two sensitive species to habitat loss (agouti and red brocket deer). Habitat quality alone was the best predictor of habitat use for two of the less sensitive species (white lipped peccary and collared peccary). Habitat use for two species was not explained by any covariate (tapir and gray brocket deer).
Conclusions
Conservation programs should incorporate both habitat quality and amount when dealing with sensitive species and prioritize habitat quality management when focusing in less sensitive species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The continual conversion of natural areas to anthropogenic land-use is a primary threat to biodiversity worldwide (Hansen et al. 2013). An understanding of species-habitat relationships is required to predict the impact of land-use change and inform species conservation and management actions (Fahrig 2003; Fischer and Lindenmayer 2007; Desbiez et al. 2009a). To date, habitat modeling in fragmented landscapes has relied on landscape composition and configuration metrics based on the patch-corridor-matrix and heterogeneous mosaic theoretical frameworks (Boscolo et al. 2016; Presley et al. 2019). Some authors have argued that the diversity of landscape effects on biodiversity can be explained simply by the amount of habitat in the landscape (Fahrig 2013). However, the importance of habitat quality and its relationship to habitat amount has not been sufficiently explored (Mortelliti et al. 2010). There is a growing interest in this topic because improving local habitat quality in remaining patches may be a promising solution in areas where increasing native vegetation cover is not viable (Baguette et al. 2013).
Species distributions in fragmented landscapes are driven by multiple, dependent ecological processes functioning simultaneously Fischer and Lindenmayer 2007; Fahrig 2017). Thus, the habitat use of species is often shaped by a tradeoff between costs (e.g., energy spent to move, avoid predation and competition) and benefits (e.g., availability of food resources, water, salt, and breeding habitat) (Driscoll et al. 2013). Previous studies have shown positive effects of habitat amount on mammalian species occurrence and richness (Melo et al. 2017; Regolin et al. 2017). However, these results may be overly simplistic and/or biased, because habitat amount does not equate to habitat quality (Fischer and Lindenmayer 2007). Habitat amount is the total area covered by a specific habitat type within the landscape (Fahrig 2013; e.g., vegetation or land-cover types), and habitat quality is the ability of the environment to provide adequate resources and conditions for the survival of individuals and persistence of populations (Hall et al. 1997; e.g., food availability).
Measurements of landscape structure based on human perspectives of land-cover types are suitable to estimate the amount of habitat in a landscape (Fischer and Lindenmayer 2007; Fahrig 2013). However, this approach fails to clarify the role of habitat quality in species occurrence or persistence as it implies homogeneity within land-cover classes (St-Louis et al. 2009, 2014; Regolin et al. 2020). Natural heterogeneity in vegetation structure and anthropogenic degradation lead to variation in biotic and abiotic habitat conditions that define habitat quality (Mortelliti et al. 2010), and its importance in predicting species distributions has been demonstrated for marsupials and rodents (Holland and Bennett 2007), primates (Willems et al. 2009), artiodactylans (Winnie et al. 2008), carnivores (Brady et al. 2011), xenarthrans (Santos et al. 2016), and birds (St-Louis et al. 2009, 2014; Wood et al. 2013). In patchy landscapes, habitat selection involves both the amount and the quality of habitat patches (Mortelliti and Boitani 2008; Gardiner et al. 2018; Costa-Araújo et al. 2021) and varies among species (Kellner et al. 2019). An essential conservation challenge is to understand the interaction between these landscape features for species with different landscape perception (sensu Goheen et al. 2003; Hansbauer et al. 2010). Assessing the mechanisms driving habitat selection is important for designing effective conservation and management actions, particularly for endangered species that may be restricted in range due to habitat loss and/or degradation (Fahrig 2003; Fischer and Lindenmayer 2007).
Brazilian Pantanal is a naturally fragmented landscape covered mainly by native grasslands interspersed by patches of woody vegetation – forest and dense shrubland (Pott and Silva 2015). High vegetation productivity is driven by a seasonal flood regime, which allow for an abundance of wildlife, including stable populations of species that are threatened in other biomes (Alho et al. 2011) such as, the lowland tapir, Tapirus terrestris (Linnaeus, 1758) (Trolle et al. 2008) and the white lipped peccary, Tayassu pecari (Link, 1795) (Keuroghlian and Eaton 2008). There are few protected areas in the Pantanal as most of the land (95%) is privately owned and operated as cattle ranches; the main economic activity in the region for the last two centuries (Harris et al. 2005). The cattle graze on natural grasslands and find complementary food sources in woody vegetation patches (e.g., fruits and leaves), which also provide relief from the hot temperatures found in the grasslands. Within woody vegetation patches, cattle also degrade the habitat of native mammal species by trampling plant seedlings and shrubs that are important food resources (Desbiez et al. 2009a). To improve cattle productivity, native vegetation has been recently replaced by pastures of exotic, invasive African grasses (Brachiaria (Trin.) Griseb.); however, the magnitude of the effects of habitat degradation and habitat loss on native species in Pantanal have not been well documented (but see Desbiez et al. 2009a; Dorado-Rodrigues et al. 2015; Eaton et al. 2017; Silveira et al. 2018). Although the sustainability agenda for the Pantanal proposed by a group of scientific experts recognizes that intensification of cattle production leads to habitat degradation, it does not include the improvement of habitat quality as a strategy for wildlife conservation (Tomas et al. 2019).
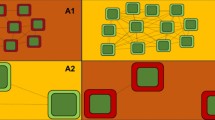
In this study, we evaluate the relative importance of habitat amount and quality on habitat use by six medium to large-bodied herbivores and frugivorous mammals in the Brazilian Pantanal wetland: lowland tapir (T. terrestris), agouti (Dasyprocyta azarae Lichtenstein, 1823), red brocket deer and the gray brocket deer (Mazama americana (Erxleben, 1777) and Mazama gouazoubira (Fischer, 1814)), white lipped peccary and collared peccary (T. pecari and Dicotyles tajacu (Linnaeus, 1758), respectively). We aim to assess how species habitat use is shaped by a potential interaction between habitat amount and habitat quality in the region. We predict that in areas with little habitat, species use may be relatively invariant to changes in habitat quality (Fig. 1A). However, at intermediate amounts of habitat, an increase in habitat quality will improve a species probability of use because quality can compensate for the amount of habitat (Fig. 1A). Finally, in areas with high habitat amount, a decrease in quality habitat will reduce species use, but not as dramatically (Fig. 1A). We also expect that the contribution of habitat quality to the habitat use will vary according to the species sensitivity to habitat modifications; habitat quality should be more important for the most sensitive species (Fig. 1B). For example, we expect that strongest interactions between habitat quantity and quality for sensitive species such as lowland tapir and agouti and few or no interaction for less sensitive species such as collared peccary and gray brocket deer.
Expected interactive effects of the amount and quality of habitat on habitat use by six medium to large-bodied herbivores and frugivores mammalian species in the Brazilian Pantanal Wetland (A). Gradient of species sensitivity to habitat modifications: gray brocket deer, collared peccary, white lipped peccary, red brocket deer, agouti, and tapir (B)
Methods
Study area
We conducted our study in the Pantanal biome, the world’s largest wetland, in the Nhecolândia subregion, Mato Grosso do Sul State, Brazil (Fig. 2). Vegetation is a mosaic of flooded and non-flooded grasslands, forest, and cerrado interspersed by seasonal and perennial lakes with freshwater or ‘salines’—lakes with alkaline and brackish waters (Rodela et al. 2008). Floristic composition is mainly from the Cerrado biome, with influences of Atlantic Forest, Amazon, and Chaco (Pott and Silva 2015). The mean annual temperature is 26 °C. The Pantanal is a periodically flood wetland. The average annual rainfall is 1100 mm, but is highly concentrated (60–80%) in the wet season (between December and May) when grasslands flood and lakes reach their highest water level. The dry season occurs from June to November. Our study area consisted of five private ranches that graze cattle (Bos taurus Linnaeus, 1758, Bovidae) at low densities (0.25–0.35 head ha−1). The study area extent is roughly 14,600 hectares, of which 54% is grassland, 22% cerrado, 21% forest, 0.03% lakes, and less than 0.01% salines (Rodela et al. 2008). Native wildlife hunting is forbidden but hunting of feral pigs (Sus scrofa Linnaeus, 1758, Suidae), an exotic species introduced about 200 years ago, is permitted (Desbiez et al. 2009b).
a Land-use and land-cover thematic map of the study landscapes in Nhecolândia subregion within Brazilian Pantanal wetland (modified from Rodela et al. 2008), b Location of Pantanal wetland within Brazil, c Location of the study area within Pantanal wetland, and d aerial photograph of the study area (Rodela et al. 2008)
Focal species
We selected six native mammalian herbivores and frugivores species because previous studies suggest that these species are adversely affected by habitat loss and degradation due to their narrow food resource requirements (Swihart et al. 2003; Kellner et al. 2019). We also choose these species because they differ in landscape perception and represent a gradient of sensitivity to habitat modifications from the less sensitive to the most sensitive: gray brocket deer, collared peccary, white lipped peccary, red brocket deer, agouti, and tapir (Fig. 1B).
Gray brocket deer is widely distributed in a diversity of forest and cerrado cover types and occurs in patches of native vegetation in agricultural landscapes, which suggests it is tolerant to anthropogenic modifications (Black-Décima et al. 2009). Collared peccary inhabits a great diversity of native vegetation types, including tropical rainforest, cerrado, semi-arid, and is tolerant to anthropogenic disturbances. White lipped peccary preferentially inhabits humid and dense pristine forests, but it is also found in dry forests near water bodies (Mayer and Wetzel 1987). Red brocket deer typically occur deep in the forest interior (Varela et al. 2009). Tapir and agouti mainly occupy native forests associated with perennial water bodies (Padilla and Dowler 1994; Desbiez et al. 2009a; Santos-Filho et al. 2012).
Camera trap sampling design
We sampled mammals from March to November 2008 using camera traps (Tigrinus®, Timbó, Santa Catarina State, Brazil) at 51 stations within the study area (Fig. 2) chosen to represent a gradient of woody vegetation cover (forest or shrubland cover). We installed one un-baited camera trap 30–40 cm above the ground within each selected woody patch. We did not install devices in grasslands because previous experience revealed that camera traps failed to operate under extreme hot weather (LG Oliveira-Santos, personal observation). Stations were systematically placed 1–2 km apart (a systematic random sample) to insure independence among sampling points, based on mean species home range area (i.e. 500 hectares, Canãs 2010; Fragoso 1999; Silvius and Fragoso 2003; Keuroghlian et al. 2004, 2014; Jorge and Peres 2005; Grotta-Neto et al. 2019). Cameras were programmed to operate 24 h a day and recorded the date and time of each photograph. The sampling effort totaled 3060 camera-trap days (30 days*51 stations*2 seasons). Camera-traps sampled each station during 60 days; 30 days during the dry season and 30 days during the wet season.
Habitat quality variables
We measured habitat quality at each station using local environmental variables and recorded the distance to the nearest waterbodies (freshwater and saline lakes). At each station, we established two perpendicular 50-m transects centered on each camera. At 0.5-m intervals along each transect (50-m transect length/0.5-m interval × 2 transects = 200 points per station), we counted the number of habitat quality variables that occurred at the point on the transect line: (i) acuri palm trees (Attalea phalerata (Mart. ex Spreng.), Aracaceae), (ii) shrubs at three heights (ground level < 0.1 m, 0.1–0.5 m, and 0.5–1.0 m), (iii) caraguata bromeliads (Bromelia antiacantha Bertoloni 1824, Bromeliaceae), and (iv) specified bare ground, when none of the considered variables were present on the transect line.
Acuri is a large-seeded palm tree that dominates the understory and produces fruits year-round, which are consumed by the tapir and both peccaries, and it is the main food resource of the agouti (Desbiez et al. 2009b; Cid et al. 2013; Negrelle 2015). We considered shrub abundance as a proxy for the structural complexity of vegetation (St-Louis et al. 2009, 2014; Brady et al. 2011). Higher levels of structural complexity are associated with higher food resources for the herbivores and lower predation risk (Brady et al. 2011; Driscoll et al. 2013). Caraguata is a thorny bromeliad that occurs in high-density on the forest floor in areas of high solar radiation (Antunes et al. 2016). We hypothesized that bromeliads will affect the detectability of all six species at occupied stations because they can act as a barrier to movement for some species and facilitate escape routes for others (Antunes et al. 2016).
Distance to lakes and habitat amount
To estimate the distance-based metrics, we calculated the Euclidean distance from the camera to the nearest perennial or seasonal freshwater lake (distance to lake), and to the nearest saline (distance to saline) using the LSMetrics (see software details below). Habitat use (occupancy) by tapir and agouti is expected to be higher at camera stations near freshwater lakes, and proximity to saline may increase habitat use by peccaries and deer, species that seek mineral supplementation (Tobler et al. 2009).
We used the land cover map generated by Rodela et al. (2008), who interpreted and classified Landsat 7 images at a 1:20,000 cartographic ratio in 12 classes. Mapping was also supported by aerial photography interpretation (scale 1: 15,000) and field validation (Rodela et al. 2008). We converted land-use and land cover maps from vector (.shp) to 5-m matrix format (.tif) using QGIS (QGIS Development Team 2019). We used the ‘raster’ R package (Hijmans 2017) to reclassify the original 12 land-use and land cover classes into 5 categories (Fig. 2; Table S1): (i) forest – Cerradão and seasonal forest and carandá tree patches), (ii) shrubland – cerrado shrubland, cerrado stricto sensu, (iii) grassland – grasslands, saline beach, saline field, and vazante, (iv) lakes – perennial or seasonal lakes, and (v) perennial saline lakes (Table S1).
We used LandScape Metrics (LSMetrics), an open-source free package (Niebuhr et al., In prep., https://github.com/LEEClab/LS_METRICS/wiki), to calculate the following landscape structure metrics in relation to the amount of habitat within a specified spatial window: (i) forest cover – percentage of forest in the landscape, and (ii) cerrado cover – percentage of cerrado in the landscape. We calculated these composition-based metrics at 10 moving window extents: 4 ha (200 × 200 m), 16 ha (400 × 400 m), 36 ha (600 × 600 m), 64 ha (800 × 800 m), 100 ha (1000 × 1000 m), 144 ha (1200 × 1200 m), 196 ha (1400 × 1400 m), 256 ha (1600 × 1600 m), 324 ha (1800 × 1800 m), and 400 ha (2000 × 2000 m).
Multicollinearity analysis
We evaluated multicollinearity of our habitat amount and quality variables using Pearson’s correlation (r) and eliminated variables when |r|≥ 0.70 (Dormann et al. 2013). First, we verified weak correlation between local habitat quality variables (|r|< 0.40; Fig. S1). Next, we calculated pairwise correlations for the 10 window extents for each landscape metric (habitat amount; Figs. S2 and S3). Not surprisingly, there was high correlation between moving window sizes, so we selected one extent of Forest cover (144 ha) and one extent of Cerrado cover (144 ha). When selecting the landscape extension, we consider the range amplitude of values of habitat amount (minimum to maximum) and the frequency of the stations in that range, selecting the extension that resulted in a frequency distribution as uniform as possible. We also avoided landscapes overlap as suggested by Holland et al. (2004). Finally, we verified that there was low multicollinearity between the eight habitat quality variables and the two habitat amount extents (|r|< 0.55; Fig. S4). The range, mean, and standard deviation of the final covariate set are presented in Table S2.
Species detection data and occupancy modelling
Our camera traps operated for 30 days per season (dry and wet) and we defined a survey as a 5-day period; accordingly, each camera station had 6 surveys (occasions) per season, totaling 12 surveys per station. We compiled detection histories for each of our native mammal species using the functions ‘cameraOperation’ and ‘detectionHistory’ of the camtrapR package (Niedballa et al. 2016).
We tested possible changes in each species distribution between seasons by fitting three dynamic (multiseason) occupancy models: (1) one model where colonization and extinction probabilities were fixed to zero, representing no change in the species’ distribution, (2) a model where colonization and extinction were modeled as a random process, and (3) a model where colonization and extinction probabilities were first-order Markov processes (MacKenzie et al. 2017). We determined the most parsimonious model structure based on the relative difference in Akaike’s Information Criterion (ΔAIC). We found no evidence that species’ distribution changed between seasons for the gray brocket deer (Table S3), thus for this species we used data from all 12 surveys in a single-season occupancy analysis. The other five species showed evidence of distributional change between seasons (Table S3), so we used a single-season occupancy model with 6 surveys and used the season as a covariate to determine if there are major differences in species occurrence or detection probability among the seasons (Table S4).
Single-season occupancy models include two parameters: (1) occupancy (ψ), the probability that the target species used a camera station during the season, and (2) detection probability (p), the probability of detecting the target species during a survey, given the station was used by the species. For each of our 6 mammalian species, we developed a candidate set of models based on natural history of each species (Table S4). We used a two-step modeling approach (Morin et al. 2020); first, we fit all the hypothesized detection probability structures using a constant occupancy structure, ψ(.). Specifically, we explored structures where species detection probability varied spatially according to the abundance of bromeliads (p(bromeliad), temporally among seasons (dry or wet, p(season)), or was constant among all stations and seasons, p(.) (Table S4). Retaining supported detection probability structures (ΔAIC or ΔQAIC < 2) we fit all hypothesized occupancy structures (Table S4). Specifically, we modelled occupancy probability (habitat use) as univariate functions of habitat amount (forest or cerrado cover), season (dry or wet, when applicable), or habitat quality (local environmental variables, distance to lake, or distance to salines). We also considered all additive and interactive combinations of habitat amount and season or habitat amount and habitat quality variables (two-way interactions, Table S6). All the covariates were standardized [(value-mean value)/standard deviation]. We also included a null model where neither occupancy nor detection probability vary with our measured covariates (intercept only). We used the parametric bootstrap procedure developed by MacKenzie and Bailey (2004) to assess goodness of fit and estimate overdispersion parameter (\(\hat{c}\)) for each species. We conducted 5000 parametric bootstraps to evaluate the performance of the most general model for each species. We fit all models using the R packages ‘unmarked’ (Fiske and Chandler 2011) and ‘AICcmodavg’ (Mazerolle 2020). We compared models in each candidate set based on Akaike Information Criterion or Quasi-AIC (ΔAIC or ΔQAIC), and report model weights (w), model fit (negative log-likelihood value), and the number of parameters (Burnham and Anderson 2002; Arnold 2010). We reported estimated coefficients for covariate effects (on the logit scale), and associated standard errors and confidence intervals for well-supported models (Burnham and Anderson 2002). We identified uninformative parameters when: (1) the addition of a given effect (covariate) did not improve model fit and (2) when the estimated effect was near zero and/or imprecise (Burnham and Anderson 2002; Arnold 2010).
Results
The parametric goodness of fit test showed little or no evidence of lack of fit for four of the six species (\(\hat{c}\) ≤ 1.4), but we did find evidence of overdispersion for tapir (\(\hat{c}\) = 2.16; p-value = 0.02) and gray brocket deer (\(\hat{c}\) = 3.17; p-value = 0.04) and thus used Quasi-AIC for model selection for these two species.
Tapir occupancy models
Tapir used roughly half of the study area (\(\hat{\psi } =\) 0.57 for all stations) and habitat use was not strongly influenced by any of our habitat covariates (Table S6). There was some evidence that habitat use was driven by the amount of habitat (forest cover), but the effects of this variable was imprecise (\(\hat{\beta }_{{forest}} = 0.49,~\widehat{{SE}}(\hat{\beta }_{{forest}} ) = 0.27\); Table S6) and the null model was the best-supported among the competing models. The detectability of the tapir was similar across stations and seasons (Table S5, \(\hat{p} =\) 0.25; \(\widehat{{SE}} =\) 0.03).
Agouti occupancy models
The best-supported model demonstrated that agouti habitat use was driven by an interaction between habitat amount (forest cover) and quality (distance to lake) (Figs. 3 and S5; w = 0.25, Table S6). There was also evidence that agouti habitat use was higher during the wet season (\(\hat{\psi } = 0.77\); \(\widehat{{SE}} =\) 0.06) and lower, or more concentrated during the dry season (\(\hat{\psi } = 0.47\); \(\widehat{{SE}} =\) 0.08; w = 0.24, Table S6). Species habitat use was explained to a lesser extent by habitat quality alone (abundance of acuri palm trees) (Fig. 4A, w = 0.17, Table S6). The top model predicted divergent agouti responses to habitat quality conditional on the amount of forest cover; in landscapes with high forest cover, agouti habitat use was high near lakes, but under low and medium habitat cover, habitat use was higher farther from lakes. The third-best model showed that agouti´s habitat use was positively related to acuri palm abundance, which is the main food source. The agouti detectability was different between the two seasons, with higher detection probability during wet season (\(\hat{p} =\) 0.53; \(\widehat{{SE}} =\) 0.03) compared to the dry season (Table S5, \(\hat{p} =\) 0.29; \(\widehat{{SE}} =\) 0.05).
Interactive effects of habitat amount and habitat quality when estimating mammal probability of use within Pantanal wetland, Brazil. Estimated habitat use (occupancy) for agouti (A) and red brocket deer (B) were interactive functions of forest cover and distance to freshwater and saline lakes, respectively. Relationships are given for low forest cover (17%; 0.33 quantile), medium (23%; 0.66 quantile), and high (57%; 1 quantile). Grey shading is the standard error
Plots of predicted mammal species habitat use (occupancy) probability within Pantanal wetland, Brazil as a function of a single habitat quality covariate. Estimated habitat use for agouti was function of abundance of acuri palm tree (A). Estimated habitat use for red brocket der was function of distance to saline (B). Estimated habitat use for white lipped peccary was function of abundance of high shrubs (C). Estimated habitat use for collared peccary was function of distance to lake (D). The points represent the occupancy estimates for the 51 stations. Grey shading is the standard error
Red brocket deer occupancy models
The best supported model (w = 0.36) suggested an interaction between habitat amount (forest cover) and quality (distance to salines). Proximity to salines increased habitat use by red brocket deer but forest cover mediated its importance (Fig. 3B and S5B). Distance to saline was more important in high forest cover landscapes than in low and medium forest amounts. The second best supported model also indicated the importance of the proximity to salines (Fig. 4B, w = 0.35, Table S6). Red brocket deer habitat use was higher at stations near salt water lakes \(\left( {\hat{\psi } = 0.90} \right)\) and declined as distance to saline increased (Fig. 4B). The detectability of the red brocket deer did not differ across stations and seasons (Table S5, \(\hat{p} = 0\).12; \(\widehat{{SE}} =\) 0.02).
White lipped peccary occupancy models
Habitat use by white lipped peccaries was most influenced by a single habitat quality variable, but contrary to our predictions, peccary occurrence decreased with the abundance of high shrubs (Table S6, Fig. 4C, w = 0.32). The second best supported model (w = 0.20) suggested an additive effect of habitat amount (forest cover) and habitat quality (abundance of high shrub) on habitat use, but the former variable was a ‘pretending variable’ (sensu Arnold 2010) as its inclusion did not improve model fit (Table S6). Local bromeliad abundance reduced detection probability for peccaries from 0.32 (\(\widehat{{SE}} =\) 0.06) at used stations with no bromeliads to 0.04 (\(\widehat{{SE}} =\) 0.03) for used stations with a high abundance of bromeliads (Table S5).
Collared peccary occupancy models
Habitat use by collared peccaries was also influenced by a single habitat quality, but contrary to our predictions, collared peccary use increased with distance from freshwater lakes (Table S6, Fig. 4D, w = 0.58). Similar to our findings for white lipped peccary, habitat amount (cerrado cover) was an uninformative parameter (i.e. ‘pretending variable’, Arnold 2010). The detectability of the collared peccary was similar across stations and seasons (Table S5, \(\hat{p} =\) 0.33; \(\widehat{{SE}} =\) 0.03).
Gray brocket deer occupancy models
Gray brocket deer used most of the study area (\(\hat{\psi } >\) 0.85 for all stations) and accordingly, habitat use was not strongly influenced by any of our habitat covariates (Table S6). There was some evidence that habitat use was higher at stations further from salines or with fewer bromeliads, but the effects of these habitat quality covariates were imprecise (Table S6). The detection probability of gray brocket deer was similar across all used stations and seasons (Table S5, \(\hat{p} =\) 0.22, \(\widehat{{SE}} =\) 0.02).
Discussion
In general, our results suggest that habitat quality is more important than habitat amount in determining species habitat use in a naturally fragmented landscape. We found that habitat quality alone was the best predictor of habitat use for two of the six species (i.e., white lipped peccary and collared peccary) and habitat amount was influential only when considered in conjunction with habitat quality covariates and only for two of the most sensitive species (i.e., agouti and red brocket deer). Habitat use by species that are more tolerant of habitat modification was better modeled by habitat quality covariates alone. Habitat use for two species was not explained by any covariate (i.e., tapir and gray brocket deer).
Only a subset of habitat quality covariates seemed important: those related to (1) distance to waterbodies (either freshwater or saline) and (2) abundance of high shrub. The influence of these habitat quality covariates on habitat use (positive, negligible, or negative) differed across species and sometimes interactively with habitat amount (agouti and red brocket deer). The abundance of acuri palm tree was only important for the one species for which it is the main food source (agouti). Abundance of low shrubs and bromeliads did not affect habitat use for any species. The only habitat amount covariate that was influential was forest cover and only when considered in conjunction with habitat quality covariates.
Interaction between habitat amount and quality in fragmented landscapes
Our results indicated that habitat quality and habitat amount interact to increase habitat use for two of the most sensitive species. This finding corroborates the importance of cost-benefits tradeoffs on species habitat selection. Contrary to our expectations, the contribution of habitat quality to species use was not highest at intermediate levels of habitat amount, but the influence of habitat quality depended on habitat amount (Fig. 3). Surprisingly the effects of habitat quality on species-specific probability of use was divergent (negative or positive) across a gradient of habitat amount (e.g., agouti results).
The idea that habitat quality likely influences species’ distribution in fragmented landscapes has been supported in some works that modeled biodiversity using only habitat quality measurements (e.g., Holland and Bennett 2007; Winnie et al. 2008; St-Louis et al. 2009; Willems et al. 2009; Brady et al. 2011; Wood et al. 2013). Other studies have compared the explanation power among metrics of habitat composition and quality, and found habitat quality can overcome habitat composition influences (e.g., St-Louis et al. 2014; Rocha et al. 2017; Santos et al. 2016; Regolin et al. 2020). Nonetheless, habitat loss and habitat degradation are dependent ecological processes acting simultaneously (Fischer and Lindenmayer 2007; Fahrig 2017) and few studies have shown the joint effects of habitat amount and quality on species distribution patterns in fragmented landscapes.
The pattern we found for two of the most sensitive species are in accordance with previous studies that suggest that species occurrence is determined by both the amount and the quality of remnant habitat patches. For instance, Mortelliti and Boitani (2008) found that patch use by carnivores (the badger Meles meles (Linnaeus, 1758) and the beech marten Martes foina (Erxleben, 1777)) was driven by additive effects of landscape structure and food resources in the Province of Siena, Italy. Their results suggest that within certain structural limits, species occurrence probability increases in small and isolated habitat patches with relative high amounts of resources; however, these species were absence in the smallest and most isolated patches, despite availability of resources. Gardiner et al. (2018) assessed the occupancy pattern of a medium-sized marsupial (the eastern bettong Bettongia gaimardi (Desmarest, 1822)) in an agricultural landscape of Tasmania, Australia. They found that species occurrence is determined by the amount of woodland cover and habitat quality, indicated by density of regenerating stems. Similarly, Costa-Araújo et al. (2021) revealed that the occurrence of the vulnerable titi monkey (Callicebus melanochir (Wied-Neuwied, 1820)) in mainly driven by patch area in the Brazilian Atlantic Forest, but improved habitat quality increases the species occurrence in small patches (< 100 ha). Collectively, all three studies suggest that species’ responses are driven mostly by habitat amount with additive effect of quality. To our knowledge, our study is the first to assess interactive effects of habitat quality and amount to predict species distributions in fragmented landscapes.
Understanding the underlying mechanisms of the role of habitat quality in habitat modeling is one of the main challenges of Landscape Ecology (Mortelliti et al. 2010). For example, it is unclear if habitat quality effects are species-trait dependent or whether habitat quality matters only within certain spatial arrangements (Mortelliti et al. 2010). The relationship between species sensitivity and the importance of habitat quality on species-specific habitat use contrasted our expectations. Habitat quality metrics were important to the habitat use by four species, but in association with habitat amount for just two of the most sensitive species. The landscape we have evaluated is immersed in a relatively well preserved area, where habitat amount might not be a limiting resource for the majority of the studied species at the spatial extent at which we performed the landscape analyzes. Thus the less sensitive species select areas associated with habitat quality, while two sensitive species (i.e., agouti and red brocket deer) consider how habitat quality interacts with habitat amount. To advance in this topic, future studies should increase the range of habitat cover gradient (e.g. 5–95%) to include variegated landscapes and/or evaluate ecosystems that are not naturally fragmented (e.g., the Brazilian Atlantic Forest or the Amazon). Additionally, future studies would benefit from the inclusion of species of different ecological groups (e.g., insectivores, omnivores, and carnivores) to assess how the relation between habitat quality and habitat amount changes across a gradient species-related traits.
Species-specific findings
Our findings revealed that tapir habitat use was not influenced any habitat covariate, contrary to our expectations, and differed from previous research in other regions. As a forest dwelling specialist (Padilla and Dowler 1994), tapirs require forest patches to forage, breed, and move through the landscape. In the Brazilian Atlantic Forest, tapirs preferably use sites near water resources with high density of palms in the southeast (Ferreguetti et al. 2018), and frequently used floodplains in the south (Vidolin et al. 2009). Tapirs occurred close to salt licks in the Chaco and Chiquitano dry forests of Bolivia (Noss et al. 2003) as well as in the Peruvian Amazon (Tobler et al. 2009). Recently, Paolucci et al. (2019) recorded tapirs using burned forests twice as often as undisturbed and closed canopy forests in the Amazon/Cerrado ecotone in Brazil. It is possible that the varied findings in previous studies are due to differences in the limiting factors across regions. For example, while water bodies and forest patches are very important to tapir occupancy (Padilla and Dowler 1994) they are not limiting in our ecosystem. The study region is highly forest with many lakes that are easily accessible, thus tapir habitat use is relatively high (> 50%) and consistent throughout the study area.
The high habitat use by agouti in highly forested landscapes near water sources is in accordance with preceding works. Agouti habitat use was higher within forest patches in the Pantanal (Desbiez et al. 2009a) and Santos-Filho et al. (2012) recorded agoutis exclusively in riparian forests in the Brazilian Cerrado. In the Atlantic Forest, red-rumped agouti (Dasyprocta leporina (Linnaeus, 1758)) occurrence was explained mainly by proximity to water (Ferreguetti et al. 2015; 2017; 2018). However, habitat use by agoutis has been poorly studied, which obscures the interpretation of our interactive model. The third best model showed that agouti´s occupancy was positively related to acuri palm abundance, as demonstrated by a previous work at the same site (Cid et al. 2013). Acuri palm fruits are the key food resources to agouti especially during the dry season (Cid et al. 2013).
The scientific literature lacks detailed information about red brocket deer habitat use. Some studies suggest the species is a forest dwelling specialist throughout its distribution. For instance, the red brocket deer preferably inhabits forest in the Brazilian Pantanal (Desbiez et al. 2009a), in the Tambopata region, south-eastern Peruvian Amazon (Tobler et al 2009), and in the Bolivian Chiquitano forest (Rivero et al. 2005). We found the species preferably inhabits high forest landscapes close to salines in Pantanal. The species must visit these natural salt sources for mineral supplementation because its sodium requirements cannot be met solely through its diet (Tobler et al. 2009), which is frugivore-herbivore (Gayot et al. 2004). Our results reinforce forest dependence for red brocket deer occupancy and are in accordance with Tobler et al. (2009) who found the species frequently using salt licks in the Peruvian Amazon.
White lipped peccaries are restricted to well-preserved forest across the species range (Altrichter et al. 2019). For instance, Reyna-Hurtado and Tanner (2005) found the species selecting medium subperennial forest and low-subperennial-flooded forest in Calakmul Forest, Campeche, Mexico. In the southern Atlantic Forest, Brazil, white lipped peccaries use mainly Araucaria forest and floodplains (Vidolin et al. 2009). The preservation of high quality forest patches is the main conservation strategy for the species persistence in the Pantanal (Keuroghlian et al. 2009) and in the Atlantic Forest (Keuroghlian and Eaton 2008). These studies define habitat quality in terms of fruit richness and availability, while we estimated food source abundance by counting acuri palm trees (including fruiting and non-fruiting individuals) and shrubs. Our results suggested white-lipped peccary use was not influenced by acuri abundance, but was negative effected by the abundance of high shrubs. Peccary herds move mostly through trails within forest patches with abundance availability of fruits and rest in bare ground areas or low height vegetation (Mayer and Wetzel 1987). Thus, the species avoids areas with high concentration of bromeliads and high shrubs because they serve as barriers to movement or are inadequate for resting.
Collared peccaries ability to use areas distant from lakes can be explained by its low water requirement and the species diet. The species kidneys have high capacity to concentrate urine, reducing the necessity of water ingestion (Garbor et al. 1997). The collared peccaries feed on resources of nonfloodable vegetation (Desbiez et al. 2009b) and consequently the species inhabits Cerrado patches and forest edges in the Pantanal (Desbiez et al. 2009a). Our results agree with previous finding from the Amazon, where collared peccaries occurred almost exclusively in terra-firme forest, avoiding wetlands and riverine vegetation (Fragoso 1999).
Although the gray brocket deer occurs primarily in edges between cerrado and forest habitats in Pantanal (Desbiez et al. 2009a; Grotta-Neto et al. 2019), our finding suggest the species is widely distributed in the study area. Our results corroborate preceding works in the Bolivian Chaco (Rivero et al. 2005) and in the Atlantic Forest (Ferregueti et al. 2015), where gray brocket deer were commonly recorded and widespread throughout those areas.
Conservation implications
Our study revealed that for a sensitive species, habitat use is determined by the interaction of both the amount and the quality of habitat patches. That is, species response to habitat quality depends on the habitat amount. Landscape management for this and other sensitive species could benefit by identify the range of forest cover over which habitat quality improvements have the biggest effects. These habitat cover thresholds are probably species-specific and vary among regions across the species distribution range. The observed patterns for the less sensitive species showed that habitat use is driven by habitat quality covariates, suggesting when habitat cover is not a limiting factor, species distributions can be predicted by habitat quality alone. We recommend that species conservation programs incorporate both habitat quality and amount when dealing with sensitive species and prioritize habitat quality management when focusing in less sensitive species.
References
Alho CJR, Camargo G, Fischer E (2011) Terrestrial and aquatic mammals of the Pantanal. Braz J Biol 71:297–310
Altrichter M, Taber A, Beck H et al (2019) Range-wide declines of a key Neotropical ecosystem architect, the Near Threatened white-lipped peccary Tayassu pecari. Oryx 46:87–98. https://doi.org/10.1017/S0030605311000421
Antunes PC, Oliveira-Santos LGR, Tomas WM et al (2016) Disentangling the effects of habitat, food, and intraspecific competition on resource selection by the spiny rat, Thrichomys fosteri. J Mammal 97:1738–1744. https://doi.org/10.1093/jmammal/gyw140
Arnold TW (2010) Uninformative parameters and model selection using akaike ’ s information criterion. J Wildl Manage 74:1175–1178. https://doi.org/10.2193/2009-367
Baguette M, Blanchet S, Legrand D et al (2013) Individual dispersal, landscape connectivity and ecological networks. Biol Rev 88:310–326. https://doi.org/10.1111/brv.12000
Boscolo D, Ferreira PA, Lopes LE (2016) Da Matriz a Matiz - Em Busca de uma Abordagem funcional para a Ecologia de Paisagens. Filos e História Da Biol 11:157–187
Black-Décima P, Rossi RV, Vigliotti A, Cartes JL, Maffei L, Duarte MJB, González S, Juli JP (2009) Brown Brocket Deer Mazama gouazoubira (Fischer 1814). In: Neotropical Cervidology. Biology and Medicine of Latin American Deer. Jaboticabal, São Paulo, Brasil. Funep/IUCN, 393 p.
Brady MJ, Mcalpine CA, Possingham HP et al (2011) Matrix is important for mammals in landscapes with small amounts of native forest habitat. Landsc Ecol 26:617–628. https://doi.org/10.1007/s10980-011-9602-6
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Cañas, LFS (2010) Uso do espaço e atividade de Tapirus terrestris em uma área do Pantanal sul. PhD dissertation. Universidade Federal do Mato Grosso do Sul, Campo Grande, Brasil.
Cid B, Oliveira-santos LG, Mourão G (2013) Seasonal habitat use of agoutis (Dasyprocta azarae) is driven by the palm Attalea phalerata in Brazilian Pantanal. Biotropica 45:380–385
Costa-Araújo R, Regolin AL, Martello F, Souza-Alves J, Hrbek T, Ribeiro MC (2021) Occurrence and conservation of the vulnerable titi monkey Callicebus melanochir in fragmented landscapes of the Atlantic forest hotspot. Oryx. https://doi.org/10.1017/S0030605319001522
Desbiez ALJ, Bodmer RE, Santos SA (2009a) Wildlife habitat selection and sustainable resources management in a Neotropical wetland. Int J Biodivers Conserv 1:11–20
Desbiez ALJ, Santos SA, Keuroghlian A, Bodmer RE (2009b) Niche Partitioning Among White-Lipped Peccaries (Tayassu pecari), Collared Peccaries (Pecari tajacu), and Feral Pigs (Sus Scrofa). J Mammal 90:119–128. https://doi.org/10.1644/08-MAMM-A-038.1
Dorado-Rodrigues TF, Maria V, Layme G et al (2015) Effects of shrub encroachment on the anuran community in periodically flooded grasslands of the largest Neotropical wetland. Austral Ecol 40:547–557. https://doi.org/10.1111/aec.12222
Dormann CF, Elith J, Bacher S et al (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x
Driscoll DA, Banks SC, Barton PS et al (2013) Conceptual domain of the matrix in fragmented landscapes. Trends Ecol Evol 28:605–613. https://doi.org/10.1016/j.tree.2013.06.010
Eaton DP, Keuroghlian A, Santos MAC et al (2017) Citizen scientists help unravel the nature of cattle impacts on native mammals and birds visiting fruiting tress in Brazil´s southern Pantanal. Biol Conserv 208:29–39. https://doi.org/10.1016/j.biocon.2016.09.010
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515. https://doi.org/10.1146/annurev.ecolsys.34.011802.132419
Fahrig L (2013) Rethinking patch size and isolation effects: The habitat amount hypothesis. J Biogeogr 40:1649–1663. https://doi.org/10.1111/jbi.12130
Fahrig L (2017) Ecological responses to habitat fragmentation Per Se. Annu Rev Ecol Evol Syst 48:1–23. https://doi.org/10.1146/annurev-ecolsys-110316-022612
Ferregueti AC, Tomás WM, Bergallo HG (2015) Density, occupancy, and activity pattern of two sympatric deer (Mazama) in the Atlantic Forest, Brazil. J Mammal 96:1245–1254. https://doi.org/10.1093/jmammal/gyv132
Ferregueti AC, Tomás WM, Bergallo HG (2017) Density, occupancy, and detectability of lowland tapirs, Tapirus terrestris, in Vale Natural Reserve, southeastern Brazil. J Mammal 98:114–123. https://doi.org/10.1093/jmammal/gyw118
Ferregueti AC, Tomas WM, Bergallo HG (2018) Density, habitat use, and daily activity patterns of the Red-rumped Agouti (Dasyprocta leporina) in the Atlantic Forest, Brazil. Stud Neotrop Fauna Environ 53:143–151. https://doi.org/10.1080/01650521.2018.1434743
Fiske I, Chandler R (2011) unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. J Stat Softw 43(10):1–23
Fischer J, Lindenmayer DB (2007) Landscape modification and habitat fragmentation: a synthesis. Glob Ecol Biogeogr 16:265–280. https://doi.org/10.1111/j.1466-8238.2006.00287.x
Fragoso JMV (1999) Perception of scale and resource partitioning by peccaries: behavioral causes and ecological implications. J Mammal 80:993–1003
Garbor T, Hellgren E, Silvy N (1997) Renal morphology of sympatric suiforms: implications for competition. J Mammal 78:1089–1095
Gardiner R, Bain G, Hamer R et al (2018) Habitat amount and quality, not patch size, determine persistence of a woodland-dependent mammal in an agricultural landscape. Landsc Ecol 33:1837–1849. https://doi.org/10.1007/s10980-018-0722-0
Gayot M, Henry O, Dubost G, Sabatier D (2004) Comparative diet of the two forest cervids of the genus Mazama in French Guiana. J Trop Ecol 20:31–43. https://doi.org/10.1017/S0266467404006157
Goheen JR, Swihart RK, Gehring TM, Miller MS (2003) Forces structuring tree squirrel communities in landscapes fragmented by agriculture: species differences in perceptions of forest connectivity and carrying capacity. Oikos 102:95–103
Grotta-Neto F, Peres PHF, Piovezan U et al (2019) Influential factors on gray brocket deer (Mazama gouazoubira) activity and movement in the Pantanal, Brazil. J Mammal 100:454–463. https://doi.org/10.1093/jmammal/gyz056
Hall LS, Krausman PR, Morrison ML (1997) The habitat concept and a plea for standard terminology. Wildl Soc Bull 25:173–182. https://doi.org/10.2307/3783301
Hansbauer MM, Storch I, Knauer F et al (2010) Landscape perception by forest understory birds in the Atlantic Rainforest: black-and-white versus shades of grey. Landsc Ecol 25:407–417. https://doi.org/10.1007/s10980-009-9418-9
Hansen MC, Potapov PV, Moore R et al (2013) High-Resolution Global Maps of of 21st-century forest cover change. Science 134:850–854
Harris MB, Tomas W, Mourão G et al (2005) Safeguarding the Pantanal wetlands: threats and conservation initiatives. Conserv Biol 19:714–720
Hijmans RJ (2017) Raster: geographic data analysis and modeling. R package version 2.6-7. https://CRAN.R-project.org/package=raster
Holland GJ, Bennett AF (2007) Occurrence of small mammals in a fragmented landscape: the role of vegetation heterogeneity. Wildl Res 34:387–397. https://doi.org/10.1071/WR07061
Holland JD, Bert DG, Fahrig L (2004) Determining the spatial scale of species ’ response to habitat. Bioscience 54:227–233
Jorge ML, Peres CA (2005) Population density and home range size of red-rumped agoutis (Dasyprocta leporina) within and outside a natural Brazil nut stand in southeastern Amazonia. Biotropica 37:317–321
Kellner KF, Duchamp JE, Swihart RK (2019) Niche breadth and vertebrate sensitivity to habitat modification: signals from multiple taxa across replicated landscapes. Biodivers Conserv 28:2647–2667. https://doi.org/10.1007/s10531-019-01785-w
Keuroghlian A, Eaton DP (2008) Importance of rare habitats and riparian zones in a tropical forest fragment: preferential use by Tayassu pecari, a wide- ranging frugivore. J Zool 275:283–293. https://doi.org/10.1111/j.1469-7998.2008.00440.x
Keuroghlian A, Eaton DP, Desbiez ALJ (2009) The response of a landscape species, white-lipped peccaries, to seasonal resource fluctuations in a tropical wetland, the Brazilian pantanal. Int J Biodivers Conserv 1:87–97
Keuroghlian A, Eaton DP, Longland WS (2004) Area use by white-lipped and collared peccaries (Tayassu pecari and Tayassu tajacu) in a tropical forest fragment. Biol Conserv 120:415–429. https://doi.org/10.1016/j.biocon.2004.03.016
Keuroghlian A, Santos MCA, do, Eaton DP (2014) The effects of deforestation on white-lipped peccary (Tayassu pecari) home range in the southern Pantanal. Mammalia 79:491–497. https://doi.org/10.1515/mammalia-2014-0094
MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey LL, Hines JE (2017) Occupancy Estimation and Modeling - Inferring Patterns and Dynamics of Species Occurrence, 2nd ed. Academic Press.
MacKenzie DI, Bailey LL (2004) Assessing the fit of site-occupancy models. J Agric Biol Environ Stat 9:300–318. https://doi.org/10.1198/108571104X3361
Mayer JJ, Wetzel RM (1987) Tayassu Pecari. Mamm Species 293:1–7
Mazerolle MJ (2020). AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R package version 2.3-1, https://cran.r-project.org/package=AICcmodavg.
Melo GL, Sponchiado J, Cáceres NC, Fahrig L (2017) Testing the habitat amount hypothesis for South American small mammals. Biol Conserv 209:304–314. https://doi.org/10.1016/j.biocon.2017.02.031
Morin DJ, Yackulic CB, Diffendorfer JE et al (2020) Is your ad hoc model selection strategy affecting your multimodel inference? Ecosphere 11:1–16. https://doi.org/10.1002/ecs2.2997
Mortelliti A, Amori G, Boitani L (2010) The role of habitat quality in fragmented landscapes: a conceptual overview and prospectus for future research. Oecologia 163:535–547. https://doi.org/10.1007/s00442-010-1623-3
Mortelliti A, Boitani L (2008) Interaction of food resources and landscape structure in determining the probability of patch use by carnivores in fragmented landscapes. Landsc Ecol 23:285–298. https://doi.org/10.1007/s10980-007-9182-7
Negrelle RRB (2015) Attalea phalerata MART. EX SPRENG.: aspectos botânicos, ecológicos, etnobotânicos e agronômicos. Ciência Florest 25:1061–1066
Niebuhr BBS, Martello F, Ribeiro JW, Vancine MH, Muylaert RL, Campos VEW, Santos JS, Tonetti VR, Ribeiro MC (in prep) Landscape Metrics (LSMetrics): a spatially explicit tool for calculating connectivity and other ecologically-scaled landscape metrics.
Niedballa J, Sollmann R, Courtiol A, Wilting A (2016) camtrapR: an R package for efficient camera trap data management. Methods Ecol Evol 7:1457–1462
Noss AJ, Cuellar RL, Barrientos J et al (2003) A camera trapping and radio telemetry Study of Lowland Tapir (Tapirus terrestris) in bolivian dry forests. Newsl IUCN/SSC Tapir Spec Gr 12:24–32
Padilla M, Dowler RC (1994) Tapirus Terrestris Mamm Species 481:1–8
Paolucci LN, Pereira RL, Rattis L et al (2019) Lowland tapirs facilitate seed dispersal in degraded Amazonian forests. Biotropica 51:245–252. https://doi.org/10.1111/btp.12627
Pott A, Silva JS V (2015) Terrestrial and aquatic vegetation diversity of the Pantanal Wetland. In: Dynamics of the Pantanal Wetland in South America. pp 111–131
Presley SJ, Cisneros LM, Klingbeil BT, Willig MR (2019) Landscape ecology of mammals. J Mammal 100:1044–1068. https://doi.org/10.1093/jmammal/gyy169
QGIS Development Team (2019) QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org.
Regolin AL, Cherem JJ, Graipel ME et al (2017) Forest cover influences occurrence of mammalian carnivores within Brazilian Atlantic Forest. J Mammal 98:1721–1731. https://doi.org/10.1093/jmammal/gyx103
Regolin AL, Ribeiro MC, Melo GL, Sponchiado J, Campanha LF, Sugai LS, Silva TS, Cáceres NC (2020) Spatial heterogeneity and habitat configuration overcome habitat composition influences on alpha and beta mammal diversity. Biotropica. https://doi.org/10.1111/btp.12800
Reyna-Hurtado R, Tanner GW (2005) Habitat preferences of ungulates in hunted and nonhunted areas in the Calakmul Forest, Campeche, Mexico. Biotropica 37:676–685. https://doi.org/10.1111/j.1744-7429.2005.00086.x
Rivero K, Rumiz DI, Taber AB (2005) Differential habitat use by two sympatric brocket deer species (Mazama americana and M. gouazoubira) in a seasonal Chiquitano forest of Bolivia. Mammalia 69:169–183. https://doi.org/10.1515/mamm.2005.015
Rocha R, López-Baucells A, Farneda FZ, et al (2017) Consequences of a large-scale fragmentation experiment for Neotropical bats: disentangling the relative importance of local and landscape-scale effects. Landsc Ecol 32:31–45. https://doi.org/10.1007/s10980-016-0425-3
Rodela LG, Ravaglia SASLAPA, Mazin V, de Neto JPQ (2008) Mapeamento de Unidades de Paisagem em Nível de Fazenda, Pantanal da Nhecolândia. Bol Pesqui e Desenvolv 83:1–23
Santos-Filho M, Frieiro-Costa F, Ignácio ARA, Silva MNF (2012) Use of habitats by non-volant small mammals in Cerrado in Central Brazil. Brazilian J Biol 72:893–902
Santos PM, Chiarello AG, Ribeiro MC, Ribeiro JW, Paglia AP (2016) Local and landscape influences on the habitat occupancy of the endangered maned sloth Bradypus torquatus within fragmentedlandscapes. Mamm Biol 81:447–454. https://doi.org/10.1016/j.mambio.2016.06.003
Silveira M, Moraes W, Fischer E, Oscar M (2018) Habitat occupancy by Artibeus planirostris bats in the Pantanal wetland, Brazil. Mamm Biol 91:1–6. https://doi.org/10.1016/j.mambio.2018.03.003
Silvius K, Fragoso JV (2003) Red-rumped agouti (Dasyprocta leporina) home range use in an Amazonian Forest: implications for the aggregated distribution of forest trees. Biotropica 35:74–83
St-Louis V, Pidgeon AM, Clayton MK et al (2009) Satellite image texture and a vegetation index predict avian biodiversity in the Chihuahuan Desert of New Mexico. Ecography (cop) 32:468–480. https://doi.org/10.1111/j.1600-0587.2008.05512.x
St-Louis V, Pidgeon AM, Kuemmerle T et al (2014) Modelling avian biodiversity using raw, unclassified satellite imagery. Philos Trans R Soc London Ser B 369:20130197. https://doi.org/10.1098/rstb.2013.0197
Swihart RK, Gehring TM, Kolozsvary MB (2003) Responses of “resistant” vertebrates to habitat loss and fragmentation: the importance of niche breadth and range boundaries. Divers Distrib 9:1–18
Tobler MW, Carrillo-Percastegui SE, Powell G (2009) Habitat use, activity patterns and use of mineral licks by five species of ungulate in south-eastern Peru. J Trop Ecol 25:261–270. https://doi.org/10.1017/S0266467409005896
Tomas WM, Roque FDO, Morato RG et al (2019) Sustainability agenda for the Pantanal Wetland: perspectives on a collaborative interface for science, policy, and decision-making. Trop Conserv Sci 12:1–30. https://doi.org/10.1177/1940082919872634
Trolle M, Noss AJ, Cordeiro JLP, Oliveira LF (2008) Brazilian Tapir density in the Pantanal: a comparison of systematic camera-trapping and line-transect surveys. Biotropica 40:211–217
Varela DM, Trovati RG, Guzmán KR, Rossi RV, Duarte JMB (2009) Red Brocket Deer Mazama americana (Erxleben 1777). In: Neotropical Cervidology. Biology and Medicine of Latin American Deer. Jaboticabal, São Paulo, Brasil. Funep/IUCN, 393 p.
Vidolin GP, Biondi D, Wandembruck A (2009) Seletividade de habitats pela anta (Tapirus terrestris) e pelo queixada (Tayassu pecari) na Floresta com Araucária. Sci for Sci 37:447–458
Willems EP, Barton RA, Hill RA (2009) Remotely sensed productivity, regional home range selection, and local range use by an omnivorous primate. Behav Ecol 20:985–992. https://doi.org/10.1093/beheco/arp087
Winnie JA Jr, Cross P, Getz W (2008) Habitat quality and heterogeneity influence distribution and behavior in African buffalo (Syncerus caffer). Ecology 89:1457–1468. https://doi.org/10.1890/07-0772.1
Wood EM, Pidgeon AM, Radeloff VC, Keuler NS (2013) Image texture predicts avian density and species richness. PLoS ONE 8:e63211. https://doi.org/10.1371/journal.pone.0063211
Acknowledgements
We are grateful to A Feuka, B Hardy, F Melo, JE Oshima, V Arroyo-Rodriguez, A Uezu, ML Jorge, M Benchimol, and anonymous reviewers for the manuscript revision.
Funding
ALR has had a doctoral scholarship (CNPq #153423/2016-1 and CAPES PDSE #88881.362065/2019-01). MCR was funded by FAPESP (#2013/50421-2), (CAPES/PROCAD #88881.068425/2014-01) and receives research grant from CNPq (#312045/2013-1; #312292/2016-3). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (Capes)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
All authors conceived study aim and hypothesis. ALR wrote the manuscript with inputs from LLB. LGOS designed data collection and carried out field work. ALR quantified landscape structure indices. ALR and LLB analyzed the data. All the authors revised the manuscript. Proof reading by LLB.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Regolin, A.L., Oliveira-Santos, L.G., Ribeiro, M.C. et al. Habitat quality, not habitat amount, drives mammalian habitat use in the Brazilian Pantanal. Landscape Ecol 36, 2519–2533 (2021). https://doi.org/10.1007/s10980-021-01280-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-021-01280-0