Abstract
Plastic packaging has been widely used in utensils, equipment, packaging, among others. However, the widespread use of plastic materials, which originate from non-renewable sources, has generated a global pollution crisis due to its non-biodegradable profile, thus leading the scientific community and consumers to evaluate their choices. Therefore, studies on alternatives to prevent environmental damage have been carried out. The development of biodegradable packaging, produced with raw materials from renewable sources, such as polysaccharides, proteins, and others, has shown to be a viable alternative to minimize these problems. However, many challenges arise when using biodegradable compounds as a polymer matrix; thus, techniques that allow better knowledge of the materials can contribute significantly to improving the properties of interest in these packages, with an emphasis on thermal analysis. This review addressed the use of thermal analysis and experimental conditions to evaluate biodegradable films of different compositions, with an emphasis on thermogravimetric analysis (TGA), derivative thermogravimetry (DTG), and differential scanning calorimetry (DSC). Small variations were observed for the experimental conditions, which can be associated with the different film constituents (polymer matrix, presence of nanocomposites and essential oils, etc.).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic plastics are widely used due to their versatility and convenience, as they can be used in the production of a wide variety of products [1], including the development of food packaging. The raw materials used in the production of these packages include polymers of petroleum origin, which stand out for their good mechanical and barrier properties, relatively low cost, lightness, and ease in the manufacturing process and application [2].
However, these packages are not easily biodegradable [2], accumulating in the environment, with a negative impact on fauna and flora, urban rainwater system, and human health [3, 4]. Thus, the increased awareness of the population about these impacts on the environment [5, 6], as well as the finite and fluctuating cost of petroleum resources [7, 8], has encouraged scientists and the polymer industry to develop sustainable solutions to minimize the environmental damage [9,10,11].
In this context, bioplastics are an alternative to minimize these effects. Ross, Ross, and Tighe [12]stated that the denomination bioplastic refers to a biodegradable petrochemical plastic or a plastic material obtained from natural biological resources. In this group, biodegradable films of biological origin have stood out. They can be produced from biopolymers (polymers or copolymers) obtained from renewable raw materials of plant or animal origin, such as lipids, proteins, and polysaccharides [13], or even synthesized by microorganisms.
Different from synthetic plastics, biodegradable films are assimilated by enzymatic, chemical, and microbial activities, leading to the hydrolysis of the polymer chain and consequently originating smaller fragments (monomers or dimers), with a complete composting of the material [6, 14].
For application in the food industry, these materials can also be incorporated with compounds with antimicrobial or antioxidant effects, being called active packaging or active films, providing the food with an additional protective property, thus leading to the increased shelf life of the products [15].
However, many challenges are encountered in the development of biodegradable packaging for large-scale applications [1]. Therefore, several techniques can be used to produce materials with suitable properties for applicability, including thermoanalytical techniques.
Thermal analysis is a set of techniques used to analyze a physical or chemical property of a sample and/or reaction products when subjected to a controlled temperature program [16].
These techniques have been used in industrial activities, such as chemistry, pharmaceuticals, essential oils, food, and polymers, as well as in the evaluation of biodegradable films to identify suitable processing conditions and structural characteristics of the polymer chain, focusing on adequate applicability of the material [17]. Thus, this review aimed to report the use of thermal analysis and the experimental conditions for the evaluation of biodegradable films of different compositions.
Biodegradable films and food packaging
Food packaging is a globally relevant and widely used technique for protecting and preserving food [18], besides containing, providing practicality in storage and distribution, functioning as a communication tool between the consumer and the product [19, 20], and allowing traceability of suspect food products [21].
Among the materials used in packaging, thermoplastic polymer-based materials have stood out due to several factors, including the replacement of the traditional material with plastics, the increase in population growth, and its access to the products [22].
However, the use of plastic materials has increased waste generation. According to Geyer et al. [23], from 1950 to 2015, 8300 million tons of plastic were produced worldwide, of which 4900 million tons were disposed of inappropriately. As a result, plastics tend to accumulate in the environment, with serious negative impacts [4].
In view of the problems caused by non-biodegradable plastic packaging, including the risks to human health, marine ecosystem, urban rainwater system, and the environment [3], innovative research is needed to develop and implement biodegradable materials from renewable, bio-based, or synthetic sources that can replace plastic packaging materials commonly used in the food industry [10]. Makhijani, Kumar, and Sharma [24] have reported some characteristics that differentiate synthetic plastics from biodegradable films (Fig. 1).
In this context, natural, biodegradable, and bioavailable polymers have gained popularity in the field of food packaging [18]. These biodegradable packages may be associated with compounds of natural or synthetic origin with antimicrobial, flavoring, or antioxidant action called active biodegradable packages [25].
Therefore, biodegradable films are usually composed of a polymer matrix of animal or plant (polysaccharides and proteins) origin, microbial fermentation (polyhydroxyalkanoate-PHA, polyhydroxybutyrate-PHB), synthetic biomass biopolymers (poly-lactic acid-PLA), and petroleum-derived products (polycaprolactone-PCL, polyglycolic acid-PGA, poly-butylene succinate-co-adipate-PBSA) [6, 26], plasticizing agent, polyols (glycerol, sorbitol) and solvent. When called active packaging, antimicrobial compounds or natural antioxidants (essential oils), or synthetics and emulsifiers are used to provide a better oil dispersion. Figure 2 shows the main constituents of the bio-based and biodegradable polymer matrices.
The biodegradable films can be composed of an infinity of combinations; thus, a further analysis of the material is required to better designate its applicability [26].
Makhijani, Kumar, and Sharma [24] reported the main advantages of replacing synthetic plastics with biodegradable films. The advantages include soil enrichment as they are composted into waste, prevention of animal accidents from improper handling, reduced labor costs for removing plastic waste from the environment as it is naturally degraded, and increased longevity and stability of landfills by reducing the waste production.
Thermal analysis
The thermal profile of the biodegradable films can be analyzed by thermogravimetric analysis (TGA) also known as thermogravimetric analysis (TG), derivative thermogravimetry (DTG), differential thermal analysis (DTA), and differential scanning calorimetry (DSC) [17].
The devices employed in the performance of these techniques are called calorimeters [27], which are classified according to the principle of heat measurement (accumulation or compensation); cell construction (single or double), and mode of operation (static/isothermal, scanning/dynamic and flow) [17].
Lopez, Chen and Perez [27] stated that the calorimeters and the procedures adopted for thermal analysis are similar. The instrument consists of a measuring chamber or cabinet and a computer. The measuring chamber contains a space for two small crucibles, also called pans or plates, consisting of an empty crucible (reference) and another containing the sample. The temperature programming and data processing are controlled by a computer. The temperature (isothermal or sweep), pressure, and atmospheric gas are pre-set according to the conditions adopted for the study [28].
Font [29] pointed out three requirements for thermal analysis, including the sample, the operating conditions (atmosphere, heat flow, or temperature variation), and the signal variation (mass loss, heat flow, temperature difference) versus the analysis time.
These techniques have wide application in the food industry for the evaluation of the thermal effect of food ingredients or components in a food system, starch gelatinization, and protein degradation, as well as for technological innovation to improve food characteristics or shelf life, and adulteration detection [16, 30, 31]. In the polymer industry, these techniques have[16, 31] been prominent in the characterization of biodegradable films of various compositions.
Differential thermal analysis (DTA)
DTA measures the changes associated with heat absorbed or released by the sample, and the temperature is varied at a controlled rate. The changes are observed when the constituents of the material undergo some specific transition phase (crystallization, melting, evaporation, glass transition, and conformational change), or chemical reactions (oxidation, reduction, hydrolysis) [32].
The difference in temperature between the sample and the inert reference versus time or temperature subjected to the same procedure is recorded. Thus, when the temperature of the sample is higher than that of the reference, thermal energy has been released during the phase transition, which characterizes the process as exothermic (DT > 0). When thermal energy is absorbed, the process is characterized as endothermic (DT < 0) [32, 33]. A DTA curve is the plot of the temperature difference as a function of the temperature or time at a constant temperature [32].
Thermogravimetric analysis (TGA) and derivative thermogravimetry (DTG)
Thermogravimetric analysis (TGA), also known as thermogravimetry (TG), is a thermal analysis that investigates the change in mass of a sample as a function of time or temperature. The mass change profile is recorded when the sample is subjected to a controlled heating or cooling environment [33].
In turn, derivative thermogravimetry (DTG) is a mathematical arrangement applied to thermogravimetry, in which the derivative of the mass change with time (dm/dt) is registered as a function of time and temperature. Curves corresponding to the first derivative of the TGA curve are obtained, and peaks delimit the areas as a function of the mass changes of the sample [33].
TGA and DTG are used primarily for understanding and predicting the thermal and oxidative stability of samples. The TGA curve allows understanding certain thermal events, which involves the mass loss or gain such as absorption, adsorption, desorption, vaporization, sublimation, degradation, oxidation, and reduction [34, 35], in addition to the identification of compounds that make up the materials [34].
It is an effective technique for evaluating polymeric materials such as fibers, biodegradable films, thermoplastics, and others. It can also be used to select materials for end-use applications, either in product performance and/or product quality [17].
As reported by Mansa and Zou [36], the main advantages of this technique include ease and speed of execution and the possibility of using a variable amount of sample (1–100 mg).
In the experimental procedure, the sample (1–100 mg) is placed in a crucible that is attached to the equipment, and the mass is identified using the thermobalance and compared with the reference crucible (empty). For the runs, parameters must be previously defined, such as the temperature conditions, which can be of the sweep/dynamic or isothermal type (up to 1000 °C), heating rate (from 1 to 100 °C min-1) and purge gas, and the values can vary according to the type of sample and the operational limits of the equipment. A curve resulting from the mass change (∆m) of a sample as a function of temperature (T) or time (t) is obtained, called thermogravimetric analysis curve (TGA) or thermogravimetric curve (TG) [32, 37]. For starch-based biodegradable films, mass losses usually start in a temperature range of 40–60 °C due to evaporation of water from the polymer matrix. The second degradation stage starts between 200 and 250 °C, corresponding to dehydration of the saccharide rings and evaporation of the plasticizers. The third degradation event starts from 270 to 450 °C, resulting in starch degradation. At 800 °C, there is a loss of 90–95% of the initial mass of the polymeric film [37, 38].
Changes in TG/DTG are very effective to characterize the most diverse materials. This technique allows assessing various parameters, including the degradation interval, the temperature of the materials and peaks at a high degradation rate; the presence of unexpected inert materials or different amounts than expected; analysis of the volatile matter, ash, fixed carbon, and moisture content [29]. In addition, the ignition temperature and the effect of gasifying agents on combustion, the degradation process and the effect of catalysts on the reaction, the synergistic interactions in the mixture of wastes, and the synthesis of new materials from wastes with polymers can be evaluated, among others [29].
Differential scanning calorimetry–DSC
Differential scanning calorimetry (DSC) stands out as one of the most applicable thermoanalytical methods to assess the thermal behavior of a sample [16]. The technique measures the difference between the amount of heat required to increase the temperature of a sample and the reference, as a function of a given temperature [39].
In addition to some thermodynamic variables, such as entropy variations (second-order transitions) including glass transition, and enthalpy, the analysis provides information about the phase transition temperature (melting, boiling, sublimation, solidification, crystal structure change) or dehydration, dissociation, degradation, oxidation–reduction reactions capable of generating heat changes [16].
In polymer science, DSC-based techniques stand out as they allow the characterization of polymeric materials, evaluating the behavior of the materials upon heating. Therefore, various properties such as heat capacity, glass transition temperature, crystallization temperature, and melting temperature can be determined [40].
In most cases, some reactions including phase transition, denaturation, gelatinization, dehydration, reduction, and certain degradation reactions produce endothermic effects; while, crystallization reactions, oxidation, freezing, and some degradation reactions lead to exothermic effects [39].
According to Nur, Azira, and Amim [39], there are two types of DSC measuring principles: power compensation DSC, in which the sample and a reference are heated in two separate furnace blocks; and heat flux DSC, in which the sample and a reference are heated in the same block. Although the instrumentation is fundamentally different, both provide the same information.
As also reported for TGA, some factors directly affect the DSC results, including the operational conditions (heating rate, sensor location, equipment atmosphere, and sample container composition) and sample characteristics (amount of sample, particle size, gas solubility, heat of reaction, and thermal conductivity), equipment calibration, and diluent. Therefore, all these factors must be considered to ensure good quality results [41].
Thermal analysis for the characterization of biodegradable films
TGA, DTG, and DSC are the most widely used thermal analyses in the evaluation of biodegradable films. TGA and DTG have been used by some authors to evaluate the thermal stability of different biodegradable films; while, DSC has been widely used to determine the transition enthalpy, degree of crystallinity, and thermal conductivity of polymeric materials [42].
Table 1 shows some studies using thermal analyses for the evaluation of biodegradable films. The columns show the main experimental conditions adopted by some authors, including the reference, the thermal analysis applied, equipment, purge gas, heating rate, and scanning temperature.
Fonseca-García, Jimenez-Regalado, and Aguirre-Loredo [43] used DSC to evaluate films based on maize starch (5%), chitosan (1%), poloxamer (pluronic F 127) at different concentrations (1, 3, and 5%), and glycerol (25%). The samples were packed in aluminum crucibles and the analysis conditions are described in Table 1. The results showed an important thermodynamic interaction with the increase in the pluronic F 127 concentration. The films containing 0 and 1% of pluronic F 127 presented a thermal behavior similar to that of pure starch, which was evidenced by the results of the glass transition temperature [starch (56 °C), film with 0% poloxamer (55 °C), Film with 1% poloxamer (44 °C)]; melting point [(starch (142 °C); film with 0% poloxamer (139 °C), film with 1% poloxamer (138 °C)], and enthalpy [starch (6 J g−1); film with 0% poloxamer (6.4 J g−1); Film with 1% poloxamer (8.4 J g−1). For the films containing 3 and 5% of pluronic F 127, a behavior closer to the pure pluronic was observed, which was lower when compared to the melting point, showing a strong interaction between the poloxamer and starch and chitosan. The results suggest nucleation of the poloxamer within the film matrix at concentrations of 3 and 5%.
Hosseini, Pirsa, and Farzi [44] used TGA to evaluate the effect of magnesium oxide (MgO) nanoparticles on the thermal properties of films made with modified maize starch and albumin. The results showed that the first mass loss occurred at approximately 90 °C, which was observed for all films, due to water evaporation from the films. The second mass loss was evidenced at 190 °C for pure films, and at 320 °C for films with the addition of MgO nanoparticles, which was associated with the degradation of glycerol. The results showed increased thermal stability of all starch and albumin-based films containing the MgO nanoparticles, which were stable at temperatures around 250–330 °C. Shankar, Reddy, and Rhim [62] stated that the inclusion of nanoparticles to films can increase the thermal stability of these polymers due to the increase in net charges, therefore increasing the interaction between the polymer chains, and preventing the release of volatile compounds.
Zou et al. [45] used TGA and DTG to evaluate films based on high amylose maize starch and konjac glucomannan (different concentrations) and glycerol as a plasticizing agent. The TGA curves showed three stages of mass loss, which corresponded to three degradation peaks of the DTG curves. The first stage of mass loss was at approximately 100 °C, corresponding to the loss of free water in the samples. The second stage occurred in a temperature range of 150–250 °C, which was attributed to the degradation of glycerol, noting that the glycerol degradation in the films with high amylose content was slower and at a higher temperature rate. The third stage of mass loss was observed between 250 and 350 °C, which was associated with the rupture of the hydrogen bond and degradation of polysaccharides. The DTG curves showed that the increase in the concentration of Konjac glucomannan in the films led to lower thermal stability, showing an increasing (negative) effect of this compound on the films.
Martins da Costa et al. [63] also used thermal analysis (TGA and DTG) to evaluate biodegradable films based on purple yam starch (2 g), chitosan (0, 0.5, and 1.0 g), and glycerol (2%). The authors reported higher degradation temperature for starch and pure chitosan when compared to the films, probably due to the presence of glycerol, which can interact with the acetamide group of chitosan, and reduce the mobility/interactions between the polymer chains. In addition, the plasticizer reduced the glass transition temperature of chitosan, which also affected the initial degradation temperature. The films presented three stages of mass loss, with the initial loss (up to 100 °C) related to water loss and a second loss at ~ 200 °C. The final event was characterized by the initial and final temperatures of 165 and 170, and 203 and 210 °C for the films made with 0.5 and 1.0 g of chitosan, respectively, which was associated with the glycerol degradation. The third stage occurred between 250 and 350 °C, corresponding to the degradation of organic matter of the films. The films made without chitosan presented one more stage of mass loss, which was higher when compared to the other films (324 °C), probably due to a higher affinity of glycerol for the acetamide group of chitosan when compared to starch amylose, and the weaker interactions between glycerol and starch during gelatinization.
De Lima Barizão et al. [64] produced films with ϰ- carrageenan, cassava starch, polyvinyl acetate, and glycerol. Films with ϰ- carrageenan and starch in the proportions of 100:0; 75:25; 50:50; 25:75; 0:100 were produced. Regarding the thermal stability, increasing the starch concentration led to an increase in thermal stability, evidenced by the inflection points in the DTG curves. For the films with higher ϰ- carrageenan concentration, the degradation was observed at 210 °C; while, films with a higher starch concentration exhibited higher degradation at 334 °C. The DSC curves of the films with higher starch concentration showed an endothermic peak at 234 °C, while no endothermic peaks were observed for the films with high ϰ- carrageenan concentrations (100, 75, 50). The endothermic peaks were associated with the melting of semi-crystalline starch structure; while, the exothermic peaks were related to the degradation of the film constituents.
Lopes et al. [27] developed biodegradable films with two stages of cross-linking, composed of sodium alginate (6, 7.5, and 9 g), babaçu coconut mesocarp powder (1–3 g), and glycerol (3–5 g). The pre-reticulation was performed by the addition of calcium chloride (1 g) to the film-forming solution. After drying the plates, the second stage was carried out with immersion of the films in calcium solution (second cross-linking). A total of 9 formulations were produced, and those that presented the best characteristics were evaluated, using an open aluminum crucible with approximately 500 mg of sample, subjected or not to the second cross-linking stage. The study revealed that the films (babassu coconut mesocarp powder: alginate: glycerol; F1 = 1:6:3; F6 = 3:7,5:4; and F7 = 1:9:5) subjected to two cross-linking stages showed a higher degradation temperature than those subjected only to stage 1 (pre-cross-linking). The authors also stated that the babassu mesocarp powder may have contributed to thermal stability, with a similar effect to biodegradable films with the inclusion of fibers. The DSC curves showed an endothermic peak at 70 °C for all formulations, which was associated with the dehydration process of the sample. In addition, all films showed an exothermic peak at 220 °C, which was related to the rapid degradation of the material.
Nordin et al. [49] investigated the effect of thymol and glycerol on the thermal stability (TGA) of maize starch films. For the analysis, aluminum crucibles with approximately 10 g of sample were used. For starch-based films, the first degradation event was observed at 50–150 °C, due to water loss from the samples (moisture). After that, the thermal stability of the samples was practically unchanged up to 270 °C. The second degradation event occurred at 286–328 °C, representing the higher starch degradation. In contrast, the thermal degradation of films composed of starch and glycerol and starch/glycerol/thymol occurred in three stages, with the first stage characterized by water evaporation (115 °C), the second degradation at 190–250 °C was attributed to the glycerol degradation, and a higher degradation rate was observed in the range of 300–345 °C, corresponding to the degradation of starch molecules, characterized by a drastic mass reduction. The DTG curves showed that the peak temperature of the films plasticized with glycerol was higher when compared to the films without the addition of glycerol. The authors reported a 25 and 50% degradation of the starch-glycerol films, and starch/glycerol/thymol films at a lower temperature when compared to starch films and starch-thymol films, probably due to the presence of the plasticizer and water molecules in the samples. This event was observed in the DTG curves at 200 and 250 °C for the starch-glycerol films and starch/glycerol/thymol films, respectively, showing higher thermal stability of the starch/glycerol/thymol films due to the good interaction between the film constituents. The last thermal event, indicating the final residue of the films (%), occurred at 500 °C, with a higher mass observed for the starch films; while, the starch/glycerol/thymol films showed a lower percentage, due to the volatilization of the plasticizer and thymol.
Riaz et al. [50]used thermogravimetric analysis to evaluate active biodegradable films based on chitosan, Chinese chive extract (1, 3, and 5%), and glycerol, dissolved in acetic acid solution (1%), using 10 mg of sample and closed aluminum crucibles. The TGA curves showed that all films exhibited three stages of mass loss. The first stage was observed at 40–120 °C, which was associated with water loss due to breaking of hydrogen bonds, the second event was observed at 130–250 °C corresponding to glycerol degradation, and the third stage (260–400 °C) was attributed to the degradation and depolymerization of the polymer (chitosan). The films made with the chive extract exhibited higher thermal stability when compared to the control film (containing only chitosan), evidenced by the lower mass loss of these films. The DSC curves showed two endothermic peaks referring to dehydration. The DSC and TGA results showed that increasing the extract concentration in the film matrix increased its thermal stability.
Xie et al. [51] used TGA to evaluate biodegradable films made with potato peel powder (3 and 5%), bacterial cellulose (5, 10, and 15% based on the potato peel powder content), curcumin (antimicrobial agent), and glycerol (plasticizer). The TGA curves revealed three peaks of film degradation; the first at 80–100 °C referred to water evaporation, the second peak from 180 to 200 °C corresponded to the mass loss due to starch and glycerol degradation, and the third degradation occurred around 300 °C due to the degradation of hemicellulose and cellulose.
Wang et al. [65] also used thermal analysis (TGA, DTG, and DSC) to evaluate biodegradable films made with carboxymethylcellulose, mucilage from Dioscorea opposita Thunb (yam), silver nitrate, glucose, and glycerol as a plasticizer. The TGA curves showed about 10% mass loss at ~ 100 °C, equivalent to the moisture content; a second mass loss was observed in the range 160–250 °C, with a peak at ~ 228 °C, which was related to the degradation of the yam mucilage; while, the other mass loss at 293 °C was probably due to the degradation of glycerol and carboxymethylcellulose, evidenced in the most extreme peak (292 °C). The DSC curves showed an endothermic peak close to 100 °C for the formulation with carboxymethylcellulose and glycerol, related to water loss; while, an exothermic peak at 301.8 °C was associated with the degradation of glycerol and carboxymethylcellulose. The other formulations showed three exothermic peaks at ~ 100, 235, and 300 °C. Therefore, the exothermic peak at 235 °C can be considered the degradation temperature of yam starch mucilage.
Batista et al. [53]used TGA to evaluate films made with biodegradable myofibrillar proteins from fish waste, chitosan, and glycerol, optimized by the response surface methodology. The first event of mass loss was observed from 30 to 100 °C, which was correlated with the total water loss of the films (moisture). In the second phase, the degradation occurred at 259.60–375.22 and 234.13–407.70 °C for the control film and the film made using the optimization conditions, respectively, evidencing that the chitosan-based films presented higher thermal stability, probably due to the interaction between the protein and chitosan, delaying the thermal degradation.
Oliveira et al. [54] studied biodegradable films based on mucilage from Pereskia aculeata Miller (ora-pro-Nobis) and glycerol as a plasticizer agent, in different concentrations. The TGA curves showed three degradation stages, observed for all treatments. The first stage occurred between 25 and 170 °C, corresponding to the initial moisture content of the samples. The second stage was evidenced at 170–370 °C, probably due to the chemical degradation of the main constituents of the biodegradable films (polysaccharides, proteins, and plasticizers). Finally, the third stage was observed in the range of 370–500 °C, corresponding to the oxidative degradation of carbon and mineral residues. The DSC results showed that the films containing 2% of ora-pro-Nobis mucilage showed a lower mass loss, when compared to those made with 1.5% mucilage, revealing that the mucilage made the films more thermally stable.
Oliveira et al. [54] also reported DSC curves under two different conditions. In the first run, the samples were heated at a rate of 10 °C min−1, with a temperature range of 25–140 °C, maintained for 10 min. In the second run, the samples were cooled to − 40 °C and heated at the same rate and maximum scanning temperature previously reported. For the ora-pro-Nobis powder, the temperature range was 25–210 °C. This study allowed establishing the glass transition (Tg) temperature of the films. A Tg reduction was observed for the films when compared to the ora-pro-Nobis mucilage powder (151 °C), due to the reduction of the intermolecular forces of the polymers by the addition of water and plasticizer. An effect of glycerol in the films made with 1.5 g mucilage was also observed, with a small Tg reduction with increasing glycerol concentration, once the plasticizer can reduce the molecular mass and increase the free volume of the polymer. In contrast, this behavior was not observed for the films made with 2% glycerol since it acted as an anti-plasticizer agent. The authors also reported that the inclusion of ora-pro-Nobis mucilage powder provided an increase in the glass transition temperature from 54 to 68 °C, indicating greater stability of the film with the increase in mucilage concentration. The Tg was also evidenced at negative temperatures, with values ranging from − 18 to − 7 °C.
Oluwasina et al. [55] also used TGA to evaluate the effect of oxidized cassava starch on the thermal properties of biodegradable films. The films were made with cassava starch (5%), glycerol, water, and different oxidized starch concentrations (0, 20, 40, and 60%). As reported by other authors, the TGA curves revealed three stages of thermal degradation. The first degradation stage was similar to those previously reported and occurred at 0 and 100 °C, corresponding to the moisture loss of the sample, corresponding to the water added to the formulations during the production of the biodegradable films. The second stage was observed around 250 °C, which was attributed to the loss of bound water, volatile matter of lipids and carbohydrates, and glycerin (249 °C). In the third degradation stage, the mass loss was associated with the depolymerization of the cassava starch molecules (breaking the glycosidic bonds of amylose and amylopectin). In the fourth degradation stage, a degradation of the carbon component of the bioplastic was observed, which can be originated from starch and glycerol. Finally, the fifth and last stage was related to the degradation of some inorganic oxides that may have formed in the course of the thermal process.
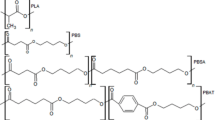
Bioplastic films prepared by Wang et al. [56] were also evaluated by thermal analysis. The films were composed of PBAT, TPS and PPC. Corn starch was modified with maleic anhydride (0.1 mass%) at 110 °C for 20 min, and 30% (w/w) liquid sorbitol was added to obtain a premix. PBAT, the premix and PPC were compounded into PBAT/TPS/PPC pellets using a twin-screw extruder. Pure and blended blown films were produced (PBAT/TPS/PPC /mass/mass /mass): PBAT-100, PPC-100, PT (PBAT/TPS)-65/35, PTP10-65/35/10, PTP20-65/35/20, PTP30-65/35/30 and PTP 40-65/35/40. The crystallization, melting and Tg of the films were determined. For PBAT/TPS/PPC films, with the increase in the PPC component, Tg increased from 27.7 to 36.0 °C, indicating a decrease in the flexibility of the PPC molecular chain. Tg, PBAT increased from 43.4 to 50.2 °C, indicating that the movement of the BT segment in PBAT is limited. The authors attributed this behavior to the reaction of MA with starch and PPC and to the limitation of intermolecular mobility. The PPC content in the films also affected the melting and crystallization of PBAT. The fusion enthalpy (ΔHm) of pure PBAT was 13.1 J g −1. After the addition of TPS and PPC, the ΔHm of the film increased to 20.3 J g−1 and then decreased.
In another study also carried out by Wang et al. [57] films composed of PBAT and thermoplastic starch (TPS) modified with the addition of MA (0.7% by mass) or TC (0.3% by mass) or MA and TC ( 0.7% by mass, 0.3% by mass) and sorbitol (30% in modified starch and 10.5% in film production), were produced by the blow extrusion method. The treatments were: 1. 100% PBAT, 2. 65% PBAT and 24.5% TPS; 3. 65% PBAT, 24.33% TPS, 0.17% MA; 4. PBAT 65% 24.43% TPS, 0.07% TC; 5. PBAT 65% 24.26% TPS, 0.07% TC and 0.17% MA. In thermal analysis, the results showed that the crystallization temperature increased by more than 20 °C with the addition of modified TPS, indicating that the addition of TPS and modified TPS promoted the crystallization of PBAT. PBAT/MA/TC/TPS had the best effect on promoting crystallization. PBAT exhibited a glass transition at around − 31.0 °C and followed by a small single melt at 123.8 °C. After the addition of TPS, the Tg of PBAT decreased to − 33.9 °C, and the melting peak decreased from 124.5 to 120.3 °C. The addition of MA promoted the reduction of PBAT Tg to − 34.3 °C, Tm increased from 124.0 to 124.6 °C. The PBAT/TC–TPS and PBAT/MA–TC–TPS films showed the same thermal behavior. However, PBAT/MA–TC–TPS showed higher ΔHm than other PBAT/starch films.
Guadagno et al. [58] developed a biodegradable thermoplastic polymer with Murexide (M) salts and commercial biodegradable vinyl alcohol (HVA) copolymer, using deionized water as a solvent. Murexide served as a self-healing filler, with the aim of imparting self-repairing capabilities to the formulated material. Three different percentages of Murexide (1%, 3% and 5% by mass) filler were dissolved in HVA to evaluate the effect of filler concentration on the thermal and self-healing properties of the resulting polymeric materials. The results of the DSC analysis highlight that increasing the amount of Murexide affects thermal events such as glass transition, crystallization and melting. TGA measurements showed that, although there is a reduction in the thermal stability of the materials in the presence of a high concentration of Murexide, the polymer remains stable up to 270 °C.
Palai et al. [59] developed blown films using PLA and corn and cassava starch plasticized with glycerol, glycidyl methacrylate (GMA) and benzoyl peroxide (BPO) as grafting agents and free radical indicator, respectively. In this study, it was observed that, in both PLA films with cassava starch and corn starch systems, all decomposition temperatures decreased significantly with increasing starch loads. Notably, PLA with a cassava starch system demonstrated superior thermal stability compared to its corn starch-containing counterpart. The blown film extrusion process used to obtain the films resulted in mechanical and thermal stress on the TPS, reducing their crystallinity and consequently affecting thermal stability. The researchers observed that the incorporation of TPS into the PLA matrix resulted in a reduction in both the Tg and Tcc of the PLA/TPS mixtures, regardless of the type of starch used. Furthermore, this reduction was more pronounced with increasing TPS load.
Quispe et al. [60] investigated the use of different concentrations (5–30%) of glycerol (G), polyglycerols (PGs), glycerol triacetate (GTA) and glycerol tributyrate (GTB) as additives for poly(3-hydroxybutyrate) (PHB) films, produced by thermocompression in a hydraulic press. DSC tests provided data such as Tm, ΔHm and Tg; while, thermogravimetric analysis determined thermal stability. In films without additives (PHB), two melting peaks were identified, related to the fusion of less and more ordered crystals, respectively, in addition to crystallization during cooling to 82.8 °C. During the second heating sweep, the Tg occurred at − 2.2 °C, and a single melting peak was identified at 176.2 °C. All additives reduced the Tm of PHB, facilitating its thermal processability, with GTA and GTB decreasing the Tg temperature, indicating a plasticizing effect. In thermal degradation, a single stage of mass loss was observed, with all plasticizers slightly decreasing the thermal stability of PHB. GTB resulted in more heat-resistant films; while, G, PGs, and GTA increased the heat sensitivity of PHB.
Poly (butylene succinate) (PBS) has also been studied for as a polymeric matrix for the production of films and food packaging. PBS is a biodegradable aliphatic polyester that stands out for its high flexibility, excellent impact resistance and robust chemical and thermal properties [66, 67]. Aziman et al.[61] produced films using PBS and tapioca starch (TPS) as a polymeric matrix, adopting the melt-blown technique. Treatments included pure PBS films, PBS/TPS films (with 40 mass% TPS mixed with 60 mass% PBS pellets), and films with addition of 1.5 and 3% BM particles. The films were named PBS 1.5% BM, PBS 3% BM, PBS/TPS 1.5% BM, and PBS/TPS 3% BM. In the thermal stability analysis, the PBS 1.5% BM and PBS 3% BM films showed a slight improvement in the decomposition onset temperature (T-on), compared to pure PBS. The introduction of TPS resulted in a significant reduction in T-on for all TPS-filled PBS films. However, PBS/TPS 1.5% BM and PBS/TPS 3% BM films showed an improvement in T-on compared to the PBS/TPS film. The initial peaks were presented at 326.9, 318.0 and 311.8 °C, for PBS/TPS, PBS/TPS 1.5% BM and PBS/TPS 3% BM films, respectively. While the peak decomposition temperature was found to be in the range of 399–404 °C for each film sample. In DSC analysis, the PBS 1.5% BM film had a higher Tc; while, the PBS 3% BM film had a lower Tc compared to pure PBS. This suggests an improvement in polymer solidification behavior for the PBS 3% BM film. The introduction of BM filler further reduced the Tc of PBS/TPS 1.5% BM and PBS/TPS 3% BM films, indicating the role of BM particles in the crystallization of PBS polymer.
According to Table 2, the thermal analyses allowed a better knowledge of the moisture resistance property of the films (solubility and WPV), followed by the determinations of thickness, mechanical properties, FTIR, and SEM.
According to Hosseini, Pirsa, and Farzi [44], both the thermal and mechanical properties of polymers are dependent on the crystalline structure of these materials; thus, the investigation about the organizational structure of films is essential. Those authors evidenced that the modified starch films and modified starch/albumin films lost part of their crystal structure after the insertion of magnesium oxide nanoparticles, which was evidenced by the peak intensity reduction in the diffractogram. On the other hand, the presence of a broad peak at 20 = 16.5° was observed for the films made with the combination of modified starch, albumin, and magnesium oxide, which indicates a relatively amorphous structure, associated with the presence of magnesium oxide. Furthermore, two small peaks corresponding to the crystalline plates of the modified starch films were observed; thus, the addition of magnesium oxide did not lead to an overall change in the structure of the film, as it retained part of its crystalline structure.
Conclusions
The use of biodegradable films for food packaging can be a promising alternative to replace petroleum-based packaging (plastics). Numerous studies have been carried out to compose the polymer matrix of these materials, with an emphasis on polysaccharides, mainly starch from different sources, in its native or modified form, as well as chitosan, carboxymethylcellulose, among others.
However, despite all the advantages of these matrices from renewable sources, there are several challenges for a large-scale application. Therefore, alternatives have been sought to improve the barriers encountered with the use of these materials. Some studies have shown that the use of other materials (fibers, nano-sized particles, agents with antimicrobial or antioxidant effects, either natural or synthetic) have contributed to overcoming some of the limitations of these materials.
Novel techniques that better characterize biodegradable films have also been used. Recent studies about the combination of conventional techniques and thermal analysis have been performed, mainly aiming to investigate the thermal stability (TGA and DTG) and some thermodynamic properties (DSC) of the films.
The most used thermoanalytical techniques include TGA, followed by DTG and DSC. Concerning the experimental conditions, a similarity was found regarding the type of purge gas used, with a greater predominance for nitrogen, besides helium and argon; while, the heating rate of 10 °C min-1 was more predominant. A wide variation in the scanning temperature was observed, ranging from 25 to 800 °C, probably due to the complexity of the film constituents.
In general, most of the studies reported three degradation stages. The first event of mass loss is associated with water loss, followed by the second event with the degradation of the plasticizer, and the third event is characterized by polymer degradation. The DSC technique allowed the identification of thermal events (endothermic and exothermic) that favored the characterization of the biodegradable films.
Therefore, the use of thermal analysis for the evaluation of biodegradable films can be a promising alternative, which contributes to a better designation of the final use of these packages, once the technique can assess a multitude of compounds.
References
Cheng H, Chen L, McClements DJ, Yang T, Zhang Z, Ren F, et al. Starch-based biodegradable packaging materials: a review of their preparation, characterization and diverse applications in the food industry. Trends Food Sci Technol. 2021;114:70–82. https://doi.org/10.1016/j.tifs.2021.05.017.
Anugrahwidya R, Armynah B, Tahir D. Bioplastics starch-based with additional fiber and nanoparticle: characteristics and biodegradation performance: a review. J Polym Environ. 2021;29:3459–76. https://doi.org/10.1007/s10924-021-02152-z.
Sajad P, Kurush AS. A review of the applications of bioproteins in the preparation of biodegradable films and polymers. J Chem Lett. 2020;1:47–58. https://doi.org/10.22034/JCHEMLETT.2020.111200.
Brandon JA, Jones W, Ohman MD. Multidecadal increase in plastic particles in coastal ocean sediments. Sci Adv. 2019. https://doi.org/10.1126/sciadv.aax0587.
Yong H, Wang X, Zhang X, Liu Y, Qin Y, Liu J. Effects of anthocyanin-rich purple and black eggplant extracts on the physical, antioxidant and pH-sensitive properties of chitosan film. Food Hydrocoll. 2019;94:93–104. https://doi.org/10.1016/j.foodhyd.2019.03.012.
Zhong Y, Godwin P, Jin Y, Xiao H. Biodegradable polymers and green-based antimicrobial packaging materials: a mini-review. Adv Ind Eng Polym Res. 2020;3:27–35. https://doi.org/10.1016/j.aiepr.2019.11.002.
Hassannia-Kolaee M, Shahabi-Ghahfarrokhi I, Hassannia-Kolaee M. Development and characterization of a novel ecofriendly biodegradable whey protein concentrate film with nano-SiO2. Inte J Food Eng. 2018. https://doi.org/10.1515/ijfe-2017-0098.
Goudarzi V, Shahabi-Ghahfarrokhi I. Photo-producible and photo-degradable starch/TiO2 bionanocomposite as a food packaging material: development and characterization. Int J Biol Macromol. 2018;106:661–9. https://doi.org/10.1016/j.ijbiomac.2017.08.058.
Sonar CR, Al-Ghamdi S, Marti F, Tang J, Sablani SS. Performance evaluation of biobased/biodegradable films for in-package thermal pasteurization. Innov Food Sci Emerg Technol. 2020;66:102485. https://doi.org/10.1016/j.ifset.2020.102485.
Stoica M, Marian Antohi V, Laura Zlati M, Stoica D. The financial impact of replacing plastic packaging by biodegradable biopolymers—a smart solution for the food industry. J Clean Prod. 2020;277:124013. https://doi.org/10.1016/j.jclepro.2020.124013.
Musso YS, Salgado PR, Mauri AN. Smart gelatin films prepared using red cabbage (Brassica oleracea L.) extracts as solvent. Food Hydrocoll. 2019;89:674–81. https://doi.org/10.1016/j.foodhyd.2018.11.036.
Ross G, Ross S, Tighe BJ. Bioplastics. Brydson’s Plast Mater. 2017. https://doi.org/10.1016/B978-0-323-35824-8.00023-2.
Henning FG, Ito VC, Demiate IM, Lacerda LG. Non-conventional starches for biodegradable films: a review focussing on characterisation and recent applications in food packaging. Carbohydrate Polymer Technologies and Applications. 2022;4:100157. https://doi.org/10.1016/j.carpta.2021.100157.
Zoungranan Y, Lynda E, Dobi-Brice KK, Tchirioua E, Bakary C, Yannick DD. Influence of natural factors on the biodegradation of simple and composite bioplastics based on cassava starch and corn starch. J Environ Chem Eng. 2020;8:104396. https://doi.org/10.1016/j.jece.2020.104396.
Tapia-Blácido DR, da Silva Ferreira ME, Aguilar GJ, Lemos Costa DJ. Biodegradable packaging antimicrobial activity. Process Dev Polysacch-Based Biopolym Pack Appl. 2020. https://doi.org/10.1016/B978-0-12-818795-1.00009-5.
Gharanjig H, Gharanjig K, Hosseinnezhad M, Jafari SM. Differential scanning calorimetry (DSC) of nanoencapsulated food ingredients. Charact Nanoencapsulated Food Ingredients. 2020. https://doi.org/10.1016/B978-0-12-815667-4.00010-9.
Jafarzadeh S, Jafari SM. Impact of metal nanoparticles on the mechanical, barrier, optical and thermal properties of biodegradable food packaging materials. Crit Rev Food Sci Nutr. 2021;61:2640–58. https://doi.org/10.1080/10408398.2020.1783200.
Liu Y, Yu J, Copeland L, Wang S, Wang S. Gelatinization behavior of starch: reflecting beyond the endotherm measured by differential scanning calorimetry. Food Chem. 2019;284:53–9. https://doi.org/10.1016/j.foodchem.2019.01.095.
Azzi A, Battini D, Persona A, Sgarbossa F. Packaging design: general framework and research agenda. Packag Technol Sci. 2012;25:435–56. https://doi.org/10.1002/pts.993.
Schifferstein HNJ, de Boer A, Lemke M. Conveying information through food packaging: a literature review comparing legislation with consumer perception. J Funct Foods. 2021;86:104734. https://doi.org/10.1016/j.jff.2021.104734.
Bajpai P (2019) Recent trends in packaging of food products. Biobased Polymers. Elsevier. p. 139–69.
Kedzierski M, Frère D, Le Maguer G, Bruzaud S. Why is there plastic packaging in the natural environment? Understanding the roots of our individual plastic waste management behaviours. Sci Total Environ. 2020;740:139985. https://doi.org/10.1016/j.scitotenv.2020.139985.
Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017. https://doi.org/10.1126/sciadv.1700782.
Makhijani K, Kumar R, Sharma SK. Biodegradability of blended polymers: a comparison of various properties. Crit Rev Environ Sci Technol. 2015;45:1801–25. https://doi.org/10.1080/10643389.2014.970682.
Bhargava N, Sharanagat VS, Mor RS, Kumar K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: a review. Trends Food Sci Technol. 2020;105:385–401. https://doi.org/10.1016/j.tifs.2020.09.015.
Rai P, Mehrotra S, Priya S, Gnansounou E, Sharma SK. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour Technol. 2021;325:124739. https://doi.org/10.1016/j.biortech.2021.124739.
Lopez C, Cheng K, Perez J. Thermotropic phase behavior of milk sphingomyelin and role of cholesterol in the formation of the liquid ordered phase examined using SR-XRD and DSC. Chem Phys Lipids. 2018;215:46–55. https://doi.org/10.1016/j.chemphyslip.2018.07.008.
Kodal M, Karakaya N, Wis AA, Ozkoc G. Thermal properties (DSC, TMA, TGA, DTA) of rubber nanocomposites containing carbon nanofillers. Carb-Based Nanofillers Rubber Nanocompos. 2019. https://doi.org/10.1016/B978-0-12-817342-8.00011-1.
Font R. Decomposition of organic wastes: thermal analysis and evolution of volatiles. Handb Therm Anal Calorim. 2018. https://doi.org/10.1016/B978-0-444-64062-8.00001-2.
Farhan A, Hani NM. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017;64:48–58. https://doi.org/10.1016/j.foodhyd.2016.10.034.
Clas S-D, Dalton CR, Hancock BC. Differential scanning calorimetry: applications in drug development. Pharm Sci Technol Today. 1999;2:311–20. https://doi.org/10.1016/S1461-5347(99)00181-9.
Saikia P. Physical and thermal analysis of polymer. Polym Sci Innov Appl. 2020. https://doi.org/10.1016/B978-0-12-816808-0.00005-6.
Loganathan S, Valapa RB, Mishra RK, Pugazhenthi G, Thomas S. Thermogravimetric analysis for characterization of nanomaterials. Therm Rheol meas Tech Nanomater Charact. 2017. https://doi.org/10.1016/B978-0-323-46139-9.00004-9.
Kuprianov VI, Arromdee P. Combustion of peanut and tamarind shells in a conical fluidized-bed combustor: a comparative study. Bioresour Technol. 2013;140:199–210. https://doi.org/10.1016/j.biortech.2013.04.086.
Haykiri-Acma H, Yaman S, Kucukbayrak S. Comparison of the thermal reactivities of isolated lignin and holocellulose during pyrolysis. Fuel Process Technol. 2010;91:759–64. https://doi.org/10.1016/j.fuproc.2010.02.009.
Mansa R, Zou S. Thermogravimetric analysis of microplastics: a mini review. Environmental Advances. 2021;5:100117. https://doi.org/10.1016/j.envadv.2021.100117.
Yadav A, Kumar N, Upadhyay A, Pratibha RK, Anurag RK. Edible packaging from fruit processing waste a comprehensive review. Food Rev Int. 2023;39:2075–106. https://doi.org/10.1080/87559129.2021.1940198.
Basiak E, Lenart A, Debeaufort F. Effect of starch type on the physico-chemical properties of edible films. Int J Biol Macromol. 2017;98:348–56. https://doi.org/10.1016/j.ijbiomac.2017.01.122.
Azira T, Amin I. (2016) Advances in differential scanning calorimetry for food authenticity testing. Advances in Food Authenticity Testing. https://doi.org/10.1016/B978-0-08-100220-9.00012-6
Drzeżdżon J, Jacewicz D, Sielicka A, Chmurzyński L. Characterization of polymers based on differential scanning calorimetry based techniques. TrAC, Trends Anal Chem. 2019;110:51–6. https://doi.org/10.1016/j.trac.2018.10.037.
Slobozeanu AE, Bejan SE, Tudor IA, Mocioiu A-M, Motoc AM, Romero-Sanchez MD, et al. A review on differential scanning calorimetry as a tool for thermal assessment of nanostructured coatings. Manuf Rev (Les Ulis). 2021;8:1. https://doi.org/10.1051/mfreview/2020038.
Müller AJ, Michell RM. Differential scanning calorimetry of polymers. Polym Morphol. 2016. https://doi.org/10.1002/9781118892756.ch5.
Fonseca-García A, Jiménez-Regalado EJ, Aguirre-Loredo RY. Preparation of a novel biodegradable packaging film based on corn starch-chitosan and poloxamers. Carbohydr Polym. 2021;251:117009. https://doi.org/10.1016/j.carbpol.2020.117009.
Hosseini SN, Pirsa S, Farzi J. Biodegradable nano composite film based on modified starch-albumin/MgO; antibacterial, antioxidant and structural properties. Polym Test. 2021;97:107182. https://doi.org/10.1016/j.polymertesting.2021.107182.
Zou Y, Yuan C, Cui B, Liu P, Wu Z, Zhao H. Formation of high amylose corn starch/konjac glucomannan composite film with improved mechanical and barrier properties. Carbohydr Polym. 2021;251:117039. https://doi.org/10.1016/j.carbpol.2020.117039.
Costa JCM, Miki KSL, Ramos AS, Teixeira-Costa BE. Development of biodegradable films based on purple yam starch_chitosan for food application. Heliyon. 2020;6:e03718. https://doi.org/10.1016/j.heliyon.2020.e03718.
Barizão CL, Crepaldi MI, Oscar Oliveira S, Oliveira AC, Martins AF, Garcia PS, Bonafé EG. Biodegradable films based on commercial κ-carrageenan and cassava starch to achieve low production costs. Int J Biol Macromol. 2020;165:582–90. https://doi.org/10.1016/j.ijbiomac.2020.09.150.
Lopes IA, Paixao LC, da Silva LJ, Rocha AA, Barros Filho AK, Santana AA. Elaboration and characterization of biopolymer films with alginate and babassu coconut mesocarp. Carbohydr polym. 2020;234:115747. https://doi.org/10.1016/j.carbpol.2019.115747.
Nordin N, Othman SH, Rashid SA, Basha RK. Effects of glycerol and thymol on physical, mechanical, and thermal properties of corn starch films. Food Hydrocoll. 2020;106:105884. https://doi.org/10.1016/j.foodhyd.2020.105884.
Riaz A, Lagnika C, Luo H, Dai Z, Nie M, Hashim MM, et al. Chitosan-based biodegradable active food packaging film containing Chinese chive (Allium tuberosum) root extract for food application. Int J Biol Macromol. 2020;150:595–604. https://doi.org/10.1016/j.ijbiomac.2020.02.078.
Xie Y, Niu X, Yang J, Fan R, Shi J, Ullah N, et al. Active biodegradable films based on the whole potato peel incorporated with bacterial cellulose and curcumin. Int J Biol Macromol. 2020;150:480–91. https://doi.org/10.1016/j.ijbiomac.2020.01.291.
Wang R, Li X, Ren Z, Xie S, Wu Y, Chen W, Ma F, Liu X. Characterization and antibacterial properties of biodegradable films based on CMC, mucilage from Dioscorea opposita Thunb. and Ag nanoparticles. Int J Biol Macromol. 2020;163:2189–98. https://doi.org/10.1016/j.ijbiomac.2020.09.115.
Batista JTS, Araújo CS, Peixoto Joele MRS, Silva JOC, Lourenço LFH. Study of the effect of the chitosan use on the properties of biodegradable films of myofibrillar proteins of fish residues using response surface methodology. Food Packag Shelf Life. 2019;20:100306. https://doi.org/10.1016/j.fpsl.2019.100306.
Oliveira NL, Rodrigues AA, Oliveira Neves IC, Teixeira Lago AM, Borges SV, de Resende JV. Development and characterization of biodegradable films based on Pereskia aculeata Miller mucilage. Ind Crops Prod. 2019;130:499–510. https://doi.org/10.1016/j.indcrop.2019.01.014.
Oluwasina OO, Olaleye FK, Olusegun SJ, Oluwasina OO, Mohallem NDS. Influence of oxidized starch on physicomechanical, thermal properties, and atomic force micrographs of cassava starch bioplastic film. Int J Biol Macromol. 2019;135:282–93. https://doi.org/10.1016/j.ijbiomac.2019.05.150.
Wang Z, Tian H, Wang X, Yu J, Jia S, Han L, et al. Study on thermal, rheological, mechanical, morphological, and barrier properties of poly(butylene adipate-co-terephthalate)/thermoplastic starch/poly(propylene carbonate) biodegradable blown films. J Therm Anal Calorim. 2023;148:1853–65. https://doi.org/10.1007/s10973-022-11858-8.
Wang Z, Zhao L, Jin B, Jia S, Han L, Pan H, et al. Effect of maleic anhydride and titanate coupling agent as additives on the properties of poly (butylene adipate-co-terephthalate)/thermoplastic starch films. Polym Bull. 2022;79:7193–213. https://doi.org/10.1007/s00289-021-03841-4.
Guadagno L, Raimondo M, Catauro M, Sorrentino A, Calabrese E. Design of self-healing biodegradable polymers. J Therm Anal Calorim. 2022;147:5463–72. https://doi.org/10.1007/s10973-022-11202-0.
Palai B, Biswal M, Mohanty S, Nayak SK. In situ reactive compatibilization of polylactic acid (PLA) and thermoplastic starch (TPS) blends; synthesis and evaluation of extrusion blown films thereof. Ind Crops Prod. 2019;141:111748. https://doi.org/10.1016/j.indcrop.2019.111748.
Quispe MM, Lopez OV, Boina DA, Stumbé J-F, Villar MA. Glycerol-based additives of poly(3-hydroxybutyrate) films. Polym Test. 2021;93:107005. https://doi.org/10.1016/j.polymertesting.2020.107005.
Aziman N, Kian LK, Jawaid M, Sanny M, Alamery S. Morphological, structural, thermal, permeability, and antimicrobial activity of PBS and PBS/TPS films incorporated with biomaster-silver for food packaging application. Polymers (Basel). 2021;13:391. https://doi.org/10.3390/polym13030391.
Shankar S, Reddy JP, Rhim J-W, Kim H-Y. Preparation, characterization, and antimicrobial activity of chitin nanofibrils reinforced carrageenan nanocomposite films. Carbohydr Polym. 2015;117:468–75. https://doi.org/10.1016/j.carbpol.2014.10.010.
da Costa JC, Miki KS, da Silva Ramos A, Teixeira-Costa BE. Development of biodegradable films based on purple yam starch/chitosan for food application. Heliyon. 2020;6(4):e03718. https://doi.org/10.1016/j.heliyon.2020.e03718.
de Lima Barizão C, Crepaldi MI, Oscar de Oliveira S, de Oliveira AC, Martins AF, Garcia PS, Bonafé EG. Biodegradable films based on commercial κ-carrageenan and cassava starch to achieve low production costs. Int J Biol Macromol. 2020;165:582–90. https://doi.org/10.1016/j.ijbiomac.2020.09.150.
Wang K, Gao S, Shen C, Liu J, Li S, Chen J, et al. Preparation of cationic Konjac glucomannan in NaOH urea aqueous solution. Carbohydr Polym. 2018;181:736–43. https://doi.org/10.1016/j.carbpol.2017.11.084.
Soccio M, Dominici F, Quattrosoldi S, Luzi F, Munari A, Torre L, et al. PBS-Based Green Copolymer as an Efficient Compatibilizer in Thermoplastic Inedible Wheat Flour/Poly(butylene succinate) Blends. Biomacromol. 2020;21:3254–69. https://doi.org/10.1021/acs.biomac.0c00701.
Bahari K, Mitomo H, Enjoji T, Yoshii F, Makuuchi K. Radiation crosslinked poly(butylene succinate) foam and its biodegradation. Polym Degrad Stab. 1998;62:551–7. https://doi.org/10.1016/S0141-3910(98)00041-X.
Acknowledgements
The authors gratefully acknowledge the financial support of Brazil’s National Council for Scientific and Technological Development (CNPq), through Grant number 141436/2020-4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santana, R.F., Bonomo, R.C.F. Thermal analysis for evaluation of biodegradable films: a review. J Therm Anal Calorim 149, 7155–7168 (2024). https://doi.org/10.1007/s10973-024-13339-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-024-13339-6