Abstract
Antimicrobial agents are substances that, when present at low concentrations, can delay microbial growth. For many years, several powerful antimicrobial substances have been synthetized. However, because of their significant side effects, there is a growing interest nowadays to find natural alternatives to the synthetic one. The present study aims to recover extracts with antimicrobial activity from Picea abies residues using two different extraction technologies, respectively, Soxhlet and supercritical carbon dioxide. Their antimicrobial activity was tested on the growth of Enterococcus faecalis and Streptococcus thermophilus. Isothermal calorimetry was used as a technique to quantify the antimicrobial effect of the extracts. The heat flow curves, obtained during the microbial growth, were fitted by a modified Gompertz function obtaining the lag time (λ) and the maximum growth rate (μmax) parameters. High-resolution mass spectrometry was used to identify the phenolic compounds responsible for the antimicrobial activity of the extracts. Regardless to the technology used, both extracts showed similar antimicrobial activity. For both microbial strains, the addition of the extract induced longer lag times (λ), while the maximum growth rate (μmax) decreased. S. thermophilus showed a higher resistance compared to E. faecalis suggesting a different capacity to metabolize the substrate in the presence of Picea abies extracts. Catechin, dihydroquercetin, astringin and isorhapontin were the identified phenolic compounds responsible for such effect. In conclusion, the results of this study provided an exciting potential for the future in light of the shift away from artificial preservatives and the trend toward natural alternative compounds coming from sustainable sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
From ancient times, wood always played a central role in human life. Despite its use for applications like heat generation, building weapons or vehicles progressively declined during the course of human civilization, nowadays, wood still remains essential in a myriad of applications, such as buildings, furniture, papers, utensils, artworks, musical instruments and more. However, behind the use of wood, a great amount of wooden residues are generated. Often, such residues have very little economical value. Therefore, there is a growing interest to investigate new possible ways to recycle the residues into new materials.
One fascinating approach to exploit and recycle wood residues was based on the extraction of bioactive compounds [1, 2]. Barks and branches of many tree species were used from centuries as a source of flavors, fragrances or colorants. Recently, there has been a growing interest to study their potential antioxidant and antimicrobial properties [3,4,5,6]. As examples, wood extracts from chestnut and cherry were tested to control the microbial spoilage of wines [7], extracts from Endopleura uchi tree reported a high antimicrobial and cytotoxic activities [8], extracts from Eucalyptus globulus wood demonstrated high activity for inhibiting bacteria and yeast growth [9], and extracts from needles of Abies alba obtained by hydrodynamic cavitation showed an enhanced antioxidant activity [10].
Among the several tree species, the residues of Norway spruce (Picea abies) received great attention as an important source of antimicrobial compounds. This species was widely distributed in Europe. Norway spruce represented about 38% of trees in the European forests [11], and it was one of the dominant forest tree species in the Alps. Its industrial processing originated important quantities of residual materials. It was found that the bark of Norway spruce had a high content of extractives with a very good antioxidant activity from the crude polar fraction [12]. In addition, because of the high content of hemicellulose the production of oligomers with potential use in nutraceutical and pharmaceutical industries seemed to be a feasible option. In another study, it was demonstrated that Norway spruce bark extracts had a strong antimicrobial activity against human pathogens such as Staphylococcus aureus, Klebsiella pneumoniae and Pseudomonas aeruginosa [13]. Similarly, a significant antibacterial effect of Picea abies wood material against Streptococcus pneumoniae was also found [14].
Although the antimicrobial and antioxidant activity of Picea abies extracts was confirmed in several studies, what was not yet well understood is the effect of the extraction technology and the solvent used during the extraction on the resulting antimicrobial or antioxidant activity. For instance, the methanolic extract of Thymus pectinatus (Lamiaceae) did not show any antimicrobial activity. However, some antimicrobial activity was observed when the essential oil was obtained by Clevenger distillation [15]. Such differences could be linked to the solvent used for the extraction. Conversely, Salem reported that methanolic extract of Picea abies exhibited strong antibacterial and antioxidant activity [1].
Moreover, not only the solvent, but also the technology could affect the resulting functional properties of wood extracts. For instance, it was reported that pressurized methanol extraction from emblica (Phyllanthus emblica L.) plant had a superior antioxidant activity than supercritical fluid or conventional methanolic extraction [16]. However, this result was debated since the use of high temperatures was known to negatively affect the resulting antioxidant activity of the extract. Other works reported, instead, that supercritical fluid extraction, thanks to the mild temperatures and the addition of co-solvents, allowed obtaining essential oils with strong antimicrobial activity. For instance, extracts from Agarwood (Aquilaria crassna) exhibited antimicrobial activity against S. aureus and C. albicans [17]. Moreover, extracts from supercritical fluid obtained by cedarwood [18], Pinus taeda chips [19] and Eucalyptus wood industrial wastes exhibited all strong antioxidant activities [20]. Recently, the potential of supercritical fluid extracts obtained from Picea abies against the growth of Escherichia coli has also been shown [21]. However, no comparisons were carried out with other extracts obtained from an organic solvent extraction.
The studies published so far clearly evidenced the lack in knowledge and controversies regarding the effective antimicrobial activity of natural extracts obtained with different technologies such as those using organic polar solvents at high temperatures and those applying more eco-friendly approaches comprising the use of carbon dioxide as apolar solvent in supercritical state. The present manuscript aimed to fill this gap. In detail, the study focused on the antimicrobial effectiveness of Picea abies extracts obtained by two different technologies: Soxhlet extraction with ethanol and supercritical fluid extraction with carbon dioxide. The choice of these two types of extraction was based essentially on the polarity of the solvents. Ethanol–Soxhlet extraction generally led extracts composed of polar phenolic compounds. Instead, extracts obtained from carbon dioxide in its supercritical state provided essential oils rich in apolar components [22, 23]. These two extraction technologies will be applied on the same spruces of Picea abies, whose choice was mainly justified by its widespread use in building and manufacturing worldwide.
The antimicrobial activity of Picea abies extracts, obtained by supercritical fluid and ethanol–Soxhlet extraction, was then tested on two gram-positive microorganisms such as Enterococcus faecalis and Streptococcus thermophilus. These microbial strains were chosen as the first one is common in human body with high resistance to hot, salty and acidic environments, while the second one is present in several dairy products and, although nonpathogenic, its inactivation provides evidences on how a natural extract can act on a microorganism of a food-related matrix. The antimicrobial activity was monitored by isothermal calorimetry. HPLC-HRMS analysis of the extracts with and without their mixture with the microbial strains was also performed to identify the phenolic compounds responsible for the antimicrobial action.
Materials and methods
Spruce from Picea abies
Collection of Norway spruce (Picea abies) residues was carried out in the South Tyrol region (Italy). Upon arrival in the laboratory, the samples were ground to obtain a fine powder with 300–800 μm particle size (Mill-LM3100, Perten Instruments, Sweden). The final moisture content of the powder was equal to 7.8 ± 1.2, while the water activity was equal to 0.4.
Extraction from Picea abies by supercritical carbon dioxide
A high-pressure pilot plant (Superfluidi s.r.l., Padova, Italy) was used to perform the extraction with supercritical carbon dioxide from residues of Picea abies. The system comprised an extractor (1 L volume of the vessel) and two gravimetric separators. Inside the extractor, a stainless steel vessel (800 mL volume), closed with porous stainless steel mesh filters on both ends, was loaded. A high-pressure diaphragm pump (Lewa LDC–M–9XXV1, Milano, Italy) was used to pump CO2 inside the vessel. For the experiments, 80 ± 1 g of Picea abies powder was loaded in the high-pressure vessel. A first experimental plan was set up in order to define the conditions of pressure (from 10 to 30 MPa), temperature (from 35 to 50 °C) and time (10 to 180 min) to obtain the highest extraction yield. Ethanol as co-solvent with percentage equal to 10% (w/w) was added to increase the concentration of phenolic compounds in the final extracts. A low CO2 flow rate of 2 L h−1 was selected in order to ensure higher contact times between the solvent and the sample. The highest yield, expressed as a ratio between the grams of extract and those of wood powder placed in the high-pressure vessel, was equal to 3.4 ± 0.5% (w/w) and was obtained at 45 °C, 20 MPa and 120 min.
Soxhlet extraction
Soxhlet extraction was performed with ethanol as solvent. About 150 mL of ethanol was recycled over 10 g of Picea abies powder in a Soxhlet apparatus for 6 h at the boiling temperature of the solvent. The yield was equal to 2.6 ± 0.7% (w/w).
Antimicrobial activity of extracts
Microbial growth conditions
Picea abies extracts were tested on two gram-positive microorganisms: Enterococcus faecalis (ATCC 29212) and Streptococcus thermophilus (ATCC 19258). The concentration of the microbial cultures ranged from 101 to 107 CFU mL−1. The strains were stored in tryptone soy broth (TSB) at − 80 °C until needed. For experimental use, the stock cultures were maintained on tryptone soy agar (TSA) slants at 4 °C and transferred monthly.
Inhibition of microbial growth
The antimicrobial activity of Picea abies extracts was assessed on E. faecalis and S. thermophilus growth by isothermal calorimetry (Thermal Activity Monitor, Model 421 TAM III, TA Instruments). The methodology was the same applied in previous studies [21, 24, 25]. In these studies, the reliability of the methodology was assessed by the comparison with accepted standards endorsed by the National Committee for Clinical Laboratory Standards (NCCLS) such as plate count technique or disk diffusion test. To perform the experiment by isothermal calorimetry, a colony of each of the two strains was transferred to 10 mL of tryptic soy broth (TSB) and incubated at 37 and 40 °C for E. faecalis and S. thermophilus, respectively. The incubation time was set to 18 h to obtain fresh early-stationary-phase cells. The microbial suspensions with a final concentration of 108 CFU mL−1 (colony forming unit per mL) were serially diluted in TSB obtaining 105 CFU mL−1. Picea abies extracts from supercritical fluid and Soxhlet extraction were also diluted in sterile TSB. Then, they were mixed with the microbial cultures reaching a final concentration equal to 1, 3 and 5 mg mL−1. About 1 mL of the samples was transferred to 4-mL sterile stainless steel vials to start the analysis with isothermal calorimetry. The heat flow developed during the microbial growth was recorded every 10 s at 37 °C for E. faecalis and 40 °C for S. thermophilus. The experiments were performed in triplicate. From each calorimetric curve, the total heat (Qtot), produced during the microbial growth, the maximum heat flow value (ϕmax) and the time at which the maximum heat flow was recorded (tp) were calculated and the results were expressed as mean values and standard deviations.
Thermodynamic parameters of microbial by isothermal calorimetry
The heat flow curves generated during the microbial growth were analyzed calculating the fractional reaction parameter α obtained from the integral of the heat flow curve. It was calculated using the following equation:
where q(t) is the cumulative heat and Qtot is the overall heat.
By plotting α calculated from Eq. (1) as a function of the time, a sigmoidal curve was obtained. The curve was fitted by an iterative least squares function using the following modified Gompertz function:
where λ is the lag time (h), μmax is the maximum growth rate (h−1) and e is the Euler’s number.
Identification of phenolic compounds responsible for the antimicrobial activity of Picea abies extracts
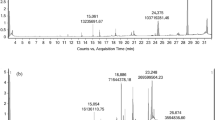
The phenolic compounds of Picea abies extracts were identified by high-performance liquid chromatography–high-performance mass spectrometry (HPLC-HRMS). A Q Exactive Orbitrap HRMS instrument (Thermo Scientific, Milano, Italy) coupled to an UltiMate 300 UHPLC instrument was used. A flow rate equal to 0.2 mL min−1 with an Accucore RP-MS LC column (100 mm × 2.1 mm i.d., 2.6 μm) with a pre-column (Thermo Scientific, Milano, Italy) was used to get the separation of the phenolic compounds. The following conditions were applied for full-MS analysis with the mass spectrometer operated in negative ionization mode: sheath gas equal to 20 (arbitrary units), aux gas equal to 5 (arbitrary units) and aux temperature of 250 °C. The spray voltage was set at ± 3.5 kV, the capillary temperature at 320 °C and RF S-lens at 65 °C. The mass ranges were selected from 100 to 1000 m z−1 with a full-MS set resolution of 70,000 at m z−1 200, AGC target at 1 × 106 and max. injection time of 175 ms. The MS2 measurements of the selected ions were performed with a resolution of 17,500 and AGC target set at 5 × 105. The phenolic compounds were identified based on their retention time and UV absorbance at 280 nm. The retention times were compared with those of the external analytical standards. Correlation of chemical compounds’ relative abundances and integration of the area under each peak were done using Compound Discoverer 2.1 software (Thermo Scientific, Milano, Italy).
To identify the phenolic compounds responsible for the inhibition activity, about 5 mg of supercritical carbon dioxide and Soxhlet extracts were added to 1 mL of TSB broth in the presence and absence of E. faecalis with a concentration of 106 CFU mL−1. This microbial strain was chosen as it is the most sensitive to the addition of Picea abies extract. The samples were incubated at 37 °C for 24 h. A third sample was also prepared by adding 5 mg of supercritical carbon dioxide and Soxhlet extracts to 1 mL of TSB broth. The three samples were analyzed by HPLC-HRMS. For extraction of the phenolic compounds, 5 mL of MeOH/water (70:30) was added to 500 mg of the sample. The mixture was vortexed for 5 min, sonicated at room temperature for 15 min, and centrifuged at 10,000 rpm for 15 min. The supernatant was filtered through 0.2-μm syringe filters before analysis. Three independent extractions were performed for each sample. The analysis was performed in triplicate, and the results were expressed as mean values and standard deviations.
Statistical analysis
The analysis of variance (ANOVA) using IBM SPSS software was carried out to detect significant differences and main effects between the values of the overall heat (Qtot), lag time (λ) and maximum growth rate (μmax) obtained with different concentrations and type of Picea abies extract added to the microbial strains. The significant differences were analyzed by the Tukey test (p < 0.01). A comparison with previous published findings confirmed the goodness of the obtained results [21, 24, 25].
Results
Microbial growth by isothermal calorimetry
Figure 1A shows the isothermal calorimetric signals (thermal power vs. time) obtained at 37 °C during the microbial growth of S. thermophilus. The inoculum concentrations ranged from 101 to 107 CFU mL−1. Figure 1B displays that the resulting cumulative heat (q) versus time (t) matched the modified Gompertz function:
where \(Q_{\text{tot}}\) is the total heat produced during the whole growth process, calculated as the area swept by the heat flow curve; λ is the lag time, which is defined as the initial period between the starting of the experiments and the onset time of the exponential growth; finally, μmax is the maximum growth rate, which is calculated as the highest slope observed along the heat flow curve. This corresponded to the maximum growth rate of the microbial strain. In addition, from the heat flow curve, it was also possible to measure the maximum heat flow value (ϕmax) together with the time at which such value was recorded (tp).
These two parameters were also connected with the microbial growth and were used to identify the end of the exponential growth, i.e., when substrates or oxygen requirements became limiting. All the results are reported in Table 1.
Correlation analysis between isothermal calorimetry variables
Table 2 reports the Pearson correlation coefficients (r) between the variables reported in Table 1. This provides information about the relationships between variables. The strongest correlation was observed between the logarithm of the microbial load concentrations and either the lag time (λ) or the peak time (tp), respectively, with r = − 0.995, n = 7, p = 0.01 and r = − 0.990, n = 7, p = 0.01. Higher microbial loads (from 101 to 107 CFU mL−1) corresponded to shorter λ or tp. In practice, within the observed range from 101 to 107 CFU mL−1 of S. thermophilus, the lag time significantly increased (p < 0.01) from 2.3 ± 0.2 to 10.9 ± 0.6 h. Because of the substantial linearity of this correlation (R2 = 0.99), a linear equation: λ = − 1.39 C + 11.11 was used to determine the maximum inoculum concentration (C, expressed as log(CFU mL−1)) that could be detected. This resulted equal to 105 CFU mL−1 [24, 25]. Similar results were also obtained for the microbial growth of E. faecalis. Again, a linear trend was observed (R2 = 0.99), with equation equal to λ = − 1.59 C + 19.57. Other variables like the total heat (Qtot), the maximum growth rate (μmax, h−1) and the maximum heat flow (ϕmax, μW) values were nearly invariant to the inoculum concentration. For these reasons, the lag time was used as an index of microbial growth in all the next sections.
Antimicrobial activity of Picea abies extracts
This section investigates the antimicrobial activity of wood extracts from Picea abies. Twelve experiments were designed in double to test the antimicrobial activity of (n = 12) extracts obtained by Soxhlet extraction and by (n = 12) supercritical fluid extraction. For each extraction technology, the lag time of the two microbial strains, respectively, Streptococcus thermophilus and Enterococcus faecalis, was tested by using three doses of the extracts. Figure 2A shows the average raw heat flow signals obtained exclusively for S. thermophilus, upon the addition of increasing concentrations of Picea abies extracts obtained by supercritical carbon dioxide extraction. Similarly, Fig. 2B shows the experiments obtained with extracts from Soxhlet extraction. In both cases, when the extract was present, the microbial growth was characterized by longer lag times (λ), shorter area (Qtot) and lower maximum heat flow (ϕmax) values. Also, the maximum growth rate (μmax) decreased with higher dose of the extract. Despite all these changes, however, for the reasons explained in the previous section, the shifts on the lag time were selected as the primary index of the antimicrobial activity.
Three-way analysis of variance
With the results observed in Fig. 2, extended to both strains of E. faecalis and S. thermophilus, a three-way ANOVA was performed to examine the effect of: (1) extracts concentrations (1, 3 and 5 mg mL−1), (2) type of extraction technology (supercritical carbon dioxide vs. Soxhlet) and (3) type of microorganism (E. faecalis vs. S. thermophilus). The dependent variable for this experimental design was the lag time, selected because of its strong negative correlation with the concentration of the microbial strains (see previous sections). As before, the isothermal calorimetric signal was recorded and fitted by a modified Gompertz function. The fitting parameters are reported in Table 3.
Considering the lag time as the dependent variable, the results of ANOVA in Table 4 show that the most important effect was given by the concentration of the extract, which accounted for more than 40% of the total variance (η2). A further 16% of variance was also explained by the interaction between the extract concentration and the microbial strain. The presence of such interaction suggested that the Enterococcus strain was more sensitive to the addition of the Picea abies extract than the Streptococcus strain. In detail, at any concentration of the extract, the average lag time (in hours) observed for Enterococcus strain (M = 16.9, SD = 7.4) was significantly higher than the one from Streptococcus (M = 9.4, SD = 1.9). This comparison led to a large value of the Student parameter t (22) = 3.46 (p = 0.002). This meant in practice that the extract of Picea abies showed a remarkably higher antimicrobial activity toward Enterococcus than Streptococcus strain. Such superior effect was even more evident at higher doses of the extract. Figure 3A shows that the effect of the dose used was maximized with Enterococcus faecalis. Such strong interaction between strain and dose was confirmed with the ANOVA results (Table 3), showing a F(2,12) = 89.8, p < 0.001.
A Effect of extract concentration on the lag time of E. faecalis (orange line) and S. thermophilus (blue line); B effect on the lag time of the two microbial strains to which Picea abies extracts from SFE (orange line) and Soxhlet (blue line) were added; C effect on the lag time of extract concentrations obtained by SFE (blue line) and Soxhlet (orange line) technology. (Color figure online)
The extraction technology also played a significant role (F(1,12) = 43, p < 0.001), although its effect was small (η2 = 4%). Such effect is displayed in Fig. 3B. In this plot, the extracts obtained by supercritical fluid extraction exerted a remarkable higher effect only when used with Enterococcus strain. Apparently, extracts from supercritical fluids had some compounds, which were more effective toward Enterococcus than Streptococcus. This hypothesis was confirmed by the significance of the interaction between the microbial strains and the extraction technology (F(1,12) = 19.5, p < 0.001, η2 = 2%).
Finally, Fig. 3C does not provide any evidence that the interaction between the extraction technology and the concentration was significant. For both extraction technologies, the lag time increased with the concentration of the extract used. The ANOVA confirmed the nonsignificant interaction. It was possible to conclude that, regardless to the technology used, both extracts succeeded similarly to inhibit the growth of microbial strains.
Antimicrobial compounds in Picea abies extracts
This section explores some of the main polyphenolic compounds responsible for the antimicrobial activity of Picea abies extracts. Among the two microbial strains, E. faecalis (106 CFU mL−1) was chosen as test microorganism. This choice was based on the results reported in the previous section showing that E. faecalis was more sensitive to the addition of Picea abies extract than S. thermophilus. The microbial culture was left at 37 °C for 24 h with the addition of extracts obtained from supercritical fluid and Soxhlet extraction (5 mg mL−1). Before and after incubation, the main phenolic compounds of the extracts were analyzed by HPLC-HRMS. All the results are reported in Table 4.
Before incubation, the most representative phenolic compounds (i.e., highest area under the peaks of the chromatogram) present in the extracts were: 2-methylbenzoic acid, gallic acid, catechin, dihydroquercetin, hydroxypinoresinol and isorhapontin. Such compounds were present in both extracts, either obtained by Soxhlet or obtained by supercritical carbon dioxide technology. A control experiment was performed by incubating the extracts in the medium at 37 °C for 24 h without the presence of the microorganism. The results did not show any significant change in these phenolic compounds.
After incubation, significant changes (p < 0.05) were observed on catechin, dihidroquercetin, astringin and isorhapontin. The area under the peaks of these phenolic compounds significantly decreased after the incubation of the microbial strain with Picea abies extracts. The consumption of these compounds was likely attributable to their antimicrobial effect. Both extracts showed a consistent inhibition effect as indicated by the values reported in Table 5. For some phenolic compounds (i.e., cinnamic acid, protocatechuic acid and gallic acid), the inhibition, calculated as decreased area under the peak, was higher for the extracts obtained by supercritical carbon dioxide.
Discussion
In this study, the antimicrobial activity of extracts from residues of Picea abies obtained by supercritical fluid and Soxhlet extraction was compared. Both extracts were able to inhibit similarly the microbial growth. For both microbial strains, when the extract was present, the growth was characterized by longer lag times (λ), lower heat developed during the growth (Qtot) and consequently lower heat flow values (ϕmax). Also, the maximum growth rate (μmax) decreased with higher dose of the extracts. The microbial strain of S. thermophilus showed a higher resistance to Picea abies extracts compared to E. faecalis. This effect could be related to the capacity of S. thermophilus to form small flocks during the growth [26]. The microbial flocks reported higher resistance to the antimicrobial agent addition likely because, in this form, the colonies resulted are less exposed to the antimicrobial extract. Instead, E. faecalis developed short chains, which, apparently, left a larger number of cells in direct contact with the antimicrobial agent [27, 28]. The antimicrobial activity of Picea abies extracts against gram-positive and gram-negative bacteria and fungi has been already demonstrated in some studies [1, 29,30,31]. They showed the unambiguously antibacterial activity of Picea abies extracts obtained by hydrodistillation and solvent extraction and recognized some compounds such as quercetin, kaempferol and myricetin as responsible for the antimicrobial activity. A comprehensive study was also addressed assessing the antimicrobial effects of wood-associated polyphenols on food pathogens and spoilage organisms [32]. Polyphenolic compounds such as pinosylvin, pinosylvin monomethyl ether, astringin, piceatannol, isorhapontin, and isorhapontigenin reported high antimicrobial activity against gram-negative (Salmonella) and gram-positive bacteria (Listeria monocytogenes, Staphylococcus epidermidis, Staphylococcus aureus) and yeasts (Candida tropicalis, Saccharomyces cerevisiae). Similarly, in a recent review phenolics and extracted phenolic compounds from Scots pine (Pinus sylvestris) and Norway spruce (Picea abies) were classified based on their antibacterial activity against several microorganisms [33]. It was reported that in Norway spruce (P. abies), the major flavonoids present were quercetin, dihidroquercetin and myricetin, which also reported a high antimicrobial activity against different bacteria.
In this study, the inhibition of Picea abies extracts was associated with some phenolic compounds, i.e., catechin, dihidroquercetin, astringin and isorhapontin, which were detected in lower concentration after the incubation with the microbial cultures. The majority of phenolic compounds in plants are synthesized via the phenylpropanoid pathway. They belong to a group of phenylalanine derivatives, which have a basic C6–C3 carbon skeleton, and are antimicrobial agents naturally synthesized in the wood to respond to microorganisms attacks [33]. This clearly explains their efficient antimicrobial action if properly extracted from wood. Although toxicity information on such phenolic compounds is still scarce, several studies showed their potential as antioxidant and antimicrobial substances. As an example, among these compounds, catechin, a pharmacologically active phenols, was found to inhibit intestinal tumor formation in mice [34], to retard the oxidation of low-density lipoprotein [35] and to enhance the antifungal effect of amphotericin B against Candida albicans [36]. Similar findings have been also published testing phenolic compounds such as astringin and isorhapontin as antioxidant and antimicrobial natural substances [33]. They all supported the results of the present study confirming the efficacy of extracts containing such compounds.
Conclusions
In this study, the antimicrobial activity of extracts from residues of Picea abies obtained by supercritical fluid and Soxhlet extraction was compared. Both extracts were able to inhibit similarly the microbial growth. For both microbial strains, when the extract was present, the growth was characterized by longer lag times (λ), lower heat developed during the growth (Qtot) and consequently lower heat flow values (ϕmax). Also, the maximum growth rate values (μmax) decreased with higher dose of the extract. Catechin, dihidroquercetin, astringin and isorhapontin were some of the detected phenolic compounds responsible for such antimicrobial activity. The results of the present study represented the first attempt to test extracts from Picea abies residues, obtained by a green extraction technology such as supercritical carbon dioxide, with the capability to inhibit microorganisms’ growth.
On a more general perspective, the study provided an exciting potential for the future, especially in light of the shift away from artificial preservatives and the application of natural compounds coming from sustainable sources.
References
Salem MZM, Elansary HO, Elkelish AA, Zeidler A, Ali HM, Yessoufou K. In vitro bioactivity and antimicrobial activity of Picea abies and Larix decidua wood and bark extracts. Bioresources. 2016;11:9421–37.
Grassmann J, Hippeli S, Vollmann R, Elstner EF. Antioxidative properties of the essential oil from Pinus mugo. J Agric Food Chem. 2003;51:7576–82.
Bianchi S, Gloess AN, Kroslakova I, Mayer I, Pichelin F. Analysis of the structure of condensed tannins in water extracts from bark tissues of Norway spruce (Picea abies [Karst.]) and Silver fir (Abies alba [Mill.]) using MALDI-TOF mass spectrometry. Ind Crops Prod. 2014;61:430–7.
Kusumoto N, Zhao T, Swedjemark G, Ashitani T, Takahashi K, Borg-Karlson A. Antifungal properties of terpenoids in Picea abies against H eterobasidion parviporum. For Pathol. 2014;44:353–61.
Minova S, Sešķēna R, Voitkāne S, Metla Z, Daugavietis M, Jankevica L. Impact of pine (Pinus sylvestris L.) and spruce (Picea abies (L.) Karst.) bark extracts on important strawberry pathogens. Proc Latv Acad Sci Sect B Nat Exact Appl Sci. 2015;69:62–7.
Sahin HT, Yalcin OU. Chemical composition and utilization of conifer needles—a review. J Appl Life Sci Int. 2017;14:1–11.
Alañón ME, García-Ruiz A, Díaz-Maroto M, Pérez-Coello MS, Moreno-Arribas M. Antimicrobial and antioxidant activity of pressurized liquid extracts from oenological woods. Food Control. 2015;50:581–8.
Politi FA, de Mello JC, Migliato KF, Nepomuceno AL, Moreira RR, Pietro RC. Antimicrobial, cytotoxic and antioxidant activities and determination of the total tannin content of bark extracts Endopleura uchi. Int J Mol Sci. 2011;12:2757–68.
Cruz JM, Domínguez JM, Domínguez H, Parajó JC. Antioxidant and antimicrobial effects of extracts from hydrolysates of lignocellulosic materials. J Agric Food Chem. 2011;49:2459–64.
Albanese L, Bonetti A, D’Acqui LP, Meneguzzo F, Zabini F. Affordable production of antioxidant aqueous solutions by hydrodynamic cavitation processing of silver fir (Abies alba Mill.) needles. Foods. 2019;8:65.
Becvárová P, Horváth M, Sarapatka B, Zouhar V. Dynamics of soil organic carbon (SOC) content in stands of Norway spruce (Picea abies) in central Europe. For Biogeosci For. 2018;11:734–42.
Neiva DM, Araújo S, Gominho J, de Cássia Carneiro A, Pereira H. An integrated characterization of Picea abies industrial bark regarding chemical composition, thermal properties and polar extracts activity. PLoS ONE. 2018;13:e0208270.
Tanase C, Cosarca S, Toma F, Mare A, Cosarca A, Mare A. Antibacterial activities of spruce bark (Picea abies L.) extract and its components against human pathogens. Rev Chim. 2018;69:1462–7.
Vainio-Kaila T, Kyyhkynen A, Rautkari L, Siitonen A. Antibacterial effects of extracts of Pinus sylvestris and Picea abies against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, and Streptococcus pneumoniae. Bioresources. 2015;10:7763–71.
Vardar-Ünlü G, Candan F, Sökmen A, Daferera D, Polissiou M, Sökmen M. Antimicrobial and antioxidant activity of the essential oil and methanol extracts of Thymus pectinatus Fisch. et Mey. Var. pectinatus (Lamiaceae). J Agric Food Chem. 2003;51:63–7.
Liu X, Zhao M, Wang J, Luo W. Antimicrobial and antioxidant activity of emblica extracts obtained by supercritical carbon dioxide extraction and methanol extraction. J Food Biochem. 2009;33:307–30.
Wetwitayaklung P, Thavanapong N, Charoenteeraboon J. Chemical constituents and antimicrobial activity of essential oil and extracts of heartwood of Aquilaria crassna obtained from water distillation and supercritical fluid carbon dioxide extraction. Silpakorn Univ Sci Technol J. 2009;3:25–33.
Eller FJ, King JW. Supercritical carbon dioxide extraction of cedarwood oil: a study of extraction parameters and oil characteristics. Phytochem Anal Int J Plant Chem Biochem Tech. 2000;11:226–31.
Pasquini D, Pimenta MTB, Ferreira LH, da Silva C, Aprigio A. Extraction of lignin from sugar cane bagasse and Pinus taeda wood chips using ethanol–water mixtures and carbon dioxide at high pressures. J Supercrit Fluids. 2005;36:31–9.
González-Vila FJ, Bautista JM, Gutierrez A, Del Rio J, González A. Supercritical carbon dioxide extraction of lipids from Eucalyptus globulus wood. J Biochem Biophys Methods. 2000;43:345–51.
Haman N, Morozova K, Tonon G, Scampicchio M, Ferrentino G. Antimicrobial effect of Picea abies extracts on E. coli growth. Molecules. 2019;24:4053–63.
Cao H, Xiao JB, Xu M. Comparison of volatile components of Marchantia convoluta obtained by supercritical carbon dioxide extraction and petrol ether extraction. J Food Compos Anal. 2007;20:45–51.
Caredda A, Marongiu B, Porcedda S, Soro C. Supercritical carbon dioxide extraction and characterization of Laurus nobilis essential oil. J Agric Food Chem. 2002;50:1492–6.
Stulova I, Kabanova N, Kriščiunaite T, Adamberg K, Laht T, Vilu R. Microcalorimetric study of the growth of Streptococcus thermophilus in renneted milk. Front Microbiol. 2015;6:79.
Kabanova N, Stulova I, Vilu R. Microcalorimetric study of the growth of bacterial colonies of Lactococcus lactis IL1403 in agar gels. Food Microbiol. 2012;29:67–79.
Hardie JM, Whiley RA. Classification and overview of the genera Streptococcus and Enterococcus. J Appl Microbiol. 2003;83(S1):1S–11S.
Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Med. 2006;119:S3–10.
Davies J, Davies D. Origins and evolution of antibacterial resistance. Microbiol Mol Biol Rev. 2010;74:417–33.
Radulescu V, Saviu S, Chifiriu C, Oprea E, Ilies DC, Marutescu L, Lazar V. Chemical composition and antimicrobial activity of essential oil from shoots spruce (Picea abies L.). Rev Chim. 2011;62:69–74.
Puupponen-Pimiä R, Nohynek L, Meier C, Kähkönen M, Heinonen M, Hopia A, Oksman-Caldentey KM. Antimicrobial properties of phenolic compounds from berries. J Appl Microbiol. 2001;90:494–507.
Rauha JP, Remes S, Heinonen M, Hopia A, Kähkönen M, Kujala T, Pihlaja K, Vuorela H, Vuorela P. Antimicrobial effect of Finnish plant extracts containing flavonoids and other phenolic compounds. Int J Food Microbiol. 2000;56:3–12.
Plumed-Ferrer C, Väkeväinen K, Komulainen H, Rautiainen M, Smeds A, Raitanen J-R, Eklund P, Willför S, Alakomi H-L, Saarela M, Wright A. The antimicrobial effects of wood-associated polyphenols on food pathogens and spoilage organisms. Int J Food Microbiol. 2013;164:99–107.
Metsämuuronen S, Siren H. Bioactive phenolic compounds, metabolism and properties: a review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem Rev. 2019;18:623–64.
Weyant MJ, Carothers AM, Dannenberg AJ, Bertagnolli MM. (+)-Catechin inhibits intestinal tumor formation and suppresses focal adhesion kinase activation in the min/+mouse. Cancer Res. 2001;61:118–25.
Mangiapane H, Thomson J, Salter A, Brown S, Bell GD, White DA. The inhibition of the oxidation of low density lipoprotein by (+)-catechin, a naturally occurring flavonoid. Biochem Pharmacol. 1992;43:445–50.
Hirasawa M, Takada K. Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans. J Antimicrob Chemother. 2004;53:225–9.
Acknowledgements
This work was supported by the Project WOOD-UP (FESR1028) and financed by the European Regional Development Fund (ERDF) Investment for Growth and Jobs Programme 2014–2020. We are grateful to the Province of Bolzano for financial support (Landesregierung mittels Beschluss Nr. 1472, 07.10.2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest in relation to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferrentino, G., Haman, N., Morozova, K. et al. Phenolic compounds extracted from spruce (Picea abies) by supercritical carbon dioxide as antimicrobial agents against gram-positive bacteria assessed by isothermal calorimetry. J Therm Anal Calorim 145, 3093–3103 (2021). https://doi.org/10.1007/s10973-020-10100-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10100-7