Abstract
The present study aimed at assessing the antimicrobial properties of a water and ethanol ultrasound-assisted extraction (UAE) of dry goji berries and of lyophilised powdered pomegranate peel in vitro. Minimum inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) methods, turbidity (cell density) measurement, and well diffusion assay were used to determine the antimicrobial activity against several species of foodborne bacteria (Gram – , Escherichia coli, Salmonella typhimurium, Campylobacter jejuni), (Gram + Staphylococcus aureus, Listeria monocytogenes, Clostridium perfringens), yeasts (Yarrowia lipolytica, Metschnikowia fructicola, and Rhodotorula mucilaginosa), and fungi (Penicillium expansum, Aspergillus niger, Fusarium oxysporum, and Rhizoctonia solani). Carbohydrate and phenolic contents were measured, and DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2΄-Azino-bis-(3-ethyl-benzthiazoline-sulphonic acid)) radical scavenging assays were used for the assessment of antioxidant activity. Fourier transform infrared (FTIR) spectrums of all samples were also evaluated in order to determine their chemical profiles. The lyophilised pomegranate peel exhibited the highest antioxidant, antimicrobial, and antifungal activity among all samples, while among the goji berry samples-who had only antibacterial and very little or no antifungal activity—the lyophilised aqueous extract with the lowest content of maltodextrin (2%) and highest phenolic content, had also the highest antioxidant, antimicrobial, and antifungal activity. The antioxidant and antimicrobial bioactivities seemed to be related to the content of polyphenols, the low concentration of maltodextrin in the encapsulated lyophilised samples and the use of optimised ultrasound assisted extraction. Minimum inhibitory concentration or zones of inhibition were in many (but not all) cases lower for the aqueous extracts compared to the ethanol or ethanol/hexane extracts of goji berries. In conclusion, the lyophilized powder of pomegranate peels and the aqueous extracts of goji berries encapsulated with minimal maltodextrin content and high polyphenol content exhibited high antioxidant and antimicrobial activity which could be utilized in food preservation or plant protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, great interest has been paid to natural substances isolated or extracted from natural sources such as plants regarding their antimicrobial properties and beneficial health effects [1, 2]. Many plant species have a long history and great interest as part of widely accepted herbal formulas that have been used as preventative and curative agents in traditional medicine, for example in ancient Greek or traditional Chinese medicines [3].Two of the most important plant species considered to be functional or bioactive are Lycium barbarum (goji berry) and Punica granatum (pomegranate) [4, 5].
Lycium barbarum fruit contains bioactive components and is not only known as a medical herb but also as a functional food. Polysaccharides are the most explored for their bioactivity and are considered as the most important bioactive constituent of the fruit in terms of quantity [6, 7]. The second largest constituent is carotenoid compounds, such as zeaxanthin dipalmitate [8], while the fruits also contain vitamins like riboflavin, thiamine, and ascorbic acid [9]. Flavonoids (rutin, myricetin, quercetin, kaempferol) and phenolic acids (cafeic acid, chlorogenic acid and coumaric acid) are also major components of goji berries, which are especially liked to bioactivity and especially antioxidant and antimicrobial activity [10, 11]. The rich variety of these bioactive compounds of goji berry and their various health effects and protective properties (immune enhancing, antioxidant, hepatoprotective, hypoglycaemic, hypolipidemic, antimicrobial, and other properties) have led to the exploitation of goji berries in an emerging market of function foods (teas, yogurts, beverages, wines) and dietary supplements [12], but they could also be utilized as novel natural antimicrobials for use in food and biological phytochemicals, which is at the core of the present research. Notably, in order to design such natural antimicrobials, the exact role and the optimal concentration of bioactive molecules involved in antimicrobial activity, as well as the optimal extraction processes for obtaining these compounds will need to be addressed, which is a major aim of this work.
The extraction process and the solvents (e.g. water, alcohol) and conditions (e.g. temperature, time) that are applied are crucial for determining the final composition of bioactive compounds, and thus the antimicrobial properties of goji berries, as has been shown previously for microwave-assisted extraction [13].Ultrasound assisted extraction (UAE) is another modern extraction technique for the production of high quality bio-active extracts from natural materials, which enables the advantageous extraction of polysaccharides, polyhenols-flavonoids and others functional compounds at lower temperatures, with low energy and solvent consumption, and is considered as one the most efficient methods for extraction of fruit bioactive compounds [14].Therefore, in our research the UAE-assisted extracts of goji berries were studied for their antimicrobial activity, based on the composition of bioactive compounds (polysaccharides, phenols) achieved by each extraction.
In many cases, the bioactive plant components, after initial isolation, are encapsulated and freeze dried into powder form, in order to optimally preserve their bioactivity and facilitate their commercial applications [15].Encapsulation is a technique by which active solid, liquid, or gas compounds are introduced into a matrix or polymeric wall system (e.g. maltodextrin) to protect them from environmental conditions (light, oxygen, temperature, and water), avoiding oxidation, increasing shelf life, preserving interactions with other food components, or controlling their release for a specific place and/or time [16]. However, the ratio of the encapsulation agent is crucial for the level of bioactivity, since the use of high concentration of encapsulation agent will inevitable reduce the content of the bioactive compounds, which is a matter of concern for the present work. Another important factor concerning bioactivity is the method of drying the fruit extracts into powder form. Freeze–drying or lyophilisation, which has been applied in the present experiment, is the most preferable processes for protecting thermosensitive and unstable molecules such as fruit polyphenols, enhancing their antioxidant activity and masking undesirable color or flavor effects [17, 18].
Pomegranate P. granatum is another fruit with significant bioactive properties, which is gaining increasing attention in the last years. During the processing of fresh P. granatum for the production of fruit juice, large amounts of peel and seed are discarded as waste, representing about 50% of the total weight [19]. Therefore, pomegranate peel could be used in applications that are beneficial to food industries, since it contains greater amounts of bioactive compounds and with stronger biological activities, compared to pomegranate juice [20,21,22,23,24,25]. Among commonly consumed fruits, pomegranate peels have the highest concentration of punicalagin, a water soluble ellagitannin (polyphenol), which contains a gallic acid component linked to a glucose molecule and comprises 11–20 g/kg of the pomegranate peel powder ellagitannin [26]. Studies have shown that punicalagin hydrolyses into smaller polyphenolic compounds in the small intestine under normal physiological conditions and has antioxidant, antifungal, and antibacterial properties [27].
Polyphenols, carotenoids, flavonoids and hydrolysable tannins extracted from fruits, vegetables, herbs, and spices have been considered as potential agents for treating or preventing a wide range of infections [28, 29]. The antimicrobial mechanisms of phenolic compounds involve the reaction of phenolics with microbial cell membrane proteins and/or protein sulfhydryl groups that cause bacterial death through membrane protein precipitation and enzyme inhibition, such as glycosyl transferases, which lead to cell lysis [30,31,32]. Pomegranate peel extracts have been widely used as natural antimicrobial agents against S. aureus, Escherichia coli, Listeria monocytogenes, and Yersinia enterocolitica [32, 33] and on the Indian subcontinent food-borne diseases and urinary tract infections are conventionally treated with pomegranate peel extract [34, 35]. Due to their strong antimicrobial properties and the ease of preparing pomegranate peel extracts, pomegranate peels were used for reference as a natural antimicrobial in the present study, in order to compare the antimicrobial activities of goji berry extracts, after different extraction and encapsulation protocols applied for the goji berry fruits.
Purpose of the research work
This research was designed to evaluate the comparative antimicrobial effects of goji (L. barbarum) berry extract and pomegranate (P. granatum) peel on some species of bacteria fungi and yeast that are either food or plant pathogens or involved in food spoilage. The in vitro assessment of different concentrations and forms of the extracts (i.e. water extracts and alcohol or alcohol–hexane extracts) as potential natural antimicrobial agents for food protection was at the core of this research.
Materials and methods
Plant materials
Goji berries were collected from a 3 year old experimental plantation of L. barbarum located in the region of Thessaly, Greece and were dried in a desiccator at 45 °C for 5 days. The samples were stored under refrigeration (0–4 °C) for further analysis. The pomegranate peel was collected as a by-product from the process of producing pomegranate juice from fruit collected from a plantation also located in Thessaly region. Further treatments of pomegranate peels and goji berry fruits are described below.
Extraction procedure
Extracts were taken from the dry goji berries through the use of a UAE device (Hielscher UP400S) that included a water bath for temperature regulation, based on the following extraction conditions, which were optimized in a previous study, in order to obtain optimal antioxidant activity [5]. The extraction was performedat a temperature of 55 °C with an extraction power of 220 W/cm2 for 25 min, at a ratio of dry goji berry to extraction solution equal to 1/20. After extraction, the extract was filtered and then used for lyophilisation. Distilled water, ethanol, and/or hexane were used as extraction solvents. The type of solvent used for each goji berry extract is described in Table 1.
Encapsulation, lyophilisation, and milling of the goji berry extract and pomegranate peel
It is well-known that freeze-dried carbohydrates and proteins may exist in an amorphous state with time-dependent physical properties that affect their storage stability. An amorphous material undergoes a change from a “glassy” state to a viscous rubbery state at the glass transition temperature (Tg), which may result in structural changes such as stickiness and collapse, while increased moisture content strongly plasticises the amorphous structure and reduces the Tg [36].
After the UAE process, the goji berry extract was mixed (encapsulated) in liquid form in different volumes (14, 7, and 2% w/v) with maltodextrin DE 18 (Amylon, S.A.) before lyophilisation in order to avoid the glass transition of the lyophilisation powders. The amount of maltodextrin used for encapsulation of each extract is described in Table 1. In the sample with the lowest percentage of maltodextrin (2% w/v), 1% w/v of silicon dioxide (SiO2) nanoparticles was also added to increase Tg and allow encapsulation, by optimising the thermal properties of the polymer system. For example, it has been shown that silica additives in an epoxide resin matrix considerably increase (by 50–70 °C) the Tg of composites [37]. Two samples (No1, No3) were extracted with a solvent comprised of a mixture of 70% v/v ethanol and 30% v/v distilled water, three samples (No2, No4, Νο6) were extracted only with distilled water, and one sample (No5) was extracted with a mixture of 33% v/v of hexane, 33% v/v distilled water, and 33% v/v ethanol (Table 1). These extracts were then lyophilised in order to produce the encapsulated lyophilised powder form that was used for the evaluation of antimicrobial activity.
The lyophilisation process was performed in a Zirbus GmbH Sublimator 4 × 5 × 6 freeze dryer and involved the three steps described below in a 24 h cycle process.
-
Step 1. The product was cooled to a final temperature of – 30 °C. Duration approximately 2 h.
-
Step 2. The product was heated to a shelf temperature of 0 °C under a vacuum of 0.15 mbar. Duration approximately 12 h.
-
Step 3. The product was heated to a shelf temperature of 40 °C under a vacuum of 0.15 mbar. Approximately 10 h.
For the pomegranate peel, the peel was lyophilised according to the aforementioned conditions and mechanically milled into a powdered form using a commercial mill in order to prepare sample No7 (Table 1). All samples were sealed in opaque plastic flasks and stored at a constant temperature oven at 30 ± 0.3 °C for up to 15 days to avoid Tg degradation.
Antimicrobial extracts/samples composition and coding
Six different goji berry extracts were used (No1–No6), as well as a freeze–dried pomegranate peel sample (No7), which was used as a reference antimicrobial for comparison (Table 1).
Determination of total phenols content (TPC) of the extracts
The method described by Waterhouse [38] was used for the determination of TPC. Briefly, 20 µL of each extract was mixed with 1.58 mL water, and then with 100 µL of Folin–Ciocalteu reagent (0.2 N). Subsequently, 300 µL of Na2CO3 solution (200 g/L) was added and after 2 h of incubation in the darkthe absorbance was measured at 765 nm. TPC was calculated on the basis of the calibration curve of gallic acid and expressed as gallic acid equivalents (GAE) in mg/mL of extract. The linearity range of standard gallic acid was determined as 50–500 mg/L (R2 = 0.999). The equation of the standard curve was obtained by linear regression: y = 0.0021x - 0.0161 (data not shown), where y is absorbance at 765 nm, x is concentration of gallic acid (mg/L) and further expressed per g of dry extract. The assay was replicated three times.
Determination of total antioxidant capacity of the extracts
DPPH (2,2-diphenyl-1-picrylhydrazyl) radicalscavenging activity
The scavenging activity on DPPH radicalsby different extracts was determined following the method of Brand-Williams et al. [39]. Test solutions of different concentrations were prepared from a stock solution of each extract. DPPH (100 μM) was dissolved in ethanol and mixed with an aliquot of 50 μL of each dilution with a total volume of 1 mL (control 950 μL ethanol + 50 μL DPPH solution). The mixture was shaken vigorously and left to stand for 20 min in the dark at room temperature. After the reaction samples were centrifuged at 10,000 rpm for 10 min, absorbance was measured at 517 nm to determine the concentration of the remaining DPPH with ethanol as blank. The radical-scavenging activity was calculated as % inhibition with the following formula:
where AS is the absorbance of the solution when the sample extract has been added at a particular level and ADPPH is the absorbance of the DPPH control solution. Effective concentration calculated at 50% (EC50) values denotes the effective concentration of a sample required to decrease the absorbance at 517 nm by 50%. All measurements were performed in triplicate.
ABTS (2,2΄-Azino-bis-(3-ethyl-benzthiazoline-sulphonic acid)) radical scavenging activity
ABTS+ radical scavenging activity was measured as described by Kerasioti et al. [40]. The reaction was carried out at 1 mL final volume, containing 400 μL H2O, 500 μL ABTS (1 mM), 50 μL H2O2 (30 μM), and 50 μL of the enzyme horseradish peroxidase (HRP 6 μM). Immediately after the addition of the enzyme, the contents were mixed and incubated at room temperature in the dark for 45 min. After incubation, the tested extracts were added in different concentrations, the contents were mixed, and the absorbance was measured at 730 nm. In each experiment, the samples without HRP were used as blanks, and the samples without goji berry extract were used as controls. All experiments were carried out in triplicate and on at least two separate occasions. The ABTS+ radical scavenging activity was calculated according to the following equation:
where Abscontrol and Abssample are the absorbance values of the control and the tested sample, respectively.
Infrared spectroscopy
FTIR spectroscopy has been used successfully for qualitative and quantitative analyses of biomolecules that determine the biological activity of natural plant specimens and extracts or phytochemicals [41, 42]. Chemical bonds between light atoms (such as C–H, O–H, and N–H) generally have molecular vibrations, which can be monitored in the mid-infrared (MIR) region (400–4000 cm–1), providing reliable and reproducible measurements of the chemical composition of a biological sample [42].
In the present study, goji berry extracts and pomegranate peel FTIR measurements were performed in the mid-infrared region (4000–650 cm−1) using a Nicolet 6700 (Thermo Scientific Co.) in order to estimate the chemical profile of each sample. The FTIR spectrophotometer, configured with the attenuated total reflectance (ATR) sampling technique, deuterated triglycine sulfate (DTGS) detector, and KBr beam splitter, was controlled by OMNIC 9 software. The samples to be analysed were placed on a diamond crystal sampling plate (accessory Smart iTX) and clamped with a pointed tip. A background scan was obtained before each sample scan with an empty sample plate. In addition, the ATR crystals and pointed tip were cleaned to remove any interference from the preceding sample. A total of 32 successive scans from each sample were collected at 4 cm−1 intervals, and the averaged spectra of each sample were used for analysis. All spectra were processed following a three-step procedure (ATR correction, baseline correction, and normalization).
Microorganisms used for the determination of antimicrobial activity
Pathogenic and spoilage microorganisms used in this study were obtained from different culture collections. These included the pathogenic bacteria E. coli ATCC 8739, S. typhimurium ATCC 14028, S. aureus ATCC 6538, L. monocytogenes ATCC 19115, C. perfringens ATCC 13124, and C. jejuni ATCC 33291. The yeasts used in this study were Y. lipolytica ATCC 9773, M. fructicola CBS 8853, and R. mucilaginosa DSM 70403. Finally, the aflatoxin-producing fungus A. flavus strain 1959 was obtained from the DSM culture collection, while the rest of the fungi used in this study (P. expansum, A. niger, F. oxysporum and R. solani) were kindly provided by the Greek Benaki Phytopathological Institute culture collection. All bacterial stockcultures were grown at 37 °C for 48 h in Tryptone Soy Broth (TSB) (Neogen, USA), except for C. jejuni, which was grown in Bolton Broth, while all fungal stockcultures were grown at 25 °C (for 72 h for yeasts and 120 h for molds) in Potato Dextrose Broth (Neogen, USA), in order to be used as inoculums in the assays of antimicrobial activity.
Antimicrobial assays
MIC/MBC assay
The above mentioned pathogenic bacteria were inoculated in test tubes with 9,9 mL liquid growth medium (TSB) supplemented with the antimicrobial samples/extracts in order to determine the Minimum Inhibitory Concentration (MIC) of each sample [43]. More specifically, the test tubes with growth medium contained 0 mg/mL (control), 10, 50, 100 and 200 mg/mL of the above powder samples (No1 through No7), except that for No7 the 200 mg/mL concentration was partially insoluble and was not applied. These concentrations were chosen based on results of similar studies examining antimicrobial activity of natural antimicrobials, where a minimum of 10 mg/mL was necessary for expressing antimicrobial activity, even in non-encapsulated samples [44, 45]. One mL of paraffin was also added to the test tubes for the incubation of C. perfringens and C. jejuni in order to facilitate anaerobic and microaerophilic incubation, respectively. The test tubes were then sterilised and subsequently inoculated with 0.1 mL of 48 h grown cultures of each of the six pathogenic bacteria, resulting in a 1/100 dilution of the cell suspension, and incubated at 37 °C for 48 h in order to determine MIC, which was defined as the lowest antimicrobial sample concentration that resulted in the absence of growth (absence of cell debris, turbidity or biofilm) of the inoculated microorganism after incubation [44].
MBC was determined after inoculation of 1 mL from the MIC tube (i.e. the test tube with lowest inhibitory concentration), as well as fromall tubes of higher concentration of antimicrobials, into test tubes containing 9 mL of the same growth medium but without any antimicrobial agents. The test tubes were again incubated at 37 °C for 48 h and observed for the presence of microbial growth. MBC was the lowest concentration where no growth was observed.
Turbidity assay
The turbidity assay was performed to assesses the bacterial cell density via the measurement of cell suspension turbidity, before and after addition of the antimicrobial goji berry extracts [46]. The inoculated test tubes of the MIC assay (containing 0, 10, 50, and 100 mg/mL of extract, as described above) were used for the turbidity measurements after the end of their incubation period. One millilitre of each growth medium that exhibited microbial growth was diluted in 9 mL of distilled water and then inserted into the turbidometer. Turbidity was measured in Nephelometric Turbidity Units (NTU), using a Eutech turbidometer (TN 100, Eutech Instruments Pte Ltd) with a resolution of 0.01 NTU.
Well diffusion assay
For the determination of inhibition zones after microbial growth in petri dishes, the well diffusion assay was used, following the method described by Weinstein [43]. The seven antimicrobial samples were dissolved in phosphate buffered saline solution (PBS) with pH 7.0–7.2 at concentrations of 10, 50, 100 and 200 mg/mL, and sterilized. For sample No7, the 200 mg/mL concentration was omitted due to poor dilution, as explained above. 100 μL of a liquid culture containing ~ 106 cfu/mL of the above pathogenic bacteria was inoculated (spread) on NA with the exception of C. jejuni, which was grown in CSA. In order to have a relatively uniform cell density of ~ 106 cfu/mL in each cell suspension, the population of each microbial stock culture was measured after serial dilutions and inoculation in solid media, and where necessary, appropriate dilutions were made with Maximum Recovery Diluent (Neogen) in order to yield a ~ 106 cfu/mL final population. Escherichia coli stock culture was enumerated after inoculation in TBX agar (Neogen), S. Typhimurium was spread on XLD agar (Neogen), S. Aureus was spread on Baird Parker agar with egg yolk tellurite (Neogen), L. monocytogeneswas spread on ALOA agar (Oxoid), C. Perfringens was inoculated in TSC agar (Neogen), and C. jejuniwas spread on Campylobacter selective agar (Neogen). Similarly, an equal amount of each of the fungal cultures (Penicillium expansum, Aspergillus niger, Aspergillus flavus, Fusarium oxysporum, Rhizoctonia solani, Yarrowia lipolytica, Metschnikowia fructicola, and Rhodotorula mucilaginosa) was spread on PDA plates. In order to have a relatively uniform fungal population added in each plate, all fungal stock cultures in Potato Dextrose Broth were diluted and enumerated after inoculation in PDA, and, where necessary, dilutions where made in order to obtain a cell density of ~ 106 cfu/mL for each fungal culture that was tested in his assay.
Four cylindrical wells of 7 mm diameter were formed in each plate using a reversed sterile Pasteur pipette, and 25 μL of antimicrobial sample solution was poured into each well. The PDA plates were incubated at 25 °C for 5 days, while NA were incubated at 37 °C for 2 days and CSA plates were incubated at 37 °C for 3 days. Clostridium perfringens was incubated anaerobically using Anaerocult A sachets (Merck, USA), and Campylobacter jejuni was incubated with addition of Anerocult C sackets (Merck, USA) in anaerobic jars. All other microorganisms were incubated aerobically. Antimicrobial activity was evaluated by measuring the diameters (mm) of the inhibition zones-the clear (transparent) zone beyond each well where no growth was observed [47,48,49]. Measurements of the inhibition zones of the well-diffusion assay were carried out in triplicate from which the mean values were estimated.
Statistical analysis
Standard deviation (SD) was calculated and the averaged values along with the SDs are documented in their respective tables or figures. Statistical differences among the means, as well as interactions between the variables, used in chemical analyses were detected through one-way ANOVA followed by a Tukey’s test, and statistical significance was set at the P ≤ 0.05 level. MiniTab®17.1.0 software was used as a tool to carry out the above mentioned tests.
Results
Total polyphenol content
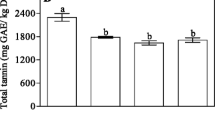
The TPC of all samples is presented in Table 2. Sample No7 has the highest concentration of total polyphenols, with 79.458 ± 4.61 (mg/g of encapsulated extract dry weight gallic acid equivalent), followed by sample No6, with 25.307 ± 1.72 mg/g polyphenol content, which is a sample extracted only with water and encapsulated with the minimum amount of maltodextrin. Lower percentages of maltodextrin in the encapsulation process apparently lead to higher percentages of dry goji berry extract and higher TPC in both ethanol-extracted and water-extracted samples.
It was generally observed that the use of distilled water as extraction solvent lead to higher polyphenolic content in the extract, while the use of ethanol and water, or the mixture of ethanol with hexane and water led to lower polyphenolic concentration in the extract, which is probably related to the water solubility of the phenolic substances in goji berry samples and the specific extraction methodology [2]. Interestingly, the substitution of part of ethanol by hexane (sample 5) diminished the extraction efficiency with regard to polyphenol content, in comparison to the use of ethanol and water and the same content of maltodextrin in sample 3 (Table 1).
Overall, the value of total polyphenol content obtained in the extracts of the present study is in agreement with previously reported values for goji berry [9, 50] and pomegranate peel [4, 51].
In vitro antioxidant activity
In this study, the six samples of goji berries were examined at concentrations of 155, 313, 625, 1250, 2500, and 5000 μg/mL, while sample No7 was examined at concentrations of 9.68, 19.37, 38.75, 77.5, 155, and 313 μg/mL due to its strong scavenging capacity of DPPH· and ABTS·+ radicals, which corresponded to an IC50 value of 25 and 4.2 (μg/mL) respectively (Table 2). Aqueous UAE samples of L. barbarum fruit appeared to have a higher antioxidant activity than those with a mixture of ethanol and water (and the same percentage of maltodextrin), and this is not in full agreement with the literature where in several cases ethanol, methanol or other organic solvents are preferable to water extraction, in terms of total polyphenol concentration and antioxidant activity of the extracts [50, 52]. This discrepancy probably lies in the extraction parameters and the use of an aqueous optimised UAE method in our study [5]. Indeed, although Pedro et al. proposed a 70% ethanol extract of goji berry as the one with highest polyphenol content and antioxidant capacity and quite higher than those of simple water extracts, their extraction process was carried out by simply stirring the sample with the solvent at room temperature [52]. In contrast, the results of Yang et al. [53] show that ultrasound assisted extraction, especially with hot subcritical water extraction at 110 °C, was the optimal extraction method for obtaining the highest polysaccharide yield and in vitro antioxidant and immune-stimulating activity, compared to simple hot water extraction or use of sonicated water at 30–40 °C. The fact that our extraction process parameters have been previously optimized for use in ultrasound assisted water extraction, may explain why we observed higher yields of total polyphenol and increased antioxidant activity in the UAE water extracts, instead of the ethanol or ethanol-hexane extracts (which may be more effective than distilled water in the absence of ultrasonication).
In our study, sample No5 exhibited the lowest radical scavenging activity, reaching the highest IC50 value, while sample No6 was the goji berry extract with the highest radical scavenging capacity using the DPPH method (Table 2). TPC showed a strong negative correlation with IC50 in the scavenging of the DPPH· radical (R2 = – 0.971 TCP/DPPH· IC50). Ιn other words, radical scavenging capacity of samples increased in parallel with TPC, which is in line with the results of previous reports [53, 54]. The Pearson correlation between the results of the scavenging of the two free radicals (DPPH·, ABTS·+) performed with Minitab 17.1software showed a value of 0.930 with a P = 0.002 and identified a strong correlation between the two methods (results not shown).
In vitro evaluation of the antimicrobial effects against bacteria, yeast, and fungi
The results of the MIC/MBC test of all samples are summarized in Table 3, while Fig. 1 shows (in diagrams 1a to 1f) the reduction in turbidity (a measure of cell density) of each tested bacteria, caused by the different antimicrobial extracts. The inhibition zones for each sample and tested microorganism (according to the well diffusion assay) are presented in Table 4. The results obtained in the present study revealed that the reference sample No7 was found to be the most active sample against bacterial, yeast, and fungal strains, while among all goji berry extracts sample No6 appears to be—in most but not all cases—the preferable antimicrobial preparation. According to the in vitro results based on MIC assay sample No7 was inhibitory against all tested microorganisms at only 10 mg/mL concentration and bacteriocidal to C. jejuni at the same concentration (Τable 3). Among goji berry extracts sample No6 (the one with the highest polyphenol content and antioxidant activity among goji berry extracts) had the lowest MIC of 50 mg/mL in all tested bacteria, except for E. coli (where MIC was equal to 100 mg/mL) (Τable 3).The comparison of sample No2 and No4 (the latter having higher percentage of maltodextrin) also revealed that in most cases the MBC for sample No2 is lower, in other words the lower the maltodextrin content, the better the bacteriocidal activity (Table 1). Similar comparison can be made between sample No1 and No3 which were the ethanol extracted goji berry samples with 7% and 14% maltodextrin, respectively. In that case, both MIC and MBC are lower for the sample with the least amount of encapsulation agent and the highest content of antioxidant polyphenols (No1).
Turbidity of the growth medium of E.coli (a), S. aureus (b), S. typhimurium (c), L. monocytogenes (d), C. perfrigens (e) and C. jejuni (f) measured by Nephelometric Turbidity Units (NTU), as an effect of the addition of six different goji berry extracts at concentrations of 10, 50, and 100 mg/mL, compared to a control (0 mg/mL) without antimicrobial extracts
Also, with regard to the type of solvent used, the choice of water versus ethanol or ethanol–hexane extraction does not seem to have clear and crucial effect on the minimum inhibitory concentration of extracts. Among samples No3, No4 and No5 the MIC of sample No3 (ethanol-extracted) is lower than the MIC of sample No4 (water-extracted) and No5 (ethanol–hexane-extracted) for L. monocytogenes, while No4 and No5 have the lowest MIC for E. coli, and other MIC values for S. aureus, S. typhimurium, C. perfringens are equal for these three samples.
According to the turbidity assay of goji berry extracts (Fig. 1a–f), sample No6 was the most effective at a concentration of only 50 mg/mL, leading to a zero increase of Nephelometric Turbidity Units (NTU) for S. aureus, L. monocytogenes, C. perfringens, E.coliand S. typhumurium,while for C. jejuni, a concentration of 100 mg/mL was required. Sample No3 (with ethanol–water solvent and the highest percentage of maltodextrin) had the lowest antimicrobial activity against E. coli, S. typhimurium and C. jejuni and sample No4 (with water solvent only and the highest percentage of maltodextrin) was also least effective against E. coli, S. aureus, C. perfringens, L. monocytogenes and C. jejuni, since a 200 mg/mL concentration was required for complete absence of turbidity (lack of growth). The comparison of samples No1 and No3 (ethanol extracts with low and high maltodextrin content, respectively) shows that when less maltodextrin was used for encapsulation, a lesser amount of extract was needed for complete absence of turbidity. A similar observation in made for the antimicrobial effect of samples No4 and No2 (water extracts with high and low maltodextrin content, respectively) where the extract with most maltodextrin (No4) had the weakest effect on reduction of turbidity and required 100 mg/mL concentration for zeroing the turbidity of E. coli, S. aureus and C. perfringens (Fig. 1).
With regard to the role of each solvent on turbidity measurements, the results were rather inconclusive. Sample No1 was slightly more effective in reducing turbidity of E. coli, S. typhimurium, S. aureus, C. perfringens but the opposite occurred for L. monocytogenes and C. jejuni, and similar observations were made when comparing samples 3, 4 and 5(Fig. 1).
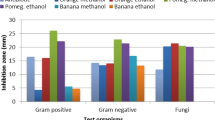
As concerns the measurements of inhibitions zones on agar plates (well diffusion assay) presented in Table 4, pomegranate was by far the most effective antimicrobial solution, exhibiting inhibition zones at 10 mg/mL (for S. typhimurium, C. jejuni) and 50 mg/mL (for E. coli, S. aureus, L. monocytogenes, C. perfringens). Among goji berry extract, sample No6 had the best inhibitory effect against S. aureus and L. monocytogenes (at only 50 mg/mL), as well as C. perfringens (at 100 mg/mL). Regarding the effect of the three different solvents, No5 (ethanol–hexane extract) had the best inhibition against E. coli (19,30 mm) and S. aureus (15,51 mm) and L. monocytogenes (16,89 mm), while No4 (water extract) had the best inhibition against S. typhimurium(18,5 mm) at 200 mg/mL concentration. Sample No3 (ethanol extract) had the lowest inhibition zones compared to No 4 and 5 (Table 4). Also, sample No2 (water extract) had clearly higher inhibition compared to No1 (ethanol extract) when used at 200 mg/mL against E. coli (19,4 mm vs. 11,59 mm), S. aureus (14.06 mm vs. 5,86 mm), S. typhimurium (9,01 mm vs. 2,69 mm) and S. perfringens (1,17 mm vs. 0 mm). Based on these results, a tentative trend of higher bacterial inhibition in water versus ethanol extracts can be proposed (Table 4). Overall, the most sensitive microorganisms towards goji berry extracts (based on size of inhibition zone at a low concentration) appeared to be E. coli, S. aureus, which are in agreement with the reports of Fit et al. [55] and Asanica et al. [56] respectively, as well as L. monocytogenes, as shown in this study (Table 4). Interestingly, C. jejuni also appears with very short or no inhibition zones in this well diffusion assay (Table 4), although it was relatively sensitive to goji berry extracts, based on the previous two methods of MIC–MBC and cell suspension turbidity. This may be attributed to the fact that for the well diffusion assay a very selective and growth-enhancing medium (Campylobacter Selective agar) was used, instead of the least selective and growth-stimulating Nutrient agar that was used for all other bacteria.
All fungi studied in this experiment did not exhibit any inhibition zones caused by goji berry extracts, with the sole exception of F. oxysporum, which had an inhibition of 4,00 mm only at 200 mg/mL of sample No6. Pomegranate solution, on the other hand, caused significant inhibition to all studied fungi even at 10 mg/mL, which was comparable to the inhibition against the studied bacteria. At this relatively low concentration, sample No7 was the only sample to inhibit S.typhimurium (7.30 mm), L. monocytogenes (5 mm), C. jejuni (9.39 mm), R. mucilaginosa (9.32 mm), Y. lipolytica (2.5 mm) and F. oxysporum (3 mm).
FTIR spectroscopy
The importance of IR spectroscopy as a fingerprint technique for the qualitative analysis arises from the fact that there are no two bioactive compounds having the same FTIR spectra.The functional groups and the structure characterization of all peaks are presented in Table 5. Typical FTIR absorbance spectra of the L. barbarum encapsulated samples and pomegranate peel (No7) are presented in Fig. 2.
This study was focused on the absorbance differences in the fingerprint region as shown in Fig. 3c and more especially in the carbonyl and carboxyl frequency regions as shown in Fig. 3d, where it was found that the intensity of the peaks correlated (Pearson R2= – 96.4) with the antioxidant and antimicrobial activity of the extracts in Fig. 4. In the carbonyl and carboxyl region, sample No7 had the most intense peaks compared with the other samples, centered at ~ 1723.59, 1604.51, and 1516.28 cm−1, with intensities of 0.49, 0.37, and 0.09, respectively. Sample No6 also had three peaks in this area, with peaks at ~ 1729.86, 1660.44, and 1521.10 cm−1 with lower intensities of 0.17, 0.19, and 0.07, respectively, as shown in Table 5. The difference in TPC between the two samples may be explained by the intensity differences of the peaks around 1720 cm−1 which are attributed to the stretching vibrations of the carboxyls groups (polyphenolic acids and mainly gallagic acid in pomegranates peels) [57].
Also, another difference is in the infrared absorption between the peaks at ~ 1604.51 (sample No7) and 1660.44 cm−1 (sample No6) that are attributed to the presence of the ellagitannin/punicalagin in pomegranate peel [58] and flavonoids, mainly quercetin in goji berries [59].
Discussion
According to Wu et al. [60], many compounds extracted from plant materials have shown promise for inhibiting bacterial pathogens and spoilage fungi when applied to certain foods. However, many different extraction and drying methods exist for their isolation, which makes it hard to compare between experiments and isolate extracts with maximum antimicrobial activity. The latter is also variable and dependent on the tested microorganisms, so a variety of target microorganisms and methods should be used in order to draw safe conclusions. This study is the one of the first reports comparing the antimicrobial activity of UAE extracts of goji berry fruits in relation to type of solvent, amount of maltodextrin used for encapsulation into soluble powder, and polyphenol content and antioxidant properties, by applying different methods for measuring both antioxidant and antimicrobial capacity.
In this experiment it was observed that aqueous UAE samples from L. barbarum fruit exhibited higher antioxidant activity than those extracted with a mixture of ethanol (or ethanol–hexane) and water. These results differ from those of previous research performed by Benchennouf et al. [50], where a Soxhlet extraction procedure was followed under boiling temperature and drying was accomplished by rotary evaporator, instead of freeze–drying. Τhis discrepancy is probably due to the use of ultrasound technology in our study during the extraction and the use of optimal extraction parameters for aqueous extraction in our study (ratio of water to dry goji berry, extraction temperature, ultrasound power, and extraction time), which was based on a previously optimized extraction model [5]. This indicates that optimization of extraction parameters is crucial before comparing the antimicrobial activity of extracts and choosing the most preferable solvent.
Solution of pomegranate peel showed the highest antimicrobial activity on all tested microorganisms and were used as a reference natural antimicrobial (Tables 3 and 4). From Table 2 it can be deduced that the pomegranate peel had an up to 50-fold higher antioxidant activity than the goji berry samples, based on the scavenging of DPPH· and ABTS·+ radicals. These results are in line with the studies of Barathikannan et al. [64]. and John et al. [19] who evaluate the pomegranate peels and also showed that the antimicrobial effect of the samples depended on the polyphenolic content, the antioxidant activity, and the type of microorganism tested.
The closest to this strongly antimicrobial sample was sample No6 of goji berry extracts. This showed the highest antioxidant activity and TPC (Table 2), which was accompanied with the best antimicrobial activity of all goji berry extracts, with minor exception for some microorganisms (Tables 3 and 4, Fig. 1). This correlation between TPC—antioxidant activity and antimicrobial activity is also in agreement with the studies of Mocan et al. [61], who assessed the antimicrobial activity of goji berry leaf extracts and found a positive correlation of TPC and antioxidant activity with the antimicrobial activity of extracts of L. chinense and L. barbarum. Similar results were also reported for L. shawii and other herbal extracts [62, 63].
Generally, our results illustrate that the amount of encapsulation agent (e.g. maltodextrin) used for encapsulating goji berry (or other plant) extracts is very crucial to the final antimicrobial activity. If very little maltodextrin is used, then encapsulation may not be successful or complete, but a high percentage of maltodextrin reduces the concentration of bioactive molecules. Therefore, we observed that in most cases antimicrobial activity was higher when maltodextrin content was reduced from 14 to 7% (Tables 3 and 4, Fig. 1) and it was optimal in sample No6 which was encapsulated with a minimum 2% maltodextrin, thanks to the anti-cacking properties of SiO2. Also, in many cases it appeared that water extracts with higher TPC also had higher antimicrobial activity (Table 4), although this trend was not very clear for all samples and all microorganisms and the turbidity measurement were variable in this regard (Fig. 1).
The strong peak at ~ 1100 cm−1, which appears in the FTIR spectrum presented in Fig. 4 is attributed to the presence of glycoconjugated polysaccharides, while a similar peak is presented by Legaz et al. [65] in the FTIR analysis of an 870 kD glycoprotein fraction isolated from sugarcane juice attributed to their ether bond between fructose and galactitol units. The broad peak at ~ 1522 cm−1 is attributed to amide II (N–H) band, while the amide I (carbonyl) band emerges at ~ 1654 cm−1 [66].
The intensity of the band at ~ 1720 cm−1, which characterizes the carboxyl group, shows a strong positive correlation with the antioxidant activity and this results is in agreement with the already known literature data where the organic acids (citric, malic, oxalic and quinic) and polyphenolic acids (caffeic, chlorogenic, coumaric and ferulic acids) are the most important bioactive compounds of the fruits in relation to antioxidant activity [57].
Furthermore, the IR spectra revealed a peak around 3315 cm−1 for the hydroxyl group, and two bands at 2850 and 2924 cm−1, which are assigned to the C–H stretching vibrations [67]. Sample No5 with the use of hexane as solvent had the lowest TPC and radical scavenging capacity, achieving the highest IC50 value. These results are in accordance-with the results of a previous study of the antioxidant and antimicrobial activities of Lycium shawii fruits extracts and the reported low activities with the same solvent [62].
Conclusion
Many reports are available on the antimicrobial and antioxidant properties of plant extracts. Some of these studies have helped in identifying the active components responsible for these activities, especially with regard to the development of drugs and nutraceuticals.
However, very limited research is available on the antifungal and antibacterial properties of goji berries extracts and especially water soluble extracts which are cheaper than alcohol extracts, consumer and environmentally friendly and could facilitate the commercial development of unique antimicrobial formulations for use in the food industry.
The results of this study clearly indicate that UAE aqueous extracts of L. barbarum fruit encapsulated with minimal amounts of maltodextrin exhibited an important antioxidant, antibacterial and antifungal activity, which may be linked to enhanced concentration of polyphenol molecules, as evidenced by FTIR spectra of the antimicrobial samples.
Further, this study showed that freeze-dried pomegranate peel, which is a by-product of the pomegranate juice industry, despite its low solubility at high concentrations, is a very efficient antioxidant and antimicrobial substance with very strong and broad antimicrobial activity (much higher compared to UAE goji berry extracts), and could be utilized in the production of added-value natural antimicrobials for food or even plant biological protection.
References
S. Leontopoulos, K. Petrotos, V. Anatolioti, P. Skenderidis, S. Tsilfoglou, I. Vagelas, Int. J. Food Biosyst. Eng. 6, 23 (2017)
P. Skenderidis, E. Kerasioti, E. Karkanta, D. Stagos, D. Kouretas, K. Petrotos, C. Hadjichristodoulou, A. Tsakalof, Toxicol. Rep. 5, 251–257 (2018)
M. Protti, I. Gualandi, R. Mandrioli, S. Zappoli, D. Tonelli, L. Mercolini, J. Pharm. Biomed. Anal. 143, 252 (2017)
C.N. Aguilar, A. Aguilera-Carbo, A. Robledo, J. Ventura, R. Belmares, D. Martinez, R. Rodríguez-Herrera, J. Contreras, Food Technol. Biotechnol. 46, 218 (2008)
P. Skenderidis, K. Petrotos, I. Giavasis, C. Hadjichristodoulou, A. Tsakalof, J. Food Process Eng 40(5), e12522 (2016)
H. Amagase, B. Sun, C. Borek, Nutr. Res. 29, 19 (2009)
K. Le, F. Chiu, K. Ng, Food Chem. 105, 353 (2007)
O. Potterat, Planta Med. 76, 7 (2010)
D. Qian, Y. Zhao, G. Yang, L. Huang, Molecules 22, 911 (2017)
A. Mocan, L. Vlase, D.C. Vodnar, A.M. Gheldiu, R. Oprean, G. Crisan, Molecules 20(8), 15060–15071 (2015). https://doi.org/10.3390/molecules200815060
B. Kulczyński, A. Gramza-Michałowska, Pol. J. Food Nutr. Sci. 66, 67 (2016)
H. Amagase, N.R. Farnsworth, Food Res. Int. 44, 1702 (2011)
A.P. Carvalho, M. Mendes, M.M. Moreira, D. Cruz, J.M. Magalhães, M.F. Barroso, M.J. Ramalhosa, A. Duarte, L. Guido, A.M. Gomes, C.D. Matos, Int. J. Food Sci. Technol. 51, 1401 (2016)
K. Vilkhu, R. Mawson, L. Simons, D. Bates, Innov. Food Sci. Emerg. Technol. 9, 161 (2008)
S. Leontopoulos, P. Skenderidis, H. Kalorizou, K. Petrotos, J. Food Biosys. Eng. 7, 1 (2017)
P. Robert, C. Fredes, Molecules (Basel, Switz.) 20, 5875 (2015)
A. Munin, F. Edwards-Lévy, Pharmaceutics 3, 793 (2011)
K.B. Petrotos, F.K. Karkanta, P.E. Gkoutsidis, I. Giavasis, N. Papatheodorou, A. C. Ntontos 6, 170 (2012)
K.M.M. John, A.A. Bhagwat, D.L. Luthria, Food Chem. 235, 145 (2017)
A. Malik, F. Afaq, S. Sarfaraz, V.M. Adhami, D.N. Syed, H. Mukhtar, Proc. Natl. Acad. Sci. U.S.A. 102, 14813 (2005)
Y. Li, C. Guo, J. Yang, J. Wei, J. Xu, S. Cheng, Food Chem. 96, 254 (2006)
M. Hajimahmoodi, M.R. Oveisi, N. Sadeghi, B. Jannat, M. Hadjibabaie, E. Farahani, M.R. Akrami, R. Namdar, Pak. J. Biol. Sci. 11, 1600 (2008)
S. Gozlekci, O. Saracoglu, E. Onursal, M. Ozgen, Pharmacogn. Mag. 7, 161 (2011)
A. Sood, M. Gupta, Food Biosci. 12, 100 (2015)
Z. Kalaycıoğlu, F.B. Erim, Food Chem. 221, 496 (2017)
M. Çam, Y. Hışıl, Food Chem. 123, 878 (2010)
U.A. Fischer, R. Carle, D.R. Kammerer, Food Chem. 127, 807 (2011)
T. Ismail, P. Sestili, S. Akhtar, J. Ethnopharmacol. 143, 397 (2012)
S.V. Leontopoulos, P. Skenderidis, V. Anatolioti, M.I. Kokkora, S. Tsilfoglou, K.B. Petrotos, I. Vagelas, J. Food Biosyst. Eng. 6(1), 38 (2017)
E. Haslam, J. Nat. Prod. 59, 205 (1996)
S. Naz, R. Siddiqi, S. Ahmad, S.A. Rasool, S.A. Sayeed, J. Food Sci. 72, M341 (2007)
L.C. Braga, J.W. Shupp, C. Cummings, M. Jett, J.A. Takahashi, L.S. Carmo, E. Chartone-Souza, A.M.A. Nascimento, J. Ethnopharmacol. 96, 335 (2005)
N.S. Al-Zoreky, Int. J. Food Microbiol. 134, 244 (2009)
G.M. El-Sherbini, K.M. Ibrahim, E.T. El Sherbiny, N.M. Abdel-Hady, T.A. Morsy, J. Egypt. Soc. Parasitol. 40, 229 (2010)
O.M. Albarri, I. Var, A. Boushihassal, M. Meral, C. Önlen, M.H. Mohamed, F. Köksal, J. Biotechnol. Sci. Res. 3(6), 175 (2017)
Y. Roos, M. Karel, Biotechnol. Prog. 6, 159 (1990)
O.V. Alekseeva, A.V. Noskov, S.S. Guseinov, A.V. Agafonov, Prot. Met. Phys. Chem. Surf. 51, 253 (2015)
A.L. Waterhouse, Current Protocols in Food Analytical Chemistry (Wiley, Hoboken, 2001)
W. Brand-Williams, M.E. Cuvelier, C. Berset, Food Sci. Technol. 28, 25 (1995)
E. Kerasioti, D. Stagos, A. Priftis, S. Aivazidis, A.M. Tsatsakis, A.W. Hayes, D. Kouretas, Food Chem. 155, 271 (2014)
J.C. Boulet, P. Williams, T. Doco, Carbohydr. Polym. 69, 79 (2007)
S. Lohumi, C. Mo, J.-S. Kang, S.-J. Hong, B.-K. Cho, J. Biosyst. Eng. 38, 312 (2013)
CLSI, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically Approved Standard, 9th edn. (CLSI, Wayne, 2012)
S.V. Leontopoulos, I. Giavasis, K. Petrotos, M. Kokkora, C. Makridis, Agric. Agric. Sci. Proc. 4, 327 (2015)
N.G. Baydar, O. Sagdic, G. Ozkan, S. Cetin, Int. J. Food Sci. Technol. 41(7), 799 (2006)
P. Dalgaard, T. Ross, L. Kamperman, K. Neumeyer, T.A. McMeekin, Int. J. Food Microbiol. 23, 391 (1994)
B. Shan, Y. Cai, J.D. Brooks, H. Corke, Int. J. Food Microbiol. 117, 112 (2007)
S.V. Leontopoulos, K.B. Petrotos, M.I. Kokkora, I. Giavasis, C. Papaioannou, Desalin. Water Treat. 57, 20646 (2016)
C. Valgas, S.M. de Souza, E.F.A. Smânia, A. Smânia Jr., Braz. J. Microbiol. 38, 369 (2007)
A. Benchennouf, S. Grigorakis, S. Loupassaki, Pharm. Biol. 55, 596 (2017)
N. Ullah, J. Ali, F.A. Khan, M. Khurram, A. Hussain, Middle-East J. Sci. Res. 11, 396 (2012)
A.C. Pedro, J.B.B. Maurer, S.F. Zawadzki-Baggio, S. Ávila, G.M. Maciel, C.W. Haminiuk, Ind. Crops Prod. 112, 90 (2018)
R.F. Yang, C. Zhao, X. Chen, S.W. Chan, J.Y. Wu, J. funct. foods 17, 903 (2015)
D. Donno, G.L. Beccaro, M.G. Mellano, A.K. Cerutti, G. Bounous, J. funct. foods 18, 1070 (2015)
N.I. Fit, F. Chirila, G. Nadas, E. Pall, R. Muresan, Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Vet. Med. 70, 72–613 (2013)
A. Asănică, C. Manole, V. Tudor, A. Dobre, R.I. Teodorescu, AgroLife Sci. J. 5(1), 15 (2016)
H. Vardin, A. Tay, B. Ozen, L. Mauer, Food Chem. 108, 742 (2008)
L. Zuo, S. Sun, Q. Zhou, J. Tao, I. Noda, J. Pharm. Biomed. Anal. 30, 1491 (2003)
D. Mlambo, Detection of Quercetin using Polymer Coated Quartz Crystal Microbalance and the Modification of á-Zirconium Phosphate to Develop a Sorbent for Organic Pollutant Removal. Doctoral dissertation, Marquette University (2010). Retrieved from https://epublications.marquette.edu/dissertations_mu/77/
D. Wu, S. Lam, K. Cheong, F. Wei, P. Lin, Z. Long, X. Lv, J. Zhao, S. Ma, S. Li, J. Pharm. Biomed. Anal. 129, 210 (2016)
A. Mocan, L. Vlase, D.C. Vodnar, C. Bischin, D. Hanganu, A.-M. Gheldiu, R. Oprean, R. Silaghi-Dumitrescu, G. Crisan, Molecules (Basel, Switzerland) 19, 10056 (2014)
I. Dahech, W. Farah, M. Trigui, A.B. Hssouna, H. Belghith, K.S. Belghith, F.B. Abdallah, Int. J. Biol. Macromol. 60, 328 (2013)
A. Salvat, L. Antonacci, R.H. Fortunato, E.Y. Suarez, H.M. Godoy, Phytomedicine 11, 230 (2004)
K. Barathikannan, B. Venkatadri, A. Khusro, N.A. Al-Dhabi, P. Agastian, M.V. Arasu, H.S. Choi, Y.O. Kim, BMC Complement. Altern. Med. 16, 264 (2016)
M.E. Legaz, M.M. Pedrosa, R. de Armas, C.W. Rodrı́guez, V. de los Rios, C. Vicente, Anal. Chim. Acta 372, 201 (1998)
P. Skenderidis, D. Lampakis, I. Giavasis, S. Leontopoulos, K. Petrotos, C. Hadjichristodoulou, Antioxidants 8, 60 (2019)
T. Maruyama, S. Katoh, M. Nakajima, H. Nabetani, T.P. Abbott, A. Shono, K. Satoh, J. Membr. Sci. 192, 201 (2001)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This chapter does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Skenderidis, P., Mitsagga, C., Giavasis, I. et al. The in vitro antimicrobial activity assessment of ultrasound assisted Lycium barbarum fruit extracts and pomegranate fruit peels. Food Measure 13, 2017–2031 (2019). https://doi.org/10.1007/s11694-019-00123-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-019-00123-6