Abstract
Phosphate buffered saline (PBS) and carbohydrates are used to freeze-dried biomaterials. It has been suggested that PBS reduces the freeze-concentrated glass-like transition temperature (\(T_{\text{g}}^{\prime }\)) of carbohydrate solutions and that freeze-dried solids collapse readily during freeze-drying. In order to obtain more insight into the subject, the effect of PBS on the \(T_{\text{g}}^{\prime }\) of trehalose solution was systematically investigated. The \(T_{\text{g}}^{\prime }\) of trehalose solution was found to be independent of the trehalose content. The \(T_{\text{g}}^{\prime }\) of trehalose–PBS solution, on the other hand, decreased with decreasing trehalose content. The behavior was described as a \(T_{\text{g}}^{\prime }\) curve for a trehalose–PBS pseudobinary system. The \(T_{\text{g}}^{\prime }\) curve was analyzed using the modified Gordon–Taylor equation, and the \(T_{\text{g}}^{\prime }\) of PBS was extrapolated to be 195.0 K. In order to compare the plasticizing effect of PBS, the \(T_{\text{g}}^{\prime }\) values of other carbohydrate materials including sugar alcohols (glucose, maltitol, sucrose, inulin, and maltodextrin) were also investigated. It was found that freeze-concentrated trehalose was more sensitive to the plasticizing effect of PBS than the other sugars. Finally, trehalose and trehalose–PBS solutions with varying trehalose content were freeze-dried, and their water activity (aw) and apparent volume ratio against pre-dried (frozen) solids (VFD V−1F ) were evaluated. Freeze-dried trehalose samples had low aw values and high VFD V−1F values. Freeze-dried trehalose–PBS samples, on the other hand, had significantly higher aw values and lower VFD V−1F values than freeze-dried trehalose samples depending on the trehalose content. These results corresponded to the \(T_{\text{g}}^{\prime }\) and initial solute content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Freeze-drying is often used to store unstable biomaterials (e.g., proteins and bacteria) at room temperature for extended periods of time. Some biomaterials are irreversibly damaged during freeze-drying and subsequent storage, and their biological activity gradually decreases. In order to prevent the destabilization of biomaterials, the effect of various stabilizers has been investigated [1, 2]. It is known that non-reduced disaccharides (sucrose and trehalose) have a stabilizing effect on biomaterials [1, 3]. The stabilizing mechanism of disaccharides has been mainly explained by two phenomena. One is the “water substitution effect”. Disaccharides form hydrogen bonds with biomaterials instead of water molecules in the dehydrated state, thus structurally stabilizing the biomaterials [1, 2, 4]. The other is the “glass transition effect”. Freeze-dried disaccharides turn into a glassy state upon dehydration. Since glassy disaccharides have extremely low molecular mobilities, the biomaterials embedded in them are dynamically stabilized [1, 5]. The glass transition effect can be characterized by the glass transition temperature (Tg). At higher Tg, lower molecular mobility and greater resistance to degradation are expected at a given condition.
We previously [6] demonstrated that sucrose and trehalose could protect freeze-dried lactic acid bacteria (LAB). In the study, the freeze-dried LAB showed ready “collapse” depending on the freeze-drying conditions. Collapse is a physical deterioration in which a freeze-dried solid cannot maintain its porous structure and shrinks into a high-density solid; drying and rehydration rates are diminished by the collapse [7,8,9]. It is known that collapse occurs when the product temperature becomes higher than the freeze-concentrated glass-like transition temperature (\(T_{\text{g}}^{\prime }\)) during freeze-drying [1]. Since the freeze-concentrated phase is a rigid (glassy) state at a temperature below \(T_{\text{g}}^{\prime }\), the porous structure formed by freeze-drying can be maintained during subsequent processing. The freeze-concentrated phase becomes a soft (rubbery) state above \(T_{\text{g}}^{\prime }\), and thus, the porous structure destructs spontaneously.
As a possible explanation for why LAB containing sucrose and trehalose readily collapsed, it was suggested that co-existent phosphate buffered saline (PBS) reduced the \(T_{\text{g}}^{\prime }\) of sucrose and trehalose. PBS, which consists of NaCl, KCl, Na2HPO4, and KH2PO4, is an essential material for LAB formulation. Thorat and Suryanarayanan [10] investigated the \(T_{\text{g}}^{\prime }\) of 5% trehalose–PBS solution and 1% mannitol–PBS solution. Although they did not discuss the effect of PBS addition on the \(T_{\text{g}}^{\prime }\) of the carbohydrate solutions, it was found that the \(T_{\text{g}}^{\prime }\) was reduced by the addition of PBS by the comparison with \(T_{\text{g}}^{\prime }\) data in the existing literature [1]. Nicolajsen and Hvidt [11] investigated the \(T_{\text{g}}^{\prime }\) of trehalose–NaCl solution. Since NaCl is a primary component of PBS (more than 80%), similar effects to PBS are expected. Although there was limited experimental data, it could be confirmed that the \(T_{\text{g}}^{\prime }\) decreased with increasing NaCl concentration in the system. In addition, Hawe and Frieß [12] investigated the \(T_{\text{g}}^{\prime }\) of mannitol–sucrose–NaCl solution and demonstrated that NaCl reduced the \(T_{\text{g}}^{\prime }\) of the carbohydrate solutions in a limited range of up to 0.2% NaCl. On the other hand, there is a report that PBS reduced the Tg of trehalose under various dehydration conditions [13].
Although the suggestion that PBS reduces the \(T_{\text{g}}^{\prime }\) of trehalose is strongly supported by previous studies, the experimental effect has not been explored systematically. It is expected that systematic experimental data will provide a better \(T_{\text{g}}^{\prime }\) prediction, and thus better quality control (e.g., collapse) of freeze-dried biomaterials stabilized by the addition of PBS and carbohydrates. The purpose of this study was first to clarify the effect of PBS on the \(T_{\text{g}}^{\prime }\) of trehalose solution systematically. Second, in order to compare the plasticizing effect of PBS, the \(T_{\text{g}}^{\prime }\) values of other carbohydrate materials including sugar alcohols (glucose, maltitol, sucrose, inulin, and maltodextrin) were determined. Finally, the effect of PBS on the degree of collapse of freeze-dried solids was investigated.
Materials and methods
Samples preparation
Trehalose dihydrate was provided by Hayashibara Co., Ltd. (Okayama, Japan). Maltodextrin (dextrose equivalent = 17–21) and inulin (degree of polymerization = 7) were provided by San-ei Sucrochemical Co., Ltd. (Aichi, Japan). Glucose, maltitol, and sucrose were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). PBS powder was purchased from Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan).
PBS powder was added to distilled water according to the product instructions (9.6 mg mL−1) to obtain a PBS solution composed of NaCl (8.0 mg mL−1), KCl (0.2 mg mL−1), Na2HPO4 (1.15 mg mL−1), and KH2PO4 (0.2 mg mL−1). Trehalose was dissolved in distilled water or PBS solution, and 100, 200, 400, and 800 mM trehalose aqueous solutions and trehalose–PBS solutions were obtained. In addition, 400 mM trehalose–PBS solutions were prepared by using two times (19.2 mg mL−1) and ten times (96.0 mg mL−1) higher concentrations of PBS. Although the high-concentration PBS solutions cannot be used for the preparation of biomaterials, they were effective for the systematic understanding of \(T_{\text{g}}^{\prime }\) of trehalose–PBS solutions.
Other carbohydrate materials including sugar alcohols (glucose, maltitol, sucrose, inulin, and maltodextrin) were also dissolved in distilled water and/or PBS solution at various solute contents (100–400 mM for low-molecular-weight materials and 3–20% for polymers).
Differential scanning calorimetry
The \(T_{\text{g}}^{\prime }\) of the samples was investigated using differential scanning calorimetry (DSC; DSC60 Plus; Shimadzu Co., Tokyo, Japan). Alumina powder was used as a reference. The temperature was calibrated by indium (melting temperature, Tm = 429.8 K), distilled water (Tm = 273.2 K), NaCl aqueous solution (eutectic temperature, Te = 252 K), and CaCl2 aqueous solution (Te = 223.4 K). Heat flow was calibrated by indium (melting enthalpy = 28.5 J g−1). Nitrogen gas and liquid nitrogen were used as purge gas and coolant, respectively. The solution sample (approximately 20 mg) was placed in an aluminum pan, and then sealed. DSC measurements were carried out between 203 K and 298 K at cooling and heating rates of 3 K min−1. In order to prevent crystallization during cooling, glucose–PBS samples were cooled down at 5 K min−1. DSC heating curves were analyzed by software interfaced with the DSC system. The onset point of the endothermic shift observed in the DSC heating curve was determined as \(T_{\text{g}}^{\prime }\). The measurements were performed in duplicate, and the results averaged.
Freeze-drying
Trehalose solution and trehalose–PBS solution (4 mL) were placed in a 20-mL vial (diameter = 24 mm), and pre-frozen in a freezer at approximately 253 K. The frozen samples were transferred onto the sample stage (25.2 × 103 mm2) in a custom-made freeze-dryer (chamber space = 11.9 × 106 mm3 and trap temperature = ca. 221 K). The stage temperature (Tstage) was preset to 253 K and/or 263 K. Primary drying was carried out at approximately 11 Pa for 22 h. Subsequently, secondary drying was carried out at the same pressure upon increasing temperature from 253 or 263 K to 278 K at approximately 5 K h−1. The samples were hermetically enclosed immediately after vacuum release, and then stored at 298 K overnight.
Water activity of freeze-dried solids
The water activity (aw) of the freeze-dried samples was investigated using an aw sensor (Rotronic HP23-AW, Swiss). The vial containing the freeze-dried sample was set directly on the sensor, and aw was evaluated at 298 K. The measurements were performed in quadruplicate, and the results averaged.
Apparent volume ratio of the freeze-dried solids against pre-dried solids
In order to evaluate the apparent volume ratio of the freeze-dried solids against pre-dried (frozen) solids (VFD V−1F ), the diameter and height were measured by a digital caliper and a displacement meter, respectively. Since the freeze-dried solid was not a completely circular column, the lowest diameter and height were typically measured. The surface area was calculated as a circle from the diameter. The apparent volume was evaluated as the surface area multiplied by the height. The apparent volume for the freeze-dried samples was divided by that of the pre-dried (frozen) samples, and then VFD V−1F was determined. The measurements were performed in quadruplicate, and the results averaged.
Statistical analysis
Statistical analysis of aw and VFD V−1F between freeze-dried trehalose and trehalose–PBS samples was performed using a t test (p < 0.05) with KaleidaGraph software (Version 3.6, Hulinks Inc., Tokyo, Japan).
Results and discussion
\(T_{\text{g}}^{\prime }\) of trehalose–PBS pseudobinary system
Typical DSC curves for 100–400 mM trehalose solutions and 100–400 mM trehalose–PBS solutions are shown in Fig. 1a, b, respectively. The samples showed endothermic shifts reflecting the freeze-concentrated glass-like transition, and \(T_{\text{g}}^{\prime }\) was determined from the onset point of the shift. The heat capacity change induced by the freeze-concentrated glass-like transition (magnitude of the endothermic shift) increased with trehalose content. This indicates that the endothermic shift is originated mainly from trehalose. It is known that two endothermic shifts (a minor endothermic shift at low temperature and a subsequent major one) are observed in DSC curves for sugar solutions [14,15,16,17,18], with the minor endothermic shift 10–15 K lower than the major endothermic shift [19]. In this study, the minor endothermic shift was observed clearly for the 800 mM trehalose aqueous and PBS solutions, but the other samples showed no, unclear, or minor endothermic shifts. The origins of the two endothermic shifts are not completely understood, and are somewhat controversial [5, 16, 20,21,22]. Taking into account the fact that the collapse of freeze-dried solids commonly occurs at a temperature above the onset point of the major endothermic shift [1, 22, 23], the major endothermic shit is more important for the purpose of this study. Thus, the major endothermic shift was considered to be the freeze-concentrated glass-like transition [15, 24, 25], and its onset point was denoted as \(T_{\text{g}}^{\prime }\).
The effect of the trehalose content on the \(T_{\text{g}}^{\prime }\) of trehalose solution and trehalose–PBS solution is shown in Fig. 1c. The \(T_{\text{g}}^{\prime }\) values of trehalose solutions varied over a negligible range (maximally ± 0.3 K). The \(T_{\text{g}}^{\prime }\) was independent of trehalose content because \(T_{\text{g}}^{\prime }\) reflects the glass transition temperature of the freeze-concentrated phase in the system. The \(T_{\text{g}}^{\prime }\) of trehalose–PBS solution, on the other hand, decreased with decreasing trehalose content. Since the reduction in trehalose content means that the PBS ratio against trehalose increases, it is suggested that PBS has a plasticizing effect on trehalose in the freeze-concentrated state.
Typical DSC curves for 400 mM trehalose–PBS solutions with varying PBS content are shown in Fig. 2a. For comparison, DSC curve for PBS solution is also shown in there. The trehalose–PBS solutions showed a clear endothermic shift, and \(T_{\text{g}}^{\prime }\) was determined. The effect of the PBS content on the \(T_{\text{g}}^{\prime }\) of 400 mM trehalose–PBS solution is shown in Fig. 2b. As expected, the \(T_{\text{g}}^{\prime }\) decreased with increasing PBS content. PBS solution without trehalose showed an endothermic peak near 252 K at the eutectic point (Fig. 2a). The Te was in agreement with that for NaCl, because NaCl is a primary component of PBS [11, 26]. The reason why trehalose–PBS solution did not show the eutectic point of NaCl is that trehalose prevented crystallization of the PBS components during cooling and subsequent heating processes [10, 15]. The 400 mM trehalose–PBS solution prepared by using ten times (96.0 mg mL−1) showed an exothermic peak at the higher temperature than the \(T_{\text{g}}^{\prime }\) (Fig. 2a). This suggests that amorphous NaCl crystalized during the heating.
In order to characterize the \(T_{\text{g}}^{\prime }\) depression behavior of trehalose–PBS solution, the \(T_{\text{g}}^{\prime }\) was plotted against dry PBS mass per dry total solute (trehalose and PBS) mass as shown in Fig. 3. This shows a \(T_{\text{g}}^{\prime }\) curve similar to the trehalose–PBS pseudobinary system. The solid line was obtained by fitting the modified Gordon–Taylor (GT) equation (Eq. 1) to the data,
where W1 and W2 are the dry mass fractions of trehalose and PBS, \(T_{\text{g1}}^{\prime }\) and \(T_{{{\text{g}}2}}^{\prime }\) are \(T_{\text{g}}^{\prime }\) values for trehalose and PBS, and k is a constant. The original GT equation is often used to describe the Tg change for solute–water binary systems [7, 24, 27,28,29,30]. It is known that the GT equation is also useful for the \(T_{\text{g}}^{\prime }\) change for solute–solute pseudobinary systems [25]. For the fitting analysis, \(T_{\text{g1}}^{\prime }\) = 244.4 K was employed from the experimental data (Fig. 1), and the two unknown parameters (\(T_{{{\text{g}}2}}^{\prime }\) and k) were determined as fitting parameters. As a result, \(T_{{{\text{g}}2}}^{\prime }\) = 195.0 K and k = 2.07 were obtained (R2 = 0.9994). As mentioned above, \(T_{\text{g}}^{\prime }\) of PBS solution could not be determined experimentally because PBS (mainly NaCl) crystallized as a result of freeze concentration. The \(T_{\text{g}}^{\prime }\) of PBS, however, could be determined as an extrapolated value. There are limited reports on the \(T_{\text{g}}^{\prime }\) of electrolytes due to their superior eutectic ability. In the case of chloride, there are reports of \(T_{\text{g}}^{\prime }\) on CaCl2 (178 K), MnCl2 (190 K), and ZnCl2 (185 K) [23]. These \(T_{\text{g}}^{\prime }\) values were near that for PBS. Since \(T_{\text{g}}^{\prime }\) decreases with molecular and/or formula mass of solute [24], the fact that PBS had a much lower \(T_{\text{g}}^{\prime }\) than trehalose is reasonable.
\(T_{\text{g}}^{\prime }\) of other carbohydrate–PBS solutions
The \(T_{\text{g}}^{\prime }\) of the trehalose–PBS solution could be described by the modified GT equation as a pseudobinary system. Since the \(T_{\text{g}}^{\prime }\) of PBS (\(T_{{{\text{g}}2}}^{\prime }\) in Eq. 1) was determined to be 195.0 K, more brief fitting analysis can be applied hereafter. In order to compare the plasticizing effect of PBS, the \(T_{\text{g}}^{\prime }\) of other carbohydrate materials including sugar alcohols (glucose, maltitol, sucrose, inulin, and maltodextrin) were investigated.
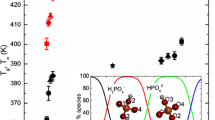
The samples showed endothermic shifts reflecting the freeze-concentrated glass-like transition as similar to trehalose–PBS solution (data not shown), and \(T_{\text{g}}^{\prime }\) was determined. There was no exothermic peak (crystallization of NaCl) higher than the \(T_{\text{g}}^{\prime }\); crystallization of NaCl was prevented by the carbohydrate materials in the range of measured PBS content. The \(T_{\text{g}}^{\prime }\) curves for the carbohydrate–PBS pseudobinary systems are shown in Fig. 4. The solid curves were obtained by fitting the modified GT equation to the data; \(T_{\text{g1}}^{\prime }\) was experimentally determined in this study and k was determined as a fitting parameter. They were in good agreement and similar to the trehalose–PBS system. The results are listed in Table 1.
\(T_{\text{g}}^{\prime }\) curve for various carbohydrate–PBS pseudobinary systems. The solid curves were obtained by fitting the GT equation to the data; \(T_{\text{g1}}^{\prime }\) was experimentally determined, \(T_{{{\text{g}}2}}^{\prime }\) was fixed to be 195.0 K, and k was treated as a fitting parameter
The k value in the modified GT equation represents the sensitivity to the plasticizing effect of PBS; the higher the k value, the greater the \(T_{\text{g}}^{\prime }\) depression caused by PBS. Glucose, maltitol, and sucrose had lower \(T_{\text{g1}}^{\prime }\) (229.7–239.3 K) and lower k (1.30) than trehalose (\(T_{\text{g1}}^{\prime }\) = 244.4 K and k = 2.07). Since there are small differences between \(T_{\text{g1}}^{\prime }\) and \(T_{{{\text{g}}2}}^{\prime }\) in the systems, the \(T_{\text{g}}^{\prime }\) depression induced by PBS will be diminished. Inulin and maltodextrin had higher \(T_{\text{g}}^{\prime }\) (250.3–256.8 K), but lower k (1.6–2.0) than trehalose. It is thought that carbohydrate polymers have a high \(T_{\text{g1}}^{\prime }\) because of strong molecular interactions induced by entanglement of the segments. The polymer entanglement cannot be unwrapped easily, and thus, inulin and dextrin are resistant to the plasticizing effect of PBS. From the comparison, it was noted that freeze-concentrated trehalose is sensitive to the plasticizing effect of PBS. This is the reason attributed to the high \(T_{\text{g}}^{\prime }\) of trehalose in spite of its low molecular weight.
Water activity and apparent volume ratio of freeze-dried trehalose samples
The effect of trehalose content on the aw of freeze-dried samples is shown in Fig. 5a (Tstage = 253 K) and b (Tstage = 263 K). The Tstage caused no or small differences in aw for both freeze-dried trehalose and trehalose–PBS samples. The aw values of freeze-dried trehalose samples were lower than 0.33, which were sufficient for freeze-dried biomaterials such LAB [6, 31,32,33,34,35,36]. The aw slightly increased with trehalose content. Since the amount of bound water having a low water vapor pressure increases with solute content, the progress of freeze-drying is diminished. Freeze-dried trehalose–PBS samples, on the other hand, had significantly higher aw than freeze-dried trehalose samples depending on the trehalose content. 200 mM and 400 mM trehalose–PBS samples had aw values lower than 0.35, and the freeze-dried porous structure was more or less maintained. 100 mM and 800 mM trehalose–PBS samples had much higher aw (above 0.45) than the other samples, and thus, the freeze-dried porous structure was completely destroyed. Since the 100 mM trehalose–PBS sample had a much lower \(T_{\text{g}}^{\prime }\) than the others, collapse during freeze-drying prevented ice sublimation. Although 800 mM trehalose–PBS had the highest \(T_{\text{g}}^{\prime }\) among the trehalose–PBS samples, freeze-drying was insufficient at the given condition. This is because the amount of bound water increased with trehalose content as mentioned above.
The effect of trehalose content on the VFD V−1F is shown in Fig. 6a (Tstage = 253 K) and b (Tstage = 263 K). There was no significant reduction in the VFD V−1F for freeze-dried trehalose samples at either Tstage, because the \(T_{\text{g}}^{\prime }\) of trehalose solution was independent of trehalose content. The VFD V−1F for freeze-dried trehalose–PBS samples, on the other hand, was lower than freeze-dried trehalose samples depending on the Tstage and trehalose content. As mentioned above, 100 mM and 800 mM trehalose–PBS samples were insufficiently dried at the given freeze-drying conditions. Since their porous structure was destroyed completely, the VFD V−1F was estimated to be zero. The VFD V−1F for 200 mM trehalose–PBS samples was significantly lower than that for 200 mM trehalose samples. This can be explained by the difference in \(T_{\text{g}}^{\prime }\) (Fig. 2); the 200 mM trehalose solution had \(T_{\text{g}}^{\prime }\) = 244 K, but the trehalose–PBS solution had \(T_{\text{g}}^{\prime }\) = 234 K. The VFD V−1F for the 200 mM trehalose–PBS sample freeze-dried at Tstage = 263 K was lower than that for the 200 mM trehalose sample freeze-dried at Tstage = 253 K. At higher Tstage, a higher sample temperature during freeze-drying is expected. Thus, the 200 mM trehalose–PBS sample freeze-dried at Tstage = 263 K will have more shrinkage. There were no significant differences in the VFD V−1F between 400 mM trehalose and trehalose–PBS samples. In addition, there was no effect of Tstage on the VFD V−1F for 400 mM trehalose–PBS samples. This is because the 400 mM trehalose–PBS sample had a relatively high \(T_{\text{g}}^{\prime }\) (239 K) and low aw (0.314–0.354).
In previous studies [6, 37,38,39], effect of carbohydrate materials on the biological stability of dried LAB has been extensively discussed. This study, on the other hand, demonstrated that the carbohydrate materials for freeze-dried LAB have an optimum content in a practical reason. The \(T_{\text{g}}^{\prime }\) decreases at a low carbohydrate content, but the amount of bound water increases at a high carbohydrate content; these factors affect the collapse of freeze-dried LAB. The results will be useful for the physical quality control of freeze-dried LAB with carbohydrate–PBS preparations.
Conclusions
The effect of trehalose content on the \(T_{\text{g}}^{\prime }\) of trehalose–PBS solutions was investigated systematically. A \(T_{\text{g}}^{\prime }\) curve for the trehalose–PBS pseudobinary system was constructed and compared with other carbohydrate materials including sugar alcohols. It was confirmed that not only \(T_{\text{g}}^{\prime }\) but also the amount of bound water affected by initial solute content are important criteria to prevent collapse during freeze-drying. These results will be useful for the process control of freeze-dried carbohydrate–PBS systems such as LAB formulations.
The \(T_{\text{g}}^{\prime }\) of carbohydrate–PBS solutions could be described by the modified GT equation as a pseudobinary system. The modified GT equation has three constants (\(T_{\text{g1}}^{\prime }\), \(T_{{{\text{g}}2}}^{\prime }\), and k) depending on the system. The \(T_{\text{g}}^{\prime }\) for various carbohydrates (\(T_{\text{g1}}^{\prime }\)) has already been published, and the \(T_{\text{g}}^{\prime }\) of PBS (\(T_{{{\text{g}}2}}^{\prime }\)) was determined in this study. In order to predict the \(T_{\text{g}}^{\prime }\) depression induced by PBS, it is necessary to understand the unknown parameter (k). For a better prediction of \(T_{\text{g}}^{\prime }\) for carbohydrate–PBS solutions, a primary factor characterizing k should be investigated in the future.
References
Wamg W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm. 2000;203:1–60. https://doi.org/10.1016/S0378-5173(00)00423-3.
Arakawa T, Prestrelski SJ, Kenney WC, Carpenter JF. Factors affecting short-term and long-term stabilities of proteins. Adv Drug Deliv Rev. 2001;46:307–26. https://doi.org/10.1016/S0169-409X(00)00144-7.
Crowe JH, Carpenter JF, Crowe LM, Anchordoguy TJ. Are freezing and dehydration similar stress vectors? A comparison of modes of interaction of stabilizing solutes with biomolecules. Cryobiology. 1990;27:219–31. https://doi.org/10.1016/0011-2240(90)90023-W.
Arakawa T, Kita Y, Carpenter JF. Protein-solvent interaction in pharmaceutical formulations. Pharm Res. 1991;8:285–91. https://doi.org/10.1023/A:1015825027737.
Roos YH. Food components and polymers. In: Phase transitions in foods. San Diego: Academic Press; 1995. pp. 109–156. https://doi.org/10.1016/B978-0-12-595340-5.X5000-7.
Teng D, Kawai K, Mikajiri S, Hagura Y. Stabilization of freeze-dried Lactobacillus paracasei subsp. paracasei JCM 8130T with the addition of disaccharide, polymer, and their mixture. Biosci Biotechnol Biochem. 2017;81:768–73. https://doi.org/10.1080/09168451.2017.1279852.
Bhandari BR, Howes T. Implication of glass transition for the drying and stability of dried foods. J Food Eng. 1999;40:71–9. https://doi.org/10.1016/S0260-8774(99)00039-4.
Tattini V Jr, Parra DF, de Moraes Pitombo RN. The influence of crucible material on the DSC thermal analysis compared to freeze-drying microscopy results. J Therm Anal Calorim. 2009;98:203–6. https://doi.org/10.1007/s10973-009-0317-8.
Pikal MJ, Shah S. The collapse temperature in freeze drying: dependence on measurement methodology and rate of water removal from the glassy phase. Int J Pharm. 1990;62:165–86. https://doi.org/10.1016/0378-5173(90)90231-R.
Thorat AA, Suryanarayanan R. Characterization of phosphate buffered saline (PBS) in frozen state and after freeze-drying. Pharm Res. 2019;36:98. https://doi.org/10.1007/s11095-019-2619-2.
Nicolajsen H, Hvidt A. Phase behavior of the system trehalose–NaCl–water. Cryobiol. 1994;31:199–205. https://doi.org/10.1006/cryo.1994.1024.
Hawe A, Frieß W. Impact of freezing procedure and annealing on the physico-chemical properties and the formation of mannitol hydrate in mannitol–sucrose–NaCl formulations. Eur J Pharm Biopharm. 2006;64:316–25. https://doi.org/10.1016/j.ejpb.2006.06.002.
Sitaula R, Bhowmick S. Moisture characteristics and thermophysical properties of trehalose–PBS mixtures. Cryobiology. 2006;52:369–685. https://doi.org/10.1016/j.cryobiol.2006.01.006.
Izutsu K, Rimand A, Aoyagi N, Kojima S. Effect of sodium teraborate (borax) on the thermal properties of frozen aqueous sugar and polyol solutions. Chem Pharm Bull. 2003;51:663–6. https://doi.org/10.1248/cpb.51.663.
Harnkarnsujarit N, Nakajima M, Kawai K, Watanabe M, Suzuki T. Thermal properties of freeze-concentrated sugar-phosphate solutions. Food Biophys. 2014;9:213–8. https://doi.org/10.1007/s11483-014-9335-6.
Pyne A, Suryanarayanan R. Enthalpic relaxation in frozen aqueous trehalose solutions. Thermochim Acta. 2003;405:225–34. https://doi.org/10.1016/S0040-6031(03)00193-X.
Ohkuma C, Kawai K, Viriyarattanasak C, Mahawanich T, Tantratuin S, Takai R, Suzuki T. Glass transition properties of frozen and freeze-dried surimi products: effects of sugar and moisture on the glass transition temperature. Food Hydrocoll. 2008;22:255–62. https://doi.org/10.1016/j.foodhyd.2006.11.011.
Levine H, Slade L. Thermomechanical properties of small-carbohydrate-water glasses and ‘rubbers’. Kinetically metastable systems at sub-zero temperatures. J Chem Faraday Trans. 1988;84:2619–33. https://doi.org/10.1039/F19888402619.
Roos YH. Frozen state transitions in relation to freeze drying. J Therm Anal Calorim. 1997;48:535–44. https://doi.org/10.1007/BF01979500.
Goff HD, Verepej E, Jermann D. Glass transitions in frozen sucrose solutions are influenced by solute inclusions within ice crystals. Thermochim Acta. 2003;399:43–55. https://doi.org/10.1016/S0040-6031(02)00399-4.
Champion D, Loupiac C, Russo D, Simatos D, Zanotti JM. Dynamic and sub-ambient thermal transition relationships in water–sucrose solutions. J Therm Anal Calorim. 2011;104:365–74. https://doi.org/10.1007/s10973-010-1114-0.
Shalaev EY, Frank F. Structural glass transitions and thermophysical processes in amorphous carbohydrates and their supersaturated solutions. J Chem Soc Faraday Trans. 1995;91:1511–7. https://doi.org/10.1039/FT9959101511.
Chang BS, Randall CS. Use of subambient thermal analysis to optimize protein lyophilization. Cryobiol. 1992;29:632–56. https://doi.org/10.1016/0011-2240(92)90067-C.
Kawai K, Fukami K, Thanatuksorn P, Viriyarattanasak C, Kajiwara K. Effects of moisture content, molecular weight, and crystallinity on the glass transition temperature of inulin. Carbohydr Polym. 2011;83:934–9. https://doi.org/10.1016/j.carbpol.2010.09.001.
Fukami K, Takeuchi S, Fukujyu T, Hagura Y, Kawai K. Water sorption, glass transition, and freeze-concentrated glass-like transition properties of calcium maltobionate-maltose mixtures. J Therm Anal Calorim. 2019;135:2775–81. https://doi.org/10.1007/s10973-018-7793-7.
Ito K. Freeze drying of pharmaceuticals. Eutectic temperature and collapse temperature of solute matrix upon freeze drying of three-component systems. Chem Pharm Bull. 1971;19:1095–102. https://doi.org/10.1248/cpb.19.1095.
Fukami K, Kawai K, Takeuchi S, Harada Y, Hagura H. Effect of water content on the glass transition temperature of calcium maltobionate and its application to the characterization of non-Arrhenius viscosity behavior. Food Biophys. 2016;11:410–6. https://doi.org/10.1007/s11483-016-9455-2.
Sogabe T, Kawai K, Kobayashi R, Jothi JS, Hagura Y. Effects of structure and water plasticization on the mechanical glass transition temperature and textural properties of freeze-dried trehalose solid and cookie. J Food Eng. 2018;217:101–7. https://doi.org/10.1016/j.jfoodeng.2017.08.027.
Ebara T, Hagura Y, Kawai K. Effect of water content on the glass transition and textural properties of hazelnut. J Therm Anal Calorim. 2019;135:2629–34. https://doi.org/10.1007/s10973-018-7672-2.
Espinosa-Andrews H, Rodríguez-Rodríguez R. Water state diagram and thermal properties of fructans powders. J Therm Anal Calorim. 2018;132:197–204. https://doi.org/10.1007/s10973-017-6868-1.
Champagne CP, Mondou F, Raymond Y, Roy D. Effect of polymers and storage temperature on the stability of freeze-dried lactic acid bacteria. Food Res Int. 1996;29:555–62. https://doi.org/10.1016/0963-9969(95)00050-X.
Palmfeldt J, Radstrom P, Hahn-Hägerdal B. Optimisation of initial cell concentration enhances freeze-drying tolerance of Pseudomonas chlororaphis. Cryobiology. 2003;47:21–9. https://doi.org/10.1016/S0011-2240(03)00065-8.
Nualkaekul S, Deepika G, Charalampopoulos D. Survival of freeze dried Lactobacillus plantarum in instant fruit powders and reconstituted fruit juices. Food Res Int. 2012;48:627–33. https://doi.org/10.1016/j.foodres.2012.06.003.
Tymczyszyn EE, Gerbino E, Illanes A, Gomez-Zavagloa A. Galacto-oligosaccharides as protective molecules in the preservation of Lactobacillus delbrueckii subsp. bulgaricus. Cryobiol. 2011;63:123–9. https://doi.org/10.1016/j.cryobiol.2011.01.013.
Albadran HA, Chatzifragkou A, Khutoryanskiy VV, Charalampopoulos D. Stability of probiotic Lactobacillus plantarum in dry microcapsules under accelerated storage conditions. Food Res Int. 2015;74:208–16. https://doi.org/10.1016/j.foodres.2015.05.016.
Higl B, Kurtmann L, Carlsen CU, Ratjen J, Först P, Skibsted LH, Kulozik U, Risbo J. Impact of water activity, temperature, and physical state on the storage stability of Lactobacillus paracasei ssp. paracasei freeze-dried in a lactose matrix. Biotechnol Prog. 2007;23:794–800. https://doi.org/10.1021/bp070089d.
Zhao G, Zhang G. Influences of protectants, rehydration media and storage on the viability of freeze-dried Oenococcus oeni for malolactic fermentation. World J Microbiol Biotechnol. 2009;25:1801–6. https://doi.org/10.1007/s11274-009-0080-9.
De Giulio B, Orlando P, Barba G, Coppola R, De Rosa M, Sada A, De Prisco PP, Nazzaro F. Use of alginate and cryo-protective sugars to improve the viability of lactic acid bacteria after freezing and freeze-drying. World J Microbiol Biotechnol. 2005;21:739–46. https://doi.org/10.1007/s11274-004-4735-2.
Efiuvwevwere BJO, Gorris LGM, Smid EJ, Kets EPW. Mannitol-enhanced survival of Lactococcus lactis subjected to drying. Appl Microbiol Biotechnol. 1999;51:100–4. https://doi.org/10.1007/s002530051369.
Acknowledgements
This study was financially supported by The Salt Science Research Foundation (1652). We acknowledge Hayashibara Co., Ltd. (Okayama, Japan) for providing trehalose and San-ei Sucrochemical Co., Ltd. (Aichi, Japan) for providing maltodextrin and inulin.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamamoto, Y., Hagura, Y. & Kawai, K. Freeze-concentrated glass-like transition temperature of carbohydrate–phosphate buffered saline systems and impact on collapse of freeze-dried solids. J Therm Anal Calorim 142, 809–817 (2020). https://doi.org/10.1007/s10973-020-09626-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09626-7