Abstract
The aim of this study was to investigate the influence of different crucible materials on the thermal analysis of binary systems. The thermal properties of two distinct solutions were measured both by Differential Scanning Calorimetry (DSC) and freeze-drying microscopy and the results were compared. The glass transition of the maximally freeze-concentrate (T g′) and the eutectic melting temperature (T eut) were not influenced by the crucible material. However the heat of fusion (ΔH) involved during the T eut as well as the ΔC p involved during the T g′ of the solutions were affected.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The intent of low-temperature thermal analysis is to identify the critical values for freezing and primary drying as well as the characteristics that contribute to the behavior of a product during the freeze-drying process. Also, in order to decrease the time and energy consumption, it is necessary to study the thermal properties of the product before it is freeze-dried to optimize the drying conditions while maintaining the highest quality of the product. Therefore, it is important to have precise and reliable knowledge of this parameter in order to maintain the product temperature as high as possible during the sublimation period without going beyond the collapse temperature [1]. Cooling a liquid below some temperature usually results in crystallization. However under special conditions, the same liquid undergoes an increase of the viscosity without crystallization, the glass transition that leads to glass formation. Owing to this fact, the glass may be defined as a material of a thermally arrested molecular arrangement of a liquid state. Hence, the generally used glass transition temperature, T g depends on cooling rate and thermal history [2]. During freezing step if a solute crystallizes from solution, it is well known that the maximum allowable product temperature during primary drying is the eutectic melting temperature (T eut). T eut is the temperature at which a liquid phase begins to appear in the frozen solution during warming. During freeze-drying, the temperature of the product should not reach T eut or a melt-back will occur consequently ruining the product. A more common type of freezing behavior, however, is where the solute remains amorphous during the freezing process, and the freezing-concentrated phase becomes more viscous as freezing proceeds. This continues until the temperature reaches the glass transition of the maximally freeze-concentrated (T g′), below which the amorphous phase becomes glassy [3]. With primary drying below this transition temperature the product will retain the microstructure, which was established by freezing. At some temperature above this transition, called collapse temperature (T c), the amorphous phase will undergo viscous flow during the time scale of freeze-drying, resulting in a collapsed product and general loss of the desirable properties of a freeze-dried solid [4]. It is very important to point out that T g′ is a second order transition and it represents the temperature at which mobility of amorphous phase in the maximally freeze-concentrate becomes significant in the time scale, while collapse is a dynamic phenomenon taking place in the freeze-drying front where the interstitial water in the frozen matrix becomes significantly mobile. The basis for such definition is that ion conduction in the interstitial region require the presence of mobile water. If mobile water is present, then the interstitial region cannot be completely frozen, in accordance to electrical measurements to determine the collapse temperature [5]. However T c is also often considered as close to the T g′ and it was shown to take place 1–2 °C above the glass transition temperature [6]. Differential Scanning Calorimetry (DSC) has been used as a mean of assessing physicochemical (endothermic and exothermic) behavior during freezing and warming. Calorimetrically, T g is usually defined at the midpoint of the ΔC p (T g) value. It should be emphasized, there are kinetic and thermodynamic contributions to the C p value in the glass transition region [7]. It is used to determine phase transitions that correlate the crystallization and melting of a product to various temperatures as well the T g′ of an aqueous formulation [8]. To perform DSC analysis, aqueous formulations are commonly put into an aluminum crucible. Whereas, lyophilized formulations are most often manufactured in a type I glass container. The aim of this study was to investigate the influence of different crucible materials on the results of DSC thermal analysis of two distinct solutions: 5% trehalose solution and 0.9% Sodium Chloride solution. In addition, the T c and T eut of these solutions were measured by freeze-drying microscopy and systematically compared to the T g′ and T eut measured by DSC in order to confirm (or not) in complex media the similarity of values observed in binary aqueous solutions.
Materials and methods
Chemicals
The model solutions were prepared as followed: 5% trehalose solution (m/m) of d(+)-Trehalose dihydrate (Sigma-Aldrich-USA) and a Sodium Chloride solution 0.9% (m/m) (Aster produtos médicos Ltda—Brazil).
Crucible materials (DSC pans)
Three different DSC pans were used during the analysis: 40 μL (microliter) aluminum standard crucible, 40 μL quartz crucible and 40 μL type I glass crucible, each with different thermal conductivity values (Table 1) [9]. The quartz and type I glass DSC pans were constructed following the same dimensions of the aluminum pan to prevent the influence of mass on the crucible material heat capacity during the analysis. The crucibles were made by Wiesberg—glass materials, RJ, Brazil.
Differential scanning calorimetry
DSC was used to determine the T g′ and T eut of these binary systems. The DSC used was a Mettler-822 equipped with cooler sub-ambient accessory. Temperature calibration was done using distillated water (melting point at 0 °C and ΔH fusion = 335 J g−1). For each different crucible material an empty pan of the same material was used as reference. DSC experiments were conducted under atmosphere of 50 mL min−1 nitrogen flow rate. To eliminate the influence of the fill volume on the crystallization temperature, exactly 40 μL of each solution were poured into each open crucible, and then cooled from 25 °C to −50 °C at 5 °C min−1 cooling rate followed by a 5 min isotherm to ensure temperature stability and sample equilibration. After cooling step, the samples were heated to 25 °C using a heating rate of 2 °C min−1. All T g′ were recorded as midpoint temperatures and the relative variation of the heat capacity across the glass transition (ΔC p, in J g−1 °C−1) were calculated.
Freeze-drying microscopy
Solution collapse temperatures were determined by a freeze-drying microscopy (FDCS 196, Linkam Scientific Instruments, Surrey, U.K.) equipped with a liquid nitrogen cooling system and a programmable temperature controller (TMS94, Linkam). The stage was calibrated for temperature measurement with an aqueous solution of NaCl 0.9% (m/m) (T eut of approximately −21.1 °C). The samples were frozen at −50 °C (5 °C min−1) followed by a 5 min isotherm to ensure temperature stability and sample equilibration. During the drying step the system was evacuated to a pressure of 100 mTorr (Edwards vacuum pump E2M1.5) maintained by a Pirani pressure monitor gauge. The frozen sample was heated using a 2 °C min−1 heating rate. Direct observation of freezing and freeze-drying was done by a Nikon Elipse E600 (Nikon, Japan) polarized microscope using a 10× magnification.
Results
Differential scanning calorimetry
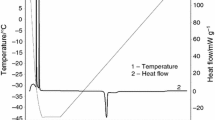
Figure 1 compares the influence of different crucible materials in the T eut of NaCl solution. The NaCl solution readily crystallizes during cooling of the frozen solution and is known to form a eutectic mixture from binary solutions. It was observed that NaCl solution T eut peak in glass (−19.96 °C) and quartz crucibles (−20.13 °C) decreased by 0.87 and 0.7 °C, respectively compared to the aluminum experiments (−20.83 °C). However, there was an almost 3-fold increase of enthalpic energy involved during the eutectic melting (ΔH eut) between the glass (ΔH eut = −10.81 J g−1) and quartz crucibles (ΔH eut = −9.84 J g−1) compared to the aluminum crucible (ΔH eut = −3.65 J g−1). This behavior could be explained due to the specific thermal conductivity differences between each crucible material (Table 1). Higher thermal conductivity (aluminum crucible) dissipated energy in shorter time did not allowing enough time to the sample response, it was confirmed by the difference of integrated area under each peak. On the other hand the crucibles (quartz and glass) that had presented lower thermal conductivity had enough time to transfer the energy to the sample increasing the integrated area under each peak (higher ΔH fusion). The same differences were observed during freezing crystallization on each sample and crucibles (data not shown). According to Willemer et al., differences on ΔH crystallization were observed studying the influence of different glass containers used during freezing in freeze-drying 1 and 5% mannitol and 10% sucrose solution. He stated that during freezing, the different forces between the walls and liquid influence the structure of the freezing product and it subcooling. The freezing speed in the quartz vials was up to 16% greater than standard vials (glass) but in the resin vials the freezing speed was 14% lower [10].
Trehalose, a non-reducing disaccharide that is retained amorphous in frozen aqueous solutions, was chosen as the model amorphous compound. There has been considerable attention to trehalose in the recent past owing to its ability to serve as a lyoprotectant. During cooling it tends to form a supersaturated freeze-concentrated solution due to incomplete crystallization of water. In this case, the lyophilization characteristics of the formulation are determined by the glass transition temperature of the maximally freeze-concentrate solute (T g′) [11].
Figure 2 shows the influence of different crucibles on the T g′ of a 5% trehalose solution. It is characterized by a discontinuity on the DSC baseline due to the change in heat capacity that occurs in the glass transition. Trehalose T g′ value (−30 °C) is in accordance with published works [12–14]. The T g′ was not influenced by the crucible material, however, a considerable difference in the specific heat capacity (ΔC p) between the crucible materials was observed during the experiments. A 4-fold increase on the ΔC p was observed for the quartz (ΔC p = 0.242 J g−1 °C−1) and glass (ΔC p = 0.275 J g−1 °C−1) compared to aluminum crucible (ΔC p = 0.069 J g−1 °C−1). This difference could be explained by the thermal conductivity and heat capacity specific for each crucible material.
Freeze-drying microscopy
The notion that T c is often considered as close to the T g′ (1–2 °C above the glass transition temperature) should be carefully considered. We observed that trehalose freeze-drying front collapsed around −25 °C, a 5 °C above the T g′ by DSC analysis (Fig. 3a). There are specific experiments using freeze-drying microscopy and DSC where differences of more than 4 °C between T c and T g′ were previous observed.
a Microscopic observations of freeze-drying 5% trehalose solution: (A) structure retention in the dried region (“not collapsed”) at –28 °C; (B) local loss of structure (“microcollapse”) at –25.2 °C; (C) overall loss of structure (“collapse”) at −23.9 °C. b Microscopic observations of freeze-drying 0.9% NaCl solution: (A) structure retention in the dried region (“not collapsed”) at –30 °C; (B) overall loss of structure (“collapse”) at –21 °C
The loss of structure for a system composed of a glassy state occurs at the freeze-drying front, and the remainder of the ice matrix retains its original structure. However when an eutectic is present in the interstitial region, the entire matrix undergoes a structural change when the sample temperature reaches the eutectic temperature. This was the case for the NaCl solution (Fig. 3b), where the T eut = −21 was closely related to the T eut determined by DSC (Fig. 2).
Discussion
DSC is a very useful tool to determine the critical temperatures (T g′ and T eut) during the lyophilization process. However DSC is performed on microliter volumes, with samples sealed in aluminum crucibles, whereas the solutions to be lyophilized are likely to be dosed in milliliter quantities in open glass vials. In this study, the thermal characterization of two distinct formulations was performed by DSC using three different crucible materials: aluminum, type I glass and quartz and the results were compared to thermal characterization trough freeze-drying microscopy. The results showed that T g′ and T eut were not influenced by the crucible material type. However, the ΔH involved in the eutectic melting and the ΔC p involved during the T g′ of the formulations were affected. This behavior could be explained by the differences in the specific heat capacity and thermal conductivity of each material used in this work. The aluminum crucible dissipated the energy involved in each thermal event faster than type I glass and quartz. During solution crystallization, it could influence the ice crystal size distribution and morphology. The aluminum crucible normally used in DSC thermal analysis does not represent the real values involved during the eutectic melting and the glass transition temperature occurred during the lyophilization of a formulation in glass vials.
According to the results obtained, both techniques, freeze-drying microscopy and DSC, showed good agreement between them, however more care should be taken regarding the notion that T c is often considered 1–2 °C above the T g′.
References
Liapis AI, Pikal MJ, Bruttini R. Research and development needs and opportunities in freeze drying. Dry Technol. 1996;14(6):1265–300.
Danch A. Some comments on nature of the structural relaxation and glass transition. J Therm Anal Calorim. 2008;91(3):733–6.
Levine H, Slade L. Thermomechanical properties of small-carbohydrate-water glasses and rubbers. J Chem Soc. 1988;1(84):2619–33.
Knopp SA, Nail SL. The relationship between the TMDSC curve of frozen sucrose solutions and collapse during freeze-drying. J Therm Anal Calorim. 1998;54:659–72.
Jenning TA. Lyophilization: Introduction and basic principles. Colorado, USA: Interpharm Press; 1999. p. 102–3.
Pikal MJ, Shah S. The collapse temperature in freeze-drying: dependence on measurement methodology and rate of water removal from the glassy state. Int J Pharm. 1990;62:165–86.
Danch A. The glass transition, finite size effect. J Therm Anal Calorim. 2006;84:663–8.
Cannon AJ, Trappler EH. The influence of lyophilization on the polymorphic behavior of mannitol. PDA J Pharm Sci Technol. 2000;54:13–22.
Young HD. Univers Phys. 8th ed. New York: Addison-Wesley; 1991. p.1356
Willemer H, Spallek M, Auchter-Krummel P, Heinz J. Freeze-drying. Georg-Wilhelm Oetjen and Peter Haseley, Wiley-Vch GmbH & Co.; 2004. p. 90.
Curtil A, Pegg DE, Wilson A. Freeze drying of cardiac valves in preparation for cellular repopulation. Cryobiology. 1997;34:13–22.
Miller DP, Pablo JJ, Corti H. Thermophysical properties of trehalose and its concentrated aqueous solutions. Pharm Res. 1997;14:578–90.
Mazur P. Cryobiology. In: Meryman HT, editor. Physical and chemical basis of injury in single-celled microorganisms subjected to freezing and thawing. London: Academic Press; 1966. pp 213–315.
Pyne A, Surana R, Suryanarayanan R. Enthalpic relaxation in frozen aqueous trehalose solutions. Thermochim Acta. 2003;405:225–34.
Acknowledgements
The authors are grateful to FAPESP (The State of Sao Paulo Research Foundation) and to CNPq (National Council for Scientific and Technological Development) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tattini, V., Parra, D.F. & de Moraes Pitombo, R.N. The influence of crucible material on the DSC thermal analysis compared to freeze-drying microscopy results. J Therm Anal Calorim 98, 203–206 (2009). https://doi.org/10.1007/s10973-009-0317-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0317-8