Abstract
The synthesized phases with chemical composition \(\left[ {{\text{M}}^{2 + }_{6} {\text{Al}}_{3} \left( {\text{OH}} \right)_{18} } \right]\left[ {{\text{A}}^{ + } \left( {{\text{SO}}_{4} } \right)_{2} } \right]12{\text{H}}_{2} {\text{O}}\) (M2+ = Mn, Mg, Zn; A+ = Li, Na or K) were evaluated in relation to their thermal behavior by thermogravimetric analysis (TGA), X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR). In the shigaite (M2+ = Mn), natroglaucocerinite (M2+ = Zn) and motukoreaite (M2+ = Mg) phases, the TGA measurements indicated that all samples were dehydrated up to 200 °C in two steps, followed by dehydroxylation above 300 °C. After the thermal treatment at 1000 °C, formation of oxides/spinels were observed for the shigaite and natroglaucocerinite phases, while for motukoreaite, oxides, spinels and MgSO4 were detected. XRD indicated a reduction in the basal distance from around 11 Å for the fully hydrated phases to around 7 Å for the dehydrated phases. The thermal treatments of some samples at 100 °C, 150 °C and 200 °C indicated that in all phases, intercalated sulfate and alkaline metal ions can be dehydrated and rehydrated. As indicated by FTIR, at 200 °C sulfate could be grafted to the layers and at 300 °C, for all the phases, a stable mixture of amorphous materials was obtained, which could not be rehydrated.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
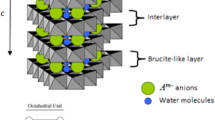
CdI2 structure occurs in a very high number of compounds, including hydroxides of divalent metals (M(OH)2 where M2+ = Mg, Ca, Mn, Fe, Co, Ni, Cd). In the structure of brucite (Mg(OH)2), Mg2+ cations are located in the center of slightly distorted octahedra surrounded by six hydroxide anions. When each octahedron Mg(OH)6 shares the edges, two-dimensional layers are produced, which are stacked in the basal direction and held together by weak bonds. Brucite-like layers are fundamental building blocks of a great variety of important hydrous phyllosilicates and layered double hydroxides (LDHs). In LDHs, some of the Mg2+ cations are isomorphically substituted by M3+ with similar cationic ratios, building positively charged layers, which need to be charge-compensated by anions. The general formula of LDH thus is \(\left[ {{\text{M}}^{2 + }_{{1 - {\text{x}}}} {\text{M}}^{3 + }_{\text{x}} \left( {\text{OH}} \right)_{2} } \right]\left( {{\text{A}}^{ - n} } \right)_{{{\text{x}}/{\text{n}}}} y{\text{H}}_{2} {\text{O}}\), where M2+ and M3+ are divalent and trivalent metal cations and \(\left( {{\text{A}}^{ - n} } \right)_{{{\text{x}}/{\text{n}}}} y{\text{H}}_{2} {\text{O}}\) is the hydrated anion. Since there is relative flexibility to formulate LDHs, the materials occur in the form of several minerals, and thousands of LDHs have been synthesized and used in scientific and industrial applications [1,2,3,4,5,6,7,8,9]. Several organic and inorganic anions have been intercalated between LDH layers, but sulfate is the least studied anion, due to some particularities like the interpolytypic transitions, formation of different phases upon hydration/dehydration and also co-intercalation of alkaline metals, especially sodium. The intercalated sulfate/sodium phases are very well known in minerals having the composition \(\left[ {{\text{M}}^{2 + }_{6} {\text{Al}}_{3} \left( {\text{OH}} \right)_{18} } \right]\left[ {{\text{A}}^{ + } \left( {{\text{SO}}_{4} } \right)_{2} } \right]12{\text{H}}_{2} {\text{O}}\), where M2+ = Mg, Zn, Mn and Fe, respectively, for the minerals motukoreaite [10], natroglaucocerinite [11], shigaite [12,13,14] and nikisherite [15]. Iron green rust \({\text{Na}}\left[ {{\text{Fe}}^{2 + }_{6} {\text{Fe}}^{3 + }_{3} \left( {{\text{SO}}_{4} } \right)_{2} \left( {\text{OH}} \right)_{18} } \right]12{\text{H}}_{2} {\text{O}}\) has also been reported to have similar structure [16, 17].
Several sulfate intercalated LDHs have also been synthesized, but the presence or absence of alkaline metals together with sulfate and the effect of water to stabilize the structure are still not totally elucidated [18,19,20,21,22,23]. Recently, our research group described the synthesis of analogues structures of some minerals containing sulfate and different alkaline metals [24], but the study of the dehydration/rehydration and thermal behavior of intercalated sulfate has not been reported. This is the objective of the present manuscript.
Experimental
All the samples were synthesized as described in a previous publication [24]. Briefly, aqueous solution of AOH 1.5 mol L−1 (A = Li+, Na+ or K+) prepared with Milli-Q water was slowly added to 100 mL of aqueous solutions containing analytical grade chemicals (more than 99% of purity) as M2+SO4 (M2+ = Mg, Zn, Mn), Al2(SO4)3 and A2SO4 (A = Li+, Na+ or K+), with molar ratios close to 6:3:1 (Table 1), and the reactions were performed at increasing pH in an automatic glass titration reactor operating at 90 °C, under N2 flow. The materials after precipitation were maintained at 90 °C for 120 h, centrifuged at 4000 rpm, and washed several times with Milli-Q water and dried at room temperature until constant weight.
After characterization, the samples were heated at 100, 150, 200 and 300 °C for 1 h in a muffle furnace and submitted to XRD and FTIR analysis. To investigate rehydrate of the samples after the XRD measurements, drops of water were added until the sample was totally wet and the samples were dried at room temperature for 24 h.
Thermogravimetric analysis (TGA) was performed with a PerkinElmer TGA 4000 under synthetic air atmosphere with a flow rate of 50 mL min−1. The samples in the form of powders were allocated in alumina crucibles and the curves obtained by a heating rate of 10 °C min−1.
The X-ray diffraction (XRD) patterns were obtained from the last samples dispersed in water after complete washing. These samples were placed in glass sample holders and after drying, they were gently pressed to avoid any diffraction peak displacements. The measurements were performed using a Shimadzu diffractometer (model XRD-6000) with CuKα radiation source of λ = 1.5418 Å, current of 30 mA, tension of 40 kV, dwell time of 2° min−1 and step of 0.02°.
Fourier-transform infrared (FTIR) spectra were obtained in the transmission mode with a Bio-Rad spectrometer (model FTS 3500 GX), in KBr disks using around 1% of each sample (w/w). A total of 32 scans were accumulated from 400 to 4000 cm−1, with the resolution of 2 cm−1.
For the quantification of the elements present in the samples, the solids were dissolved in an aqueous solution prepared with Milli-Q water, containing 1.0% v/v HNO3, and analyzed in duplicate with a Thermo Scientific simultaneous axial view ICP-OES spectrometer (model iCAP 6500). The data were treated with the ThermoiTEVA Analyst version 1.2.0.30 program, and an average values were used in the compounds’ formulations.
Results and discussion
In the TGA/DTG curves of Li-shigaite (Fig. 1A), Na-shigaite (Fig. 1B) and K-shigaite (Fig. 1C), the samples indicated two steps of water loss up to around 145–160 °C, due to different coordinated water molecules, attributed to sodium and sulfate. The TGA curve indicated at least three mass losses in the regions of 200–250 °C and 450–550 °C, attributed to the dehydroxylation of the layered structure. The last step at around 800–850 °C is attributed to the metal sulfate decomposition, release of SO3 and formation of the respective oxides/spinels. In the TGA/DTG curves of natroglaucocerinite (Fig. 1D–F), a similar profile was observed for the Li and K phases (Fig. 1D, F), but the mass losses in the range of 200–250 °C were shifted to higher temperatures for the K phase (Fig. 1F). The mass losses close to 460–570 °C observed in the shigaite phases were absent in the natroglaucocerinite and motukoreaite, due probably to the oxidation of Mn2+.
In motukoreaite (Fig. 1G–I), the thermal decomposition profile was very similar for all alkali metals, but the DTG peaks of the dehydration of the alkali metals (70, 74 and 77 °C for Li, Na and K) tent to grow in the series, as expected. The decomposition of sulfates in motukoreaite was shifted to higher temperatures in comparison with shigaite and natroglaucocerinite, making it hard to determine the experimental formula through TGA. To evaluate the composition of the solid residue, the samples were heated at 1000 °C for 30 min and the solids were recovered for the XRD analysis (Fig. 2).
In motukoreaite, MgSO4 was still present, as reported in the literature [25]. With the help of the TGA/DTG curves and the chemical compositions, as reported previously [24], the amount of water could be determined (Table 2). The experimental data based on the chemical analysis obtained in anhydrous basis were in good agreement with the expected formula (deviation around 1–2%).
If the ideal formulation of the minerals is considered as \(\left[ {{\text{M}}^{2 + }_{6} {\text{Al}}_{3} \left( {\text{OH}} \right)_{18} } \right]\left[ {{\text{A}}^{ + } \left( {{\text{SO}}_{4} } \right)_{2} } \right]12{\text{H}}_{2} {\text{O}}\), or in a reduced way \(\left[ {{\text{M}}^{2 + }_{0.667} {\text{Al}}_{0.333} \left( {\text{OH}} \right)_{2} } \right]\left[ {{\text{A}}^{ + }_{0.111} \left( {{\text{SO}}_{4} } \right)_{0.222} } \right] \, 1.333{\text{H}}_{2} {\text{O}}\), good correlations were observed between the theoretical and experimental formulas, including the water contents (Table 2).
In spite of these characteristics, the TGA/DTG curves are very similar to those reported in the literature for motukoreaite [26] and other LDHs intercalated with sulfate anions [25, 27,28,29]. This technique is frequently used to investigate the thermal stability of LDHs intercalated with different anions [30, 31].
Based on the temperatures of dehydration, the samples were heated at determined temperatures and the XRD (Fig. 3–6) and FTIR data were collected (Fig. 7).
The XRD diffraction patterns of Na-shigaite (Fig. 3), heated at 100 °C (Fig. 3b), 150 °C (Fig. 3c), 200 °C (Fig. 3d) and 300 °C (Fig. 3e), indicated that at 100 °C, the basal distance was maintained at 11.03 Å, while at 150 °C two different materials were obtained, both with very broad diffraction peaks, indicating very small crystal size and structural disorder. The first phase presented a basal distance of 11.4 Å and the second of 7.26 Å, the last attributed to the dehydrated state and sulfate grafted to the partially dehydroxylated layers. At 200 °C, the dehydrated phase predominated and even smaller basal distance was determined (6.8 Å). After placing a drop of water on the dehydrated samples and waiting 24 h to allow complete drying, the phase heated at 150 °C was totally recovered (Fig. 3f) and the phase heated at 200 °C was partially recovered, indicating the rehydration of the phase. At 300 °C, an amorphous material was obtained, indicating that at this temperature the LDH’s structure was collapsed by dehydroxylation. In spite of the reformation effect, this phase could also not be rehydrated due to the formation of stable amorphous sulfates (not shown).
In K-shigaite, the basal distance of the original phase was 11.28 Å and at 100 °C a mixture of the original material and a dehydrated material with a basal distance of 8.9 Å (Fig. 4b) was obtained. At 150 °C, the original material was present with a dehydrated phase having basal distance of 7.1 Å (Fig. 4c). At 200 °C, a disordered phase was obtained with a basal distance of 8.9 Å (Fig. 4d), while at 300 °C, an amorphous material was obtained (Fig. 4e). After hydration, the phase heated at 150 °C was totally recovered (Fig. 4f) and the phase heated at 200 °C (Fig. 4g) was also recovered but with broader diffraction peaks.
The possible explanation for the transformation of the grafted sulfate phase by hydration that occurs only in the K-shigaite, is the hydrolysis of the M–O–SO3 bonds to regenerate M–OH and SO−24 and consequently the basal distance of the hydrated sulfate.
In Li-natroglaucocerinite (basal distance of 11.15 Å) 100 °C, a phase with a basal distance of 10.1 Å was obtained (Fig. 5b). At 150 °C, a mixture of phases with a basal distances of 8.9 and 7.6 Å (Fig. 5c) was obtained. At 200 °C, a disordered phase was obtained with a basal distance of 7.2 Å (Fig. 5d), while at 300 °C, an amorphous material was obtained (Fig. 5e). After placing a drop of water on the heated samples waiting 24 h for drying, the phase heated at 150 °C was totally recovered (Fig. 5f) and the phase heated at 200 °C (Fig. 5g) was partially recovered, in the presence of a phase with basal distance of 7.3 Å.
In the case of K-motukoreaite (Fig. 6) with a basal distance of 10.87 Å, after heating at 100 and 200 °C (Fig. 6b, c), the basal distance is reduced to around 10.1 Å and after heating at 200 and 300 °C, the reduction is even higher going to around 7–7.5 Å (Fig. 6d, e).
After an attempt to rehydrate the phases heated at 150 °C and 200 °C, very broad diffraction peaks were observed close to 9 Å (Fig. 6 f, g), indicating that the original phase could be recovered. In fact, this phase has the structure irreversibly damaged even after heating at 100 °C.
Using the ideal formula \(\left[ {{\text{M}}^{2 + }_{6} {\text{Al}}_{3} \left( {\text{OH}} \right)_{18} } \right]\left[ {{\text{Na}}\left( {{\text{SO}}_{4} } \right)_{2} } \right]12{\text{H}}_{2} {\text{O}}\) as example, we can propose the decomposition profiles as indicated in Eq. 1.
*Mn3O4 for shigaite.
The FTIR spectra of Na-shigaite (Fig. 7A) revealed a broad band at 3440 cm−1, attributed to the stretching vibrations of the O–H bonds of the LDH structure and physisorbed/intercalated water molecules.
The sulfate bands can be seen at 1109 cm−1 (shoulder at 1144 and 1192 cm−1), attributed to the ν3 antisymmetric bending mode. The bands at 960 cm−1 and 600 cm−1 were also attributed, respectively, to ν1 and ν4 vibrations. Based on the splitting of the ν3 and also the active ν1 band, it can be concluded that sulfate is in a distorted octahedron due to the electrostatic effects and coordination with water molecules [32]. The bands in the region of 400 to 750 cm−1 are attributed to the M–O stretching vibrations, commonly observed in LDHs. After heating at 100 °C (Fig. 7A-b), in spite of the partial dehydration as indicated by TGA analysis (Fig. 1A) and the maintenance of the same basal distance as in the hydrated phase (Fig. 3a, b), the bands were observed almost in the same positions, indicating small structural changes. A possible explanation is the rehydration of the sample by exposure to humid air during the transport and analysis of the sample. When the sample was heated at 150 °C (Fig. 7A-c) and 200 °C (Fig. 7A-d), a change was observed, with further splitting of the ν3 band and disappearance of the ν1 band, with the appearance of a new band at 1049 cm−1.
This is a typical behavior of the change of sulfate symmetry from distorted tetrahedral to lower symmetry, attributed to the grafting of sulfate onto the partially dehydroxylated LDH layers [33,34,35]. According to Frost and co-workers, the reduction in symmetry is observed by the splitting of the ν3 and ν4 bands into two components in C3v symmetry and three components in C2v symmetry [35]. When the sample was heated at 300 °C (Fig. 7A-e), the majority of the M–O bands of the LDH were removed, but the broad sulfate bands were still present, now in the form of single metal sulfates (Fig. 7A-b), as also indicated by TGA (Fig. 1A). As already observed by XRD, the phase heated at 300 °C could not be rehydrated (Fig. 7A-h), due to the formation of stable amorphous sulfates.
Since the sample treated at 150 °C returned to the phase similar to 100 °C after contact with a drop of water and drying under air for 24 h (Fig. 7A-f), and the phase treated at 200 °C was partially recovered (the band at 960 cm−1 vanished (Fig. 7A-g)), it is likely that sulfate grafting to the layers occurs thorough cation-sulfate bond formation. The bonds occurs probaly with metals of the same layer rather than connecting two adjacent layers as sometimes suggested, which would attribute rigidity to the system, hindering the rehydration [33].
The FTIR spectra of K-shigaite phases (Fig. 7B) presented very similar behavior to that of Li-shigaite (Fig. 7A).
In the FTIR spectra of Li-natroglaucocerinite (Fig. 7C-a), contamination with carbonate was observed but was totally eliminated after the thermal treatment at 200 °C (Fig. 7C-d), close to the temperature reported for other LDHs intercalated with carbonate [36,37,38,39,40]. The small band present after hydration of the samples heated at 150 and 200 °C (Fig. 7C-f, g) indicated that probably carbonate was not intercalated but instead physisorbed to the LDH particles. The other samples behaved in a similar way as described for Na-shigaite (Fig. 7A) and K-shigaite (Fig. 7B).
In K-motukoreaite (Fig. 7D), behavior was observed similar to Li-natroglaucocerinite (Fig. 7C), but the samples after heating and after attempts to rehydrate present broad diffraction peaks indicating a great structural disorder as also indicated by X-ray diffraction (Fig. 6). As observed in Na-shigaite and K-shigaite, K-motukoreaite was free from carbonate.
Conclusions
The synthesized phases with a chemical composition \(\left[ {{\text{M}}^{2 + }_{6} {\text{Al}}_{3} \left( {\text{OH}} \right)_{18} } \right]\left[ {{\text{A}}^{ + } \left( {{\text{SO}}_{4} } \right)_{2} } \right]12{\text{H}}_{2} {\text{O}}\) (M2+ = Mn, Zn; A+ = Li, Na or K) (shigaite and natroglaucocerinite, respectively) were submitted to thermal treatment and presented several steps of dehydration, dehydroxylation of the layered double hydroxide structures and formation of oxides/spinels up to 1000 °C.
When the samples were heated at 100 °C, most of them had a very close basal distance to that of the fully hydrated phases. At 150 °C and 200 °C, the basal distances were reduced from ~ 11 to ~ 7 Å, with small fluctuations depending on the formulation, attributed to dehydration of intercalated sulfate and alkaline metals. After treatment at 200 °C, the sulfate was grafted to the partially dehydroxylated double hydroxide layers. All these samples could be partially or totally rehydrated (sometimes just by exposing the sample to the atmosphere at room temperature), except the samples heated at 300 °C, after which the material was decomposed irreversibly into a mixture of amorphous materials, which could not be rehydrated under the experimental conditions.
At 1000 °C, shigaite and natroglaucocerinite phases presented formation of oxides and cubic structure spinels, while in all \(\left[ {{\text{M}}^{2 + }_{6} {\text{Al}}_{3} \left( {\text{OH}} \right)_{18} } \right]\left[ {{\text{A}}^{ + } \left( {{\text{SO}}_{4} } \right)_{2} } \right]12{\text{H}}_{2} {\text{O}}\) (A+ = Li, Na or K) (motukoreaite) samples, the decomposition was incomplete at 1000 °C, as a mixture of MgO, MgAl2O4 and MgSO4 was detected.
References
Liu Y, Gao Y, Wang Q, Lin W. The synergistic effect of layered double hydroxides with other flame retardant additives for polymer nanocomposites: a critical review. Dalton Trans. 2018;47:14827–40.
Wu MJ, Wu JZ, Zhang J, Chen H, Zhou JZ, Qian GR, Xu ZP, Du Z, Rao QL. A review on fabricating heterostructures from layered double hydroxides for enhanced photocatalytic activities. Catal Sci Technol. 2018;8:1207–28.
Mishra G, Dash B, Pandey S. Layered double hydroxides: a brief review from fundamentals to application as evolving biomaterials. Appl Clay Sci. 2018;153:172–86.
He X, Qiu X, Hu C, Liu Y. Treatment of heavy metal ions in wastewater using layered double hydroxides: a review. J Dispers Sci Technol. 2018;39:792–801.
Baig N, Sajid M. Applications of layered double hydroxides based electrochemical sensors for determination of environmental pollutants: a review. Trends Environ Anal Chem. 2017;16:1–15.
Theiss FL, Ayoko GA, Frost RL. Synthesis of layered double hydroxides containing Mg2+, Zn2+, Ca2+ and Al3+ layer cations by co-precipitation methods—a review. Appl Surf Sci. 2016;383:200–13.
Mohapatra L, Parida K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J Mater Chem. 2016;4:10744–66.
Rives V, del Arco M, Martin C. Intercalation of drugs in layered double hydroxides and their controlled release: a review. Appl Clay Sci. 2014;88–89:239–69.
Nalawade P, Aware B, Kadam VJ, Hirlekar R. Layer double hydroxides: a review. J Sci Ind Res. 2009;68:267–72.
Rodgers KA, Chisholm JE, Davis RJ, Nelson CS. Motukoreaite, a new hydrated carbonate, sulfate, and hydroxide of magnesium and aluminum from Auckland, New Zealand. Miner Mag. 1977;41:389–90.
Witzke T, Pöllmann H, Vogel A. Struktur und synthese von [Zn8-xAlx(OH)16][(SO4)x/2+y/2.Nay(H2O)6]. Zeit Kristal. 1995;9:S252.
Cooper MA, Hawthorne FC. The crystal structure of Shigaite, [AlMn22+(OH)6]3(SO4)2.Na(H2O)6(H2O)6, a hydrotalcite-group mineral. Can Miner. 1996;34:91–7.
Peacor DR, Dunn PJ, Kato A, Wicks FJ. Shigaite, a new manganese aluminum sulfate mineral from the loi mine, Shiga, Japan. Neues Jahrb Miner Monatsh. 1985;1985:453–7.
Pring A, Slade PG, Birch WD. Shigaite from iron monarch, South Australia. Miner Mag. 1992;56:417–9.
Huminicki DMC, Hawthorne FC. The crystal structure of Nikischerite, NaFe62+Al3(SO4)2(OH)18.(H2O)12, a mineral of the Shigaite group. Can Miner. 2003;41:79–82.
Christiansen BC, Balic-Zunic T, Petit PO, Frandsen C, Mørup S, Geckeis H, Katerinopoulou A, SvaneStipp SL. Composition and structure of an iron-bearing, layered double hydroxide (LDH)—green rust sodium sulphate. Geochim Cosmochim Acta. 2009;73:3579–92.
Nickel EH, Wildman JE. Hydrohonessite—a new hydrated Ni–Fe hydroxy-sulphate mineral; Its relationship to honessite, carrboydite, and minerals of the Pyroaurite group. Miner Mag. 1981;44:333–7.
Miyata S, Okada A. Synthesis of hydrotalcite-like compounds and their physico-chemical properties—the systems Mg2+–Al3+–SO42− and Mg2+–Al3+–CrO42−. Clays Clay Miner. 1977;25:14–8.
El Malki K, de Roy A, Besse JP. New copper–chromium layered double hydroxide compound: discussion of pillaring with intercalated tetrahedral anions. Eur J Solid State Inorg Chem. 1989;26:339–51.
Khaldi M, de Roy A, Chaouch M, Besse JP. New varieties of zinc–chromium–sulfate lamellar double hydroxides. J Solid State Chem. 1997;130:66–73.
Radha S, Antonyraj CA, Kamath PV, Kannan S. Polytype transformations in the SO42−containing layered double hydroxides of zinc with aluminum and chromium: the metal hydroxide layer as a structural synthon. Z Anorg Allg Chem. 2010;636:2658–64.
Radha S, Kamat PV. Polytypism in sulfate-intercalated layered double hydroxides of Zn and M(III) (M = Al, Cr): observation of cation ordering in the metal hydroxide layers. Inorg Chem. 2013;52:4834–41.
Mostarih R, de Roy A. Thermal behavior of a zinc–chromium–sulfate lamellar double hydroxide revisited as a function of vacuum and moisture parameters. J Phys Chem Solids. 2006;67:1058–62.
Sotiles AR, Baika LM, Grassi MT, Wypych F. Cation exchange reactions in layered double hydroxides intercalated with sulfate and alkaline cations (A(H2O)6)[M+26 Al3(OH)18(SO4)2].6H2O (M+2 = Mn, Mg, Zn; A+= Li, Na, K). J Amer Chem Soc. 2019;141:531–40.
Kameda T, Yuki F, Toshiaki Y. Thermal decomposition of SO42− intercalated Mg–Al layered double hydroxide—elimination behavior of sulfur oxides. J Therm Anal Calorim. 2011;110:641–6.
Wachowiak J, Pieczka A. Motukoreaite from the Kłodawa Salt Dome, Central Poland. Miner Mag. 2016;80:277–89.
Zhang H, Wen X, Wang Y. Synthesis and characterization of sulfate and dodecylbenzenesulfonate intercalated zinc–iron layered double hydroxide by one-step coprecipitation route. J Solid State Chem. 2007;180:1636–47.
Chitrakar R, Sonoda A, Makita Y, Hirotsu T. Synthesis and bromate reduction of sulfate intercalated Fe(II)–Al(III) layered double hydroxides. Sep Purif Technol. 2011;80:652–7.
Theiss FL, Ayoko GA, Frost RL. Thermogravimetric analysis of selected layered double hydroxides. J Therm Anal Calorim. 2013;112:649–57.
Theiss FL, Palmer SJ, Ayoko GA, Frost RL. Sulfate intercalated layered double hydroxides prepared by the reformation effect. J Therm Anal Calorim. 2012;107:1123–8.
Vulic T, Reitzmann A, Ranogajec J, Marinkovic-Neducin R. The influence of synthesis method and Mg–Al–Fe content on the thermal stability of layered double hydroxides. J Therm Anal Calorim. 2012;110:227–33.
Peak D, Ford RG, Sparks DL. An in Situ ATR-FTIR investigation of sulfate bonding mechanisms on goethite. J Colloid Interface Sci. 1999;218:289–99.
Constantino VRL, Pinnavaia TJ. Basic Properties of Mg2+1-x Al3+x layered double hydroxides intercalated by carbonate, hydroxide, chloride, and sulfate anions. Inorg Chem. 1995;34:883–92.
Wright CMR, Ruengkajorn K, Kilpatrick AFR, Buffet JC, O’Hare D. Controlling the surface hydroxyl concentration by thermal treatment of layered double hydroxides. Inorg Chem. 2017;56:7842–50.
Frost RL, Theiss FL, López A, Scholz R. Vibrational spectroscopic study of the sulphate mineral glaucocerinite (Zn,Cu)10Al6(SO4)3(OH)32.18H2O—a natural layered double hydroxide. Spectrochim Acta A Mol Biomol Spectrosc. 2014;127:349–54.
Guimaraes JL, Marangoni R, Ramos LP, Wypych F. Covalent grafting of ethylene glycol into the Zn–Al–CO3 layered double hydroxide. J Colloid Interface Sci. 2000;227:445–51.
Elhalil A, Farnane M, Machrouhi A, Mahjoubi FZ, Elmoubarki R, Tounsadi H, Abdennouri M, Barka N. Effects of molar ratio and calcination temperature on the adsorption performance of Zn/Al layered double hydroxide nanoparticles in the removal of pharmaceutical pollutants. J Sci Adv Mater Devices. 2018;3:188–95.
Cheng X, Huang X, Wang X, Sun D. Influence of calcination on the adsorptive removal of phosphate by Zn–Al layered double hydroxides from excess sludge liquor. J Hazard Mater. 2010;177:516–23.
Lv L, He J, Wei M, Evans DG, Duan X. Factors influencing the removal of fluoride from aqueous solution by calcined Mg–Al–CO3 layered double hydroxides. J Hazard Mater. 2005;133:119–28.
Rani KM, Palanisamy PN. Synthesis and characterization of mesoporous, nanostructured zinc aluminium carbonate layered double hydroxides (ZAC-LDHs) and its calcined product (CZA-LDH). J Inorg Organomet Polym Mater. 2018;28:1127–35.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001, CNPq (projects 303846/2014-3, 400117/2016-9) and FINEP. Prof. Marcos Rogério Mafra performed the TGA analysis. ARS and NAGG received PhD scholarships from CAPES and CNPq, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sotiles, A.R., Gomez, N.G. & Wypych, F. Thermogravimetric analysis of layered double hydroxides intercalated with sulfate and alkaline cations [M62+Al3(OH)18][A+(SO4)2] 12H2O (M2+ = Mn, Mg, Zn; A+ = Li, Na, K). J Therm Anal Calorim 140, 1715–1723 (2020). https://doi.org/10.1007/s10973-019-08955-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08955-6