Abstract
The removal of the sulfate anion from water using synthetic hydrotalcite (Mg/Al LDH) was investigated using powder X-ray diffraction (XRD) and thermogravimetric analysis (TG). Synthetic hydrotalcite Mg6Al2(OH)16(CO3)·4H2O was prepared by the co-precipitation method from aluminum and magnesium chloride salts. The synthetic hydrotalcite was thermally activated to a maximum temperature of 380 °C. Samples of thermally activated hydrotalcite where then treated with aliquots of 1000 ppm sulfate solution. The resulting products where dried and characterized by XRD and TG. Powder XRD revealed that hydrotalcite had been successfully prepared and that the product obtained after treatment with sulfate solution also conformed well to the reference pattern of hydrotalcite. The d(003) spacing of all samples was found to be within the acceptable region for a LDH structure. TG revealed all products underwent a similar decomposition to that of hydrotalcite. It was possible to propose a reasonable mechanism for the thermal decomposition of a sulfate containing Mg/Al LDH. The similarities in the results may indicate that the reformed hydrotalcite may contain carbonate anion as well as sulfate. Further investigation is required to confirm this.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
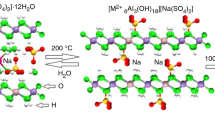
Layered double hydroxides (LHDs) are also known as hydrotalcite like materials or anionic clays. Many LDHs such as hydrotalcite, takovite, carrboydite, reevesite, honessite, pyroaurite, and iowaite occur in nature while others have been prepared synthetically in the laboratory [1–6]. LDHs are based on the brucite (Mg(OH)2) structure in which some divalent layer cations are replaced with trivalent cations (Al3+ in the case of hydrotalcite). The result of this substitution is a layered structure carrying a net positive charge. This positive charge is balanced by anions intercalated between the layers [7, 8]. LDHs follow the general formula:
where M2+ and M3+ are the divalent and trivalent layer cations respectively, 0.2 < x < 0.33 and An− is the exchangeable anion [8–11].
LDHs exhibit several unusual properties, the most interesting is known as the reformation effect. When a LDH is calcined usually at 300–500 °C interlayer water and anions as well as the hydroxyl groups are eliminated. The result is a mixed metal oxide [3, 12, 13]. This process is also known as thermal activation. When the calcined LDH is placed in solution containing anions, the LDH structure can re-form. Whether the original structure is formed depends on a number of factors, including the nature of the lattice cations and the calcination temperature [8, 14]. In this process water is adsorbed to form the hydroxyl layers, and anions present in the solution will be adsorbed into the interlayer to balance the positive charge of the layers [12]. The reformation effect already has been used to remove a range of anions from water [14–19]. In this article, we report on the removal of the sulfate anion from water under normal atmospheric conditions (not under an inert atmosphere) using synthetic hydrotalcite prepared by the co-precipitation method.
Experimental procedure
Preparation of Mg6Al2(OH)16(CO3)·4H2O
All reagents used in the experiments described in this article were AR grade. Sodium hydroxide and sodium sulfate where obtained from Ajax Finechem Pty Ltd. Magnesium chloride hexahydrate and aluminum chloride hexahydrate where obtained from Clem-Supply. Sodium carbonate was obtained from MERCK Pty Limited. All chemicals where used as received with no additional purification or pretreatment.
A large batch of Mg6Al2(OH)16(CO3)·4H2O (synthetic hydrotalcite) was prepared by the co-precipitation method for the use in this and other experiments. A mixed metal solution was prepared by dissolving aluminum chloride hexahydrate (603.6 g) and magnesium chloride hexahydrate (1524.7 g) in deionised water (10,000 cm3). The caustic solution was prepared by slowly dissolving sodium hydroxide (800.2 g) and sodium carbonate (1690.9 g) in deionised water (10,000 cm3). The mixed metal solution was added drop wise from a separating funnel to the caustic solution while stirring. The pH of the solution was monitored using a pH probe and found to average 12.2. Additional sodium hydroxide was added first in granular form then in a solution to keep the pH above 10.
As soon as the two solutions where combined the hydrotalcite formed as a white precipitate. The hydrotalcite was collected by vacuum filtration and washed with 0.1 M sodium carbonate solution (10,000 cm3). The hydrotalcite was collected and dried in an oven at approximately 80 °C. When the hydrotalcite was dry it was ground with a mortar and pestle and then homogenized by combining all the samples into a large glass dish and thoroughly mixed. The total mass of hydrotalcite obtained was 927.6 g.
Thermal activation and reformation
Approximately 150 g of the Mg/Al LDH was thermally activated in a furnace to a maximum temperature of 380 °C. The rate of heating averaged at 20 °C/min up to the set temperature, and was left at that temperature for 1 h. A 1000 ppm solution was prepared by dissolving sodium sulfate (2.2158 g) in deionised water (500 cm3). Two amounts of sample were used in this investigation, 3 and 10 g. The experiment was repeated to confirm the results.
Each sample was then treated with a 20 cm3 aliquot of the 1000 ppm sulfate solution. Each sample was then stirred for 30 min. The mixture was separated by vacuum filtration. The solids were recovered for further analysis and dried in an oven at approximately 80 °C for ~2 days. The filtrate was collected using a syringe filter (0.45 μm) to remove any remaining particulate material before further analysis.
Characterization of synthetic hydrotalcite
X-ray diffraction patterns were collected using a Philips X’pert wide angle X-Ray diffractometer, with Cu Ka radiation (1.54052 Å).
Thermal analysis was carried out using a TA® Instruments incorporated high resolution thermogravimetric analyser (series Q500) under a flowing nitrogen atmosphere (40 cm3/min). The sample (77.365 and 63.228 mg for the 1 and 10 g samples respectively) was placed in an open platinum pan and heated from room temperature at a rate of 2.50 °C/min to a maximum temperature of 1000 °C. TG and DTG curves where obtained however a mass spectrometer was not available for evolved gas analysis.
Results and discussion
Powder XRD
The Powder XRD results shown in Fig. 1 indicate that a LDH structure was successfully formed. The XRD pattern closely fits that of synthetic hydrotalcite which is the Mg/Al LDH. Sodium chloride was still present in the sample despite the washing process. There was also some indication that small amounts of sodium carbonate hydrate may be present in the precipitate. The d(003) spacing was found to be 7.81 Å, which was consistent with a LDH structure [8, 14].
Thermal activation caused a clear change in the XRD pattern as expected. The results are presented in Fig. 2. Comparison of pattern shown in Fig. 1 with that of the thermally activated LDH shown in Fig. 2 shows the LDH structure was lost with thermal activation and regenerated after treatment with sulfate solution. This observation confirms the reformation process of LDH structures. Some phases that may be present in the thermally activated hydrotalcite include NaCl, Na3Mg(CO3)2Cl, AlO(OH), and Na2Mg(CO3)2. The d(003) spacing was found to increase to 8.13 Å. After reformation in the sodium sulfate solution, the LDH structure was successfully reconstructed. Minimal change in the d(003) spacing was observed for the treated thermally activated hydrotalcites (7.83 and 7.76 Å for the 3 and 10 g samples, respectively) compared to the original hydrotalcite (7.81 Å). There did not appear to be any additional phases that formed after the reformation process compared to the original hydrotalcite sample. The peaks corresponding to sodium chloride where lower in intensity in the 3 g sample compared to the 10 g sample.
Thermal analysis
Thermogravimetric analysis was performed on samples of the hydrotalcite before thermal activation (BTA) and after the adsorption of sulfate. There are only a few possible units that could be lost from the sample as it undergoes decomposition; these include water, carbon dioxide, hydroxyl units and oxygen. As evolved mass spectrometry was not carried out it is impossible to conclusively identify any gases evolved, however as the mechanism of the thermal decomposition of hydrotalcite is described in the literature it is possible to propose a decomposition mechanism based on previous experiments [1, 9, 10, 20–27].
Six mass losses were observed during the decomposition of hydrotalcite BTA shown in Fig. 3. The first two peaks occurred at 39 and 69 °C and accounted for mass losses of 3.81 and 7.70% respectively. Due to the relatively low temperatures of these mass losses it is fairly safe to assume they correspond to weakly adsorbed water. The next mass loss at 170 °C accounted for 4.40% of the mass loss. This peak occurs at a high enough temperature for the loss of interlayer water but not high enough for the removal of the carbonate anions or hydroxyl groups.
The fourth mass loss was the largest occurring at 310 °C accounting for 20.44% of the mass loss. When the DTG curve was examined two overlapping features could be clearly distinguished one occurring at ~282 °C and a second larger feature at 310 °C. Mass losses at this temperature range can usually be attributed to loss of interlayer anions. In this case, carbonate (decarbonation) and the loss of hydroxyl groups (dehydroxylation) are proposed to have been removed at this decomposition step. These processes usually overlap and are usually the largest contributor to the mass loss in LDH structures.
A small mass loss occurred at 698 °C with 7.27% mass loss. The final mass loss at 871 °C with a percentage mass loss of 13.52 also appeared to have two overlapping features. The first larger feature occurred at 871 °C while a much smaller feature was observed at 939 °C. The temperature at which these mass losses occur is too high for dehydroxylation or decarbonation. They can most likely be attributed to decomposition of the metal oxide into a spinel phase resulting in the loss of oxygen. The following mechanism can be proposed for the thermal decomposition of the synthetic hydrotalcite [1, 10, 22–24].
The first step in the thermal decomposition involves removal of weakly adsorbed water (up to 100 °C).
The next step is elimination of interlayer water (100–200 °C).
Dehydroxylation and decarbonation then appear to occur simultaneously (200–400 °C). The hydroxyl and carbonate groups are lost as water and carbon dioxide, leaving a magnesium/aluminum oxide mixture.
Decomposition of the mixed metal oxide at temperatures above 400 °C into a spinel phase resulting in a loss of oxygen.
The TG curve of the 3 g of reformed hydrotalcite by treatment in a 1000 ppm sulfate solution is shown in Fig. 4. Three mass losses where were observed. The first mass loss occurred at ~125 °C accounting for 14.53% mass loss. A broad feature is observed in the DTG curve indicating the mass loss is likely due to loss of adsorbed water and interlayer water. The next mass loss occurred at approximately 352 °C and accounted for 28.47% mass loss. The increase in decomposition temperature, compared to BTA, may indicate that sulfate anions have been successfully intercalated. The carbonate only hydrotalcite had a decomposition temperature of only 310 °C. It is also possible that the increase in decomposition temperature may result from the reformed hydrotalcite having a different structure to that of the original. Once again the DTG curve showed two peaks overlapping at 316 and 352 °C. This mass loss was attributed to loss of interlayer anions (carbonate and sulfate) and dehydroxylation (hydroxide). The final mass loss of 3.13% occurred between 632 and 1000 °C. Two small broad peaks where observed in the DTG curve in this region.
Only four mass loss steps where observed in the TG curve of the 10 g sample of reformed hydrotalcite, the results are presented in Fig. 5. The first mass loss at 171 °C can be attributed to the loss of water. The DTG curve shows a broad feature that can likely be attributed to the loss of adsorbed water while the sharp peak at 171 °C is most likely due to the loss of interlayer water. The second and largest mass loss occurred at 319 °C accounting for 26.77%. A small peak at 368 °C was observed in the DTG curve overlapping with the larger peak at 319 °C. Comparison of this DTG curve with the DTG curve of the hydrotalcite BTA suggests that the small peak at 368 °C represents the loss of intercalated sulfate anions, while the larger peak at 319 °C is associated with the loss of intercalated carbonate anions. This mass loss is attributed to loss of interlayer anions (carbonate and sulfate) and loss of hydroxyl groups. The next mass loss at 706 °C was the smallest with a percent mass loss of only 2.52%. The final mass loss occurred at 841 °C with a percent mass loss of 5.46%. A similar mechanism can be proposed for the decomposition of the sulfate intercalated LDH.
Again the first step in the thermal decomposition is the removal of weakly adsorbed water from the surface.
The next step involves the removal of interlayer water.
The next step involves elimination of the hydroxyl groups as water. Interlayer anions (carbonate and sulfate) will be eliminated in this step most likely as sulfur dioxide and carbon dioxide resulting in the formation of the same mixed metal oxide observed for BTA.
Finally, decomposition of the metal oxide can result in the formation of a spinel phase.
Conclusions
Powder XRD indicated the d(003) spacing was within the accepted range for a LDH structure. Thermal activation was found to cause a small increase in the d(003) spacing (0.32 Å). Reformation with sulfate anions caused the d(003) spacing to resemble the d(003) of the original hydrotalcite. It is possible that the majority of sulfate anions are adsorbed on the surface of the regenerated hydrotalcite, while carbonate and a small amount of sulfate has been intercalated into the structure however further investigation is required to confirm this. When the powder XRD patterns were compared to the reference pattern of hydrotalcite a number of characteristic peaks where observed. Several peaks in the powder XRD pattern could be attributed to the presence of sodium chloride. This was not unexpected as it was the main byproduct of the synthesis method used. There results indicate that synthetic Mg/Al hydrotalcite was successfully prepared.
Comparison of the dehydroxylation and decarbonation temperature indicated that the reformed LDH has intercalated sulfate anions. This is shown by an increase in decomposition temperature of the peak between 300 and 400 °C. The carbonate only hydrotalcite had a decomposition temperature of 310 °C, while the reformed hydrotalcites from sulfate solutions had a decomposition temperature of 352 and 368 °C. All hydrotalcite samples appear to undergo decomposition through a similar mechanism. The similarity of the powder XRD and TG results may indicate that a mixed LDH containing both sulfate and carbonate anions was prepared. The intercalation of carbonate in the reformed hydrotalcites is possible as no attempts where made to prevent the intercalation of carbonate which LDHs have a high affinity for. Further investigation is required to determine the exact composition of the hydrotalcite interlayer.
References
Bouzaid J, Frost RL. Thermal decomposition of stichite. J Therm Anal Calorim. 2007;89(1):133–5.
Frost RL, Erickson KL. Decomposition of the synthetic hydrotalcites mountkeithite and honessite-a high resolution thermogravimetric analysis and infrared emission spectroscopic study. Thermochim Acta. 2004;421(1–2):51–8. doi:10.1016/j.tca.2004.04.008.
Frost RL, Palmer SJ, Grand L-M. Synthesis and thermal analysis of indium-based hydrotalcites of formula Mg6In2(CO3)(OH)16·4H2O. J Therm Anal Calorim. 2010;101(3):859–63.
Bakon KH, Palmer SJ, Frost RL. Thermal analysis of synthetic reevesite and cobalt substituted reevesite (Ni, Co)6Fe(OH)16(CO3)·4H2O. J Therm Anal Calorim. 2010;100(1):125–31.
Grand L-M, Palmer SJ, Frost RL. Synthesis and thermal stability of hydrotalcites containing gallium. J Therm Anal Calorim. 2009;. doi:10.1007/s10973-009-0456-y.
Grand L-M, Palmer SJ, Frost RL. Synthesis and thermal stability of hydrotalcites containing manganese. J Therm Anal Calorim. 2009;. doi:10.1007/s10973-009-0402-z.
Pesic L, Salipurovic S, Markovic V, Vucelic D, Kagunya W, Jones W. Thermal characteristics of a synthetic hydrotalcite-like material. J Mater Chem. 1992;2(10):1069–73. doi:10.1039/jm9920201069.
Rives V. Characterisation of layered double hydroxides and their decomposition products. Mater Chem Phys. 2002;75:19–25.
Frost RL, Palmer SJ, Spratt HJ. Thermal decomposition of hydrotalcites with variable cationic ratios. J Therm Anal Calorim. 2009;95(1):123–9.
Frost RL, Bouzaid JM, Martens WN. Thermal decomposition of the composite hydrotalcites of iowaite and woodallite. J Therm Anal Calorim. 2007;89(2):511–9.
Węgrzyn A, Rafalska-Łasocha A, Majda D, Dziembaj R, Papp H. The influence of mixed anionic composition of Mg–Al hydrotalcites on the thermal decomposition mechanism based on in situ study. J Therm Anal Calorim. 2010;99(2):443–57.
Erickson KL, Bostrom TE, Frost RL. A study of structural memory effects in synthetic hydrotalcites using environmental SEM. Mater Lett. 2004;59(2–3):226–9. doi:10.1016/j.matlet.2004.08.035.
Grand L-M, Palmer SJ, Frost RL. Synthesis and thermal stability of hydrotalcites based upon gallium. J Therm Anal Calorim. 2010;101(1):195–8. doi:10.1007/s10973-009-0456-y.
Rives V, editor. Layered double hydroxides: present and future. New York: Nova Science Pub Inc; 2001.
Das DP, Das J, Parida K. Physicochemical characterization and adsorption behavior of calcined Zn/Al hydrotalcite-like compound (HTlc) towards removal of fluoride from aqueous solution. J Colloid Interface Sci. 2002;261:213–20.
Liu R, Frost RL, Martens WN. Absorption of the selenite anion from aqueous solutions by thermally activated layered double hydroxide. Water Res. 2009;43(5):1323–9. doi:10.1016/j.watres.2008.12.030.
Liang L, Li L. Adsorption behavior of calcined layered double hydroxides towards removal of iodide contaminants. J Radioanal Nucl Chem. 2006;273(1):221–6.
Palmer SJ, Frost RL. Use of Hydrotalcites for the Removal of Toxic Anions from Aqueous Solutions. Ind Eng Chem Res. 2010;49(19):8969–76. doi:10.1021/ie101104r.
Frost RL, Musumeci AW. Nitrate absorption through hydrotalcite reformation. J Colloid Interface Sci. 2006;302(1):203–6. doi:10.1016/j.jcis.2006.06.024.
Frost R, Bouzaid J, Martens W, Kloprogge T. Thermal decomposition of the synthetic hydrotalcite woodallite. J Therm Anal Calorim. 2006;86(2):437–41.
Frost RL, Adebajo MO, Erickson KL. Raman spectroscopy of synthetic and natural iowaite. Spectrochim Acta A. 2005;61A(4):613–20. doi:10.1016/j.saa.2004.05.015.
Frost RL, Bouzaid JM, Musumeci AW, Kloprogge JT, Martens WN. Thermal decomposition of the synthetic hydrotalcite iowaite. J Therm Anal Calorim. 2006;86:437–41.
Frost RL, Erickson KL. Thermal decomposition of natural iowaite. J Therm Anal Calorim. 2004;78(2):367–73.
Frost RL, Martens WN, Erickson KL. Thermal decomposition of the hydrotalcite. J Therm Anal Calorim. 2005;82:603–8.
Frost RL, Palmer SJ, Nguyen TM. Thermal decomposition of hydrotalcite with molybdate and vandate anions in the interlayer. J Therm Anal Calorim. 2008;92(3):879–86.
Spratt HJ, Palmer SJ, Frost RL. Thermal decomposition of synthesised layered double hydroxides based upon Mg/(Fe, Cr) and carbonate. Thermochim Acta. 2008;479(1–2):1–6. doi:10.1016/j.tca.2008.08.016.
Lin Y-H, Adebajo MO, Frost RL, Kloprogge JT. Thermogravimetric analysis of hydrotalcites based on the takovite formula NixZn6-xAl2(OH)16(CO3)·4H2O. J Therm Anal Calorim. 2005;81:83–9.
Acknowledgements
The financial and infra-structure support of the Chemistry Discipline of the Faculty of Science and Technology, Queensland University of Technology is gratefully acknowledged. The Australian Research Council (ARC) is thanked for funding the instrumentation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Theiss, F.L., Palmer, S.J., Ayoko, G.A. et al. Sulfate intercalated layered double hydroxides prepared by the reformation effect. J Therm Anal Calorim 107, 1123–1128 (2012). https://doi.org/10.1007/s10973-011-1369-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1369-0