Abstract
The molar heat capacities, C P, of 1-ethyl-3-methylimidazolium tetrafluoroborate (1) + pyrrolidin-2-one or 1-methylpyrrolidin-2-one (2) + cyclopentanone or cyclohexanone (3) ternary mixtures have been measured at 293.15, 298.15, 303.15 and 308.15 K and 0.1 MPa using micro-differential scanning calorimeter. The observed C P data have been utilized to evaluate their excess heat capacities, \( (C_{\text{P}}^{\text{E}} )_{123} \) values, and same have been fitted to Redlich–Kister equation to predict ternary adjustable parameters along with their standard deviations. The Moelywn-Huggins concept (Huggins in Polymer 12:389–399, 1971) of interactions between the surfaces of constituent molecules in binary mixtures has been extended to ternary mixtures using topology of the constituent molecules to obtain expression (Graph theory) that predict correctly the \( (C_{\text{P}}^{\text{E}} )_{123} \) data of the present mixtures. The observed \( (C_{\text{P}}^{\text{E}} )_{123} \) data have also been analyzed in terms of modified Flory’s theory.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As challenge emerged in the burgeoning chemical industries and environmental pollution due to use of volatile organic solvents, researchers were confined in a box what they could and could not do. They explored ways to use new compounds like ionic liquids in place of traditional volatile and corrosive organic liquids to minimize their use. Creating new thermodynamic data on liquid mixtures containing ionic liquid due to their unique properties [1, 2] will foster new opportunities for their use in chemical industries. Heat capacity of liquid or liquid mixtures is one of the properties for use in thermal applications. Accurate heat capacity and excess heat capacity data of liquid/liquid mixtures are crucial (i) for the design and operation of heat transfer processes [3, 4] and (ii) for units such as fractionation tower used to separate mixture [5]. The sensitivity of tower’s segment is determined by an energy balance which in turn involves liquid/liquid mixture enthalpy or heat capacity data. The use of ionic liquids due to their unique properties especially low volatility or their mixtures with organic liquids can be used as alternative sources in designing of equipments or environmental treatment technologies [6–9]. Ionic liquids having imidazolium cations are considered to be efficient ionic liquids for high absorption of carbon dioxide, diesel extractive desulfurization (EDS), oxidative desulfurization (ODS) and catalytic oxidative desulfurization (ECODS) and seem to be a good alternative for diesel desulfurization [10–14]. Further, several investigations on imidazolium-based ionic liquids possessing tetrafluoroborate anion have been carried out for their use in capacitors, solar cells, fuel cells and batteries [15–20]. Pyrrolidin-2-one and 1-methyl pyrrolidin-2-one have potential to be used in the solvent extraction process for separating polar substances from nonpolar substances, petrochemicals and biological applications [21, 22]. Cyclopentanone and cyclohexanone are used as safety solvents and important intermediates for the synthesis of many organic compounds which are used in chemical, pharmaceutical and cosmetic industries [22–25]. Liquid mixtures consisting of 1-ethyl-3-methylimidazolium tetrafluoroborate, pyrrolidin-2-one, 1-methyl pyrrolidin-2-one, cyclopentanone and cyclohexanone may, therefore, comprise a class of mixtures of importance in chemical, pharmaceutical and biological industries. In continuation of our earlier studies on thermodynamic properties of binary/ternary liquid mixtures containing 1-ethyl-3-methylimidazolium tetrafluoroborate as one of the component [26–29], we report here excess heat capacity, \( (C_{\text{P}}^{\text{E}} )_{123} \), data of 1-ethyl-3-methylimidazolium tetrafluoroborate (1) + pyrrolidin-2-one or 1-methyl pyrrolidin-2-one (2) + cyclopentanone or cyclohexanone (3) ternary mixtures.
Experimental
1-Ethyl-3-methylimidazolium tetrafluoroborate [emim][BF4] (mass fraction: 0.980) was used without further purification. The water content in ionic liquid was regularly checked using Karl Fischer titration [30] and found to be less than 320 ppm. Pyrrolidin-2-one (2-Py) (mass fraction: 0.994) was purified by vacuum distillation over calcium oxide [31], 1-methyl pyrrolidin-2-one (NMP) (mass fraction: 0.992) was purified by fractional distillation under reduced pressure [32], and cyclopentanone (Fluka, mass fraction: 0.991) and cyclohexanone (Fluka, mass fraction 0.988) were purified by fractional distillation [33]. The source of liquids, along with methods of purification and final purity, is reported in Table 1. Densities, ρ, speeds of sound, u, and heat capacities, C P, of the pure liquids at studied temperatures are listed in Table 2 and also compared with literature values [23, 25, 33–53]. The ρ and u values of the purified liquids were measured using a density and sound analyzer apparatus (Anton Paar DSA 5000) in the manner as described elsewhere [54, 55]. The uncertainties in the density and speed of sound measurements are ±0.5 kg m−3 and 0.1 m s−1, respectively. Further, uncertainty in the temperature measurement is ±0.01 K.
The molar heat capacities, C P, of pure liquids and the present mixtures were measured by differential scanning calorimeter Micro DSC (Model—μDSC 7 Evo) manufactured by M/S SETARAM instrumentation, France, as described elsewhere [56]. The calibration of equipment was done by Joule effect method which in turn was controlled by SETARAM software. The joule effect calibration was checked by measuring heat of fusion of naphthalene (147.78 J g−1) [57]. The heat capacity of a liquid or their mixtures was measured in a standard batch cell (Hastalloy C276) composed of a cylinder of 6.4 mm of internal diameter and 19.5 mm height and had a capacity of containing 1 cm3 of a liquid. The reference experimental cell was filled with water (equivalent to the mass of liquid in a standard batch cell). The mole fraction of each mixture was made by measuring the masses of the components of mixtures in airtight glass bottles using an electric balance (Mettler AX-205 Delta) with an uncertainty of ±10−5 g. For a scanning sequence, the initial and final temperatures were supplied along with heating rate of 0.4 K min−1. The temperature cycle and scanning rate of isothermal level were maintained by software provided by SETARAM instrumentation. The uncertainty in measured heat capacity, C P, values is 0.3 %.The uncertainty in mole fraction is 1 × 10−4. The uncertainty in the temperature measurement is ±0.02 K.
Results
The molar heat capacities, C P, of [emim][BF4] (1) + 2-Py or NMP (2) + cyclopentanone or cyclohexanone (3) ternary mixtures are listed in Table 3. The excess heat capacities, \( (C_{\text{P}}^{\text{E}} )_{123} \), for (1 + 2 + 3) mixtures were calculated by Eq. 1
where (C P), (C P)i (i = 1 or 2 or 3) and x i (i = 1 or 2 or 3) denote molar heat capacity of the ternary mixtures, molar heat capacity and mole fraction of pure components, respectively. Such \( (C_{\text{P}}^{\text{E}} )_{123} \) values for the present (1 + 2 + 3) mixtures are listed in Table 3. The \( \left( {C_{\text{P}}^{\text{E}} } \right)_{123} \) were fitted to Redlich–Kister [58] equation
where \( (C_{\text{P}} )_{123}^{(\text{n})} (n = 0 - 2) \), etc., are characteristic parameters of binaries (1 + 2), (2 + 3) and (1 + 3) of (1 + 2 + 3) mixtures and were taken from literature [59–61]. The \( (C_{\text{P}} )_{123}^{(\text{n})} (n = 0 - 2) \), etc., are ternary adjustable parameters of the (1 + 2 + 3) ternary mixtures and were calculated by least-square optimization of these parameters. Such parameters along with standard deviations, \( \sigma (C_{\text{P}}^{\text{E}} )_{123} \), defined by Eq. 3
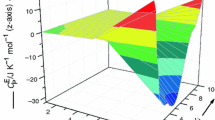
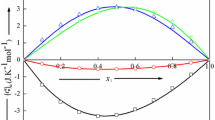
(where m is the number of data points and n is the number of adjustable parameters of Eq. 2) are listed in supporting Table 1S. Various surfaces generated by \( (C_{\text{P}}^{\text{E}} )_{123} \) data are shown in Figs. 1–4. In Fig. 1, \( (C_{\text{P}}^{\text{E}} )_{123} \) values (corresponding to 1–2 axis) were obtained by keeping x 3 constant and varying the values of x 1 and x 2 (shown as green line); \( (C_{\text{P}}^{\text{E}} )_{123} \) values (corresponding to 1–3 axis) were obtained by keeping x 2 constant and varying the values x 1 and x 3 (shown as red line).
Discussion
The excess heat capacities, \( (C_{\text{P}}^{\text{E}} )_{123} \), of the [emim][BF4] (1) + NMP or 2-Py (2) + cyclopentanone or cyclohexanone (3) mixtures are not available in literature for comparison with measured results. The \( (C_{\text{P}}^{\text{E}} )_{123} \) of the present (1 + 2 + 3) mixtures are positive over entire mole fraction of (1) and (2). The positive \( (C_{\text{P}}^{\text{E}} )_{123} \) values suggest that capability of cyclohexanone or cyclopentanone molecules to build a non-random structure in mixed state (due to interactions with [emim][BF4]:NMP or 2-Py molecular entities) is superior to the effect caused by disruption of associated NMP or 2-Py entities, and interactions between [emim][BF4]:NMP or 2-Py molecular entities [37]. The \( (C_{\text{P}}^{\text{E}} )_{123} \) values of [emim][BF4] (1) + NMP or 2-Py (2) + cyclohexanone (3) mixtures are higher than those of [emim][BF4] (1) + NMP or 2-Py (2) + cyclopentanone (3) mixtures. It may be due to reason that cyclohexanone is more basic in character than cyclopentanone [62] and also possesses chair form with almost no strain. Thus, cyclohexanone will give strong interactions and more compact structure with [emim][BF4]: NMP or 2-Py molecular entities as compared to cyclopentanone. Higher\( (C_{\text{P}}^{\text{E}} )_{123} \) for [emim][BF4] (1) + 2-Py (2) + cyclopentanone or cyclohexanone (3) mixtures than those for [emim][BF4] (1) + NMP (2) + cyclopentanone or cyclohexanone (3) mixtures may be due to lesser mixing of 2-Py with [emim][BF4] and cyclohexanone because of the preference of 2-Py molecules to hydrogen bond with themselves. The \( \left( {\frac{{\partial C_{\text{P}}^{\text{E}} }}{\partial T}} \right) \) for [emim][BF4] (1) + 2-Py (2) + cyclopentanone or cyclohexanone (3) mixtures are positive which in turn suggest strong interactions occurring in mixed state due to disruption of self-associated entities of 2-Py and ion–dipole interactions in [emim][BF4]. However, \( \left( {\frac{{\partial C_{\text{P}}^{\text{E}} }}{\partial T}} \right) \) for [emim][BF4] (1) + NMP (2) + cyclopentanone or cyclohexanone (3) mixtures are negative. Decreasing \( (C_{\text{P}}^{\text{E}} )_{123} \) values with increasing temperature can be associated with decrease of molecular interactions between like molecules compared with unlike molecules in mixed state.
The \( (C_{\text{P}}^{\text{E}} )_{123} \) data were next analyzed in terms of Graph and Flory’s theories.
Graph theory
Excess heat capacities of ternary mixtures
The analysis of excess molar volumes, V E, excess isentropic compressibilities, \( \kappa_{\text{S}}^{\text{E}} \), excess molar enthalpies, H E, and excess heat capacities, \( C_{\text{P}}^{\text{E}} \), and IR studies of [emim][BF4] (1) + 2-Py or NMP or cyclopentanone or cyclohexanone (2); 2-Py or NMP (1) + cyclopentanone or cyclohexanone (2) mixtures [37–39, 63] have shown that (1) [emim][BF4] exists as monomer; (2) 2-Py or NMP exists as associated molecular entities; and (3) cyclopentanone or cyclohexanone is characterized by dipole–dipole interactions. The [emim][BF4] (1) + 2-Py or NMP (2) + cyclopentanone or cyclohexanone (3) mixtures can, therefore, be assumed to involve processes; (a) establishment of unlike (i) 1 − 2n (n = 2), (ii) 2n − 3n (n = 2) and (iii) 1 − 3n (n = 2) contacts; (b) unlike contact formation between the constituent molecules ruptures self-association or dipole–dipole interactions with (i) 2n and (ii) 3n molecules to form 2 and 3 molecules and enhances the randomness in mixed state; (c) 1, 2 and 3 constituent molecules undergo interactions to form (i) 1:2, (ii) 2:3 and (iii) 1:3 molecular complexes, which in turn leads to non-randomness in mixed state as compared to pure state. If χ 12, χ 23, χ 13; χ 22, \( \chi_{{\text{33}}} \) and \( \chi_{12}^{{\prime }} ,\chi_{12}^{{\prime \prime }} ,\chi_{12}^{{{\prime \prime \prime }}} \) are molar interaction energy parameters for 1 − 2n, 2n − 3n, 1 − 3n unlike contacts, respectively, rupture of associated molecular entities 2n and 3n, and molecular interactions between 1, 2 and 3 constituent molecules to yield randomness and non-randomness, respectively, in mixed state, then change in thermodynamic property, \( \left( {\Delta C_{\bf{P}} } \right) \), due to processes (a) (i–iii), (b) (i–ii) and (c) (i–iii) were given [56, 64–69] by relation:
As \( v_{ 2} /v_{ 1} = ^{ 3} \xi_{ 1} /^{ 3} \xi_{ 2} \), where (\( ^{3} \xi_{i} \)), (\( ^{3} \xi_{i} \))m (i = 1 or 2 or 3), etc., are connectivity parameters of third degree of molecules in pure as well as mixed state and are defined [70] by
The \( \delta_{\text{m}}^{\nu } \), etc., values reflect the valency of the atoms forming the bond and are expressed as [71] \( \delta^{v} \) = Z m − h, where Z m is the maximum valency of the atom and h is the number of hydrogen atom attached to it. The \( ^{3} \xi \) values for the constituent molecules were taken from literature [37, 61, 63] consequently; Eq. 4 was reduced to
For the present mixtures, we assumed that \( \chi_{ 1 2} \cong \chi^{\prime}_{ 1 2} = \chi_{12}^{*} ;\chi_{ 2 3} \cong \chi^{\prime\prime}_{12} = \chi_{ 2 3}^{*} ;\chi_{ 1 3} \cong \chi^{\prime\prime\prime}_{12} = \chi_{13}^{*} \) and χ 22 ≅ χ 33 = χ * and then Eq. 6 was reduced to
Equation 7 contains four unknown χ *12 , χ *23 , χ *13 and χ *parameters. These parameters were commuted utilizing \( (C_{\text{P}}^{\text{E}} )_{123} \) data at four arbitrary compositions and then subsequently used to predict \( (C_{\text{P}}^{\text{E}} )_{123} \) data at other values of x 1 and x 2. Such \( (C_{\text{P}}^{\text{E}} )_{123} \) values are listed in Table 3 and also compared with their corresponding experimental values. The χ *12 , χ *23 , χ *13 and χ * parameters and mean deviations between \( (C_{\text{P}}^{\text{E}} )_{123} \) values and \( (C_{\text{P}}^{\text{E}} )_{123} \) values calculated by Graph theory, \( \sigma (C_{\text{P}}^{\text{E}} )_{{123\;{\text{Graph}}}} \), are also reported in supporting Table 2S. Perusal of data in Table 3 indicates that \( (C_{\text{P}}^{\text{E}} )_{123} \) values determined by Graph theory are in agreement with experimental data which in turn support various assumptions in deriving Eq. 7.
Flory’s theory
Differentiating Flory’s expression for excess molar enthalpies [72, 73] for binary and ternary mixtures with respect to the temperature, T, and excess heat capacities, \( (C_{\text{P}}^{\text{E}} )_{123} \), for ternary mixtures was expressed by
where \( \tilde{v}_{\text{i}}^{*} \),\( P_{\text{i}}^{*} \) and \( \tilde{v}_{\text{i}}^{{}} \)(i = 1 or 2 or 3) are the characteristic volume, characteristic pressure and reduced volume of pure component (i) and \( \tilde{v} \) is reduced volume of mixture and all the terms have the same significance as described elsewhere [72, 73]. The Flory parameters for the liquids under investigations are taken from literature [61, 63]. Flory assumed that interaction energy parameters, \( \chi_{12}^{**} \), etc., for sub-binaries of (1 + 2 + 3) ternary mixtures, which in turn are evaluated by using their H E data at equimolar composition, were assumed to be independent of temperature by Flory. However, Benson and D’ Arcy [74] assumed that \( \chi_{12}^{**} \), etc., parameters for binary mixtures should be a function of temperature. Consequently, \( (C_{\text{P}}^{\text{E}} )_{123} \) values for ternary mixtures were then expressed by relation
The reduced volumes, \( \tilde{v} \), and thermal coefficient, α, of ternary mixtures were calculated using
where \( V_{{\hbox{123}}}^{\hbox{E}} \) represent excess molar volumes of ternary (1 + 2 + 3) mixtures. Such \( \chi_{12}^{**} \), etc., values for the various binaries were calculated using H E value at equimolar composition that was taken from literature [37, 61, 63]. The calculated \( \left( {C_{\text{P}}^{\text{E}} } \right)_{123} \) values via Eqs. 8–11 for the present mixtures are compared with experimental values and presented in Table 3. The values of \( \chi_{12}^{**} \), etc., are recorded in supporting Table 2S. Examination of data in Table 3 indicates that Flory’s theory correctly predicts the sign of \( \left( {C_{\text{P}}^{\text{E}} } \right)_{123} \) values. However, quantitative agreement is poor. The failure of theory to correctly predict the sign of \( \left( {C_{\text{P}}^{\text{E}} } \right)_{123} \) may be due to strong interactions between unlike molecules.
Conclusions
The excess heat capacity, \( \left( {C_{\text{P}}^{\text{E}} } \right)_{123} \), values of the 1-ethyl-3-methylimidazolium tetrafluoroborate (1) + pyrrolidin-2-one or 1-methylpyrrolidin-2-one (2) + cyclopentanone or cyclohexanone (3) mixtures have been evaluated by using their molar heat capacities, \( C_{\varvec{P}} \). The \( \left( {C_{\text{P}}^{\text{E}} } \right)_{123} \) of the studied ternary mixtures are positive over entire mole fraction of (1) and (2) which in turn suggest that capability of cyclohexanone or cyclopentanone molecules to build a non-random structure in mixed state is superior to the effect caused by disruption of associated NMP or 2-Py entities, and interactions between [emim][BF4]:NMP or 2-Py molecular entities. While \( \left( {\frac{{\partial C_{\text{P}}^{\text{E}} }}{\partial T}} \right) \) for [emim][BF4] (1) + 2-Py (2) + cyclopentanone or cyclohexanone (3) mixtures are positive, those for [emim][BF4] (1) + NMP (2) + cyclopentanone or cyclohexanone (3) mixtures are negative. The \( \left( {C_{\text{P}}^{\text{E}} } \right)_{123} \) data have been analyzed in terms of Graph and Flory theories. It has been observed that \( \left( {C_{\text{P}}^{\text{E}} } \right)_{123} \) values obtained from Graph theory compare reasonably well with the experimental values.
References
Vranes MB, Dozic S, Djeric V, Gadzuric SB. Volumetric properties of binary mixtures of 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide with N-methylformamide and N, N-dimethylformamide from (293.15 to 323.15) K. J Chem Eng Data. 2013;58:1092–102.
Vranes M, Zec N, Tot A, Papovic S, Dozic S, Gadzuric S. Density, electrical conductivity, viscosity and excess properties of 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide + propylene carbonate binary mixtures. J Chem Thermodyn. 2014;68:98–108.
Zábranský M, Růžička V. Heat capacity of liquids. Critical Review 9th PPEPPD Kurashiki, Japan. 4. Pineiro, A. 2004.
Sharma VK, Solanki S, Bhagour S. Excess heat capacities of binary and ternary mixtures containing 1-ethyl-3-methylimidazolium tetrafluoroborate and anilines. J Chem Eng Data. 2014;59:1852–64.
Syed TH, Hughes TJ, Marsh KN, May EF. Isobaric heat capacity measurements of liquid methane, ethane, and propane by differential scanning calorimetry at high pressures and low temperatures. J Chem Eng Data. 2012;57:3573–80.
Lansalot-Matras C, Moreau C. Dehydration of fructose into 5-hydroxymethylfurfural in the presence of ionic liquids. Catal Commun. 2003;4:517–20.
Welton T. Room-temperature ionic liquids solvents for synthesis and catalysis. Chem Rev. 1999;99:2071–83.
Seddon KR. Ionic liquids for clean technology. J Chem Technol Biotechnol. 1997;68:351–6.
Marsh KN, Deev A, Wud ACT, Tran E, Klamt A. Room temperature ionic liquids as replacements for conventional solvents—a Review. Korean J Chem Eng. 2002;19:357–62.
Te M, Fairbridge C, Ring Z. Oxidation reactivities of dibenzothiophenes in polyoxometalate/H2O2 and formic acid/H2O2 systems. Appl Catal A. 2001;219:267–80.
Campos-Martin JM, Capel-Sanchez MC, Fierro JLG. Highly efficient deep desulfurization of fuels by chemical oxidation. Green Chem. 2004;6:557–62.
Al-Shahrani F, Xiao T, Liewellyn SA, Barri S, Jiang Z, Shi H, Martine G, Green MLH. Desulfurization of diesel via the H2O2 oxidation of aromatic sulfides to sulfones using a tungstate catalyst. Appl Catal B. 2007;23:311–6.
Zhang W, Xu K, Zhang Q, Liu D, Wu S, Verpoort F, Song XM. Oxidative desulfurization of dibenzothiophene catalyzed by ionic liquid [BMIm] HSO4. Ind Eng Chem Res. 2010;49:11760–3.
Zhao D, Liao Y, Zhang Z. Toxicity of ionic liquids. Clean. 2007;35:42–8.
Ye C, Liu W, Chen Y, Yu L. Room-temperature ionic liquids: a novel versatile lubricant. Chem Commun. 2001;21:2244–5.
Sheldon RA, Lau RM, Sorgedrager MJ, Van Rantwijk F, Seddon KR. Biocatalysis in ionic liquids. Green Chem. 2002;4:147–51.
Schöfer SH, Kaftzik WP, Kragl U. Enzyme catalysis in ionic liquids: lipase catalysed kinetic resolution of 1-phenylethanol with improved enantioselectivity. Chem Commun. 2001;5:425–6.
Wilkes JS. Properties of ionic liquid solvents for catalysis. J Mol Catal A. 2004;214:11–7.
Koch VR, Dominey LA, Nanjundiah C, Ondrechen MJ. The intrinsic anodic stability of several anions comprising solvent-free ionic liquids. J Electrochem Soc. 1996;143:798–803.
Mousavisafavi SM, Mirkhani SA, Gharagheizi F, Akbari J. A predictive quantitative structure–property relationship for glass transition temperature of 1,3-dialkyl imidazolium ionic liquids. Part 1: the linear approach. J Therm Anal Calorim. 2013;111:235–46.
Kavita T, Attri P, Venkatesu P, Rama DRS, Hofman T. Influence of temperature on thermophysical properties of ammonia ionic liquids with N-methyl-2-pyrrolidone. Thermochim Acta. 2012;545:131–40.
Kumari PG, Venkatesu P, Hofman T, Rao MVP. Excess molar enthalpies and vapour-liquid equilibrium for N-methyl-2-pyrrolidone with ketones. J Chem Eng Data. 2010;55:69–73.
Sankar MG, Ponneri V, Kumar KS, Sakamuri S. Molecular interactions between amine and cyclic ketones at different temperatures. J Therm Anal Calorim. 2014;115:1821–7.
Ciocirlan O, Teodorescu M, Dragoescu D, Iulian O, Barhala A. Densities and excess molar volumes for binary mixtures of cyclohexanone with chloroalkanes at temperatures between (288.15 and 318.15) K. J Chem Eng Data. 2010;55:968–73.
Rafiee HR, Ranjbar S, Poursalman F. Densities and viscosities of binary and ternary mixtures of cyclohexanone, 1,4-dioxane and isooctane from T = (288.15 to 313.15) K. J Chem Thermodyn. 2012;54:266–71.
Sharma VK, Bhagour S, Solanki S, Sharma D. Thermodynamic properties of ternary mixtures of 1-ethyl-3-methyl imidazolium tetrafluoroborate with 1-methyl pyrrolidin-2-one or pyrrolidin-2-one + water. Thermochim Acta. 2013;563:72–81.
Sharma VK, Bhagour S. Molecular interactions in 1-ethyl-3-methyl imidazolium tetrafluoroborate + amide mixtures: excess molar volumes and excess isentropic compressibilities and excess molar enthalpies. J Solution Chem. 2013;42:800–22.
Solanki S, Hooda N, Sharma VK. Topological investigations of binary mixtures containing ionic liquid 1-ethyl-3-methylimidazolium tetrafluoroborate and pyridine or isomeric picolines. J Chem Thermodyn. 2013;56:123–35.
Sharma VK, Solanki S, Bhagour S, Sharma D. Excess molar enthalpies of ternary mixtures containing 1-ethyl-3-methylimidazolium tetrafluoroborate and organic solvents. Thermochim Acta. 2013;569:36–41.
Scholz E. Karl Fischer Titration. Berlin: Springer-Verlag; 1984.
Garcia B, Herrera C, Leal JS. Shear viscosities of binary liquid mixtures: 2-pyrrolidinone with 1-alkanols. J Chem Eng Data. 1991;36:269–74.
Letcher TM, Lachwa J, Domanska U. The excess molar volumes and enthalpies of (N-methyl-2-pyrrolidinone + an alcohol) at T = 298.15 K and the application of the ERAS theory. J Chem Thermodyn. 2001;33:1169–79.
Riddick JA, Bunger WB, Sakano TK. Organic solvents physical properties and methods of purification. 4th ed. New York: Wiley Interscience; 1986.
Curras MR, Gomes MFC, Husson P, Padua AAH, Garcia J. Calorimetric and volumetric study on binary mixtures 2, 2, 2-trifluoroethanol + (1-butyl-3-methylimidazolium tetrafluoroborat or 1-ethyl-3-methylimidazolium tetrafluoroborate). J Chem Eng Data. 2010;55:5504–12.
Navia P, Troncoso J, Romani L. Excess magnitudes for ionic liquid binary mixtures with a common ion. J Chem Eng Data. 2007;52:1369–74.
Stoppa A, Zech O, Kunz W, Buchner R. The conductivity of imidazolium-based ionic liquids from (−35 to 195) °C. A: variation of cation’s alkyl chain. J Chem Eng Data. 2010;55:1768–73.
Sharma D, Bhagour S, Sharma VK. Thermodynamic and topological studies of 1-ethyl-3-methylimidazolium tetrafluoroborate + pyrrolidin-2-one and 1-methyl-pyrrolidin-2-one mixtures. J Chem Eng Data. 2012;57:3488–97.
Pal A, Bhardwaj RK. Excess molar volumes and viscosities for binary mixtures of 2-propoxyethanol and 2-isopropoxyethanol with 2-pyrrolidinone, N-methyl-2-pyrrolidinone, N, N-dimethylformamide, and N, N-dimethylacetamide at 298.15 K. J Chem Eng Data. 2002;47:1128–34.
Papamatthaiakis D, Aroni F, Havredaki V. Isentropic compressibilities of (amide + water) mixtures: a comparative study. J Chem Thermodyn. 2008;40:107–18.
Garcia-Abuin A, Gomez-Diaz D, Rubia MDL, Navaza JM. Density, speed of sound, viscosity, refractive index, and excess volume of N-methyl-2-pyrrolidone + ethanol (or water or ethanolamine) from T = (293.15 to 323.15) K. J Chem Eng Data. 2011;56:646–51.
Kumari PG, Radhamma M, Sekhar GC, Rao MV. Excess volumes and speeds of sound of N-methyl-2-pyrrolidinone with chloroethanes and chloroethenes at 303.15 K. J Chem Eng Data. 2002;47:425–7.
Changsheng Y, Peisheng MA, Qing Z. Excess molar volumes and viscosities of binary mixtures of p-xylene with cyclohexane, n-heptane, n-octane, sulfolane, N-methyl-2-pyrrolidinone and acetic acid at 303.15 and 323.25 K and atmospheric pressure. Chinese J Chem Eng. 2004;12:700–6.
Ciocirlan O, Teodorescu M, Dragoesce D, Iulian O, Barhala A. Densities and excess volumes for binary mixtures of cyclopentanone with chloroalkanes at T = (288.15, 298.15, 308.15 and 318.15) K. J Chem Eng Data. 2010;55:3891–5.
Dragoescu D, Teodorescu M, Barhala A. Isothermal (vapour plus liquid) equilibria and excess Gibbs free energies in some binary (cyclopentanone plus chloroalkane) mixtures at temperatures from 298.15 to 318.15 K. J Chem Thermodyn. 2007;39:1452–7.
Palaiologou MM, Arianas GK, Tsierkezos NG. Thermodynamic investigation of dimethyl sulfoxide binary mixtures at 293.15 and 313.15 K. J Solution Chem. 2006;35:1551–65.
Lange NA. Handbook of Chemistry. 11th ed. New York: Mc Graw-Hill; 1973.
Nayak JN, Aralaguppi MI, Aminabhavi TM. Density, viscosity, refractive index, and speed of sound in the binary mixtures of 1,4-dioxane + ethyl acetoacetate, + diethyl oxalate, + diethyl phthalate, or + dioctyl phthalate at 298.15, 303.15, and 308.15 K. J Chem Eng Data. 2003;48:1489–94.
Singh S, Rattan VK, Kapoor S, Kumar R, Rampal A. Thermophysical properties of binary mixtures of cyclohexane + nitrobenzene, cyclohexane + nitrobenzene, and cyclohexane + cyclohexanone at (298.15, 303.15, and 308.15) K. J Chem Eng Data. 2005;50:288–92.
George J, Sastry NV. Densities, viscosities, speed of sound, and relative permittivities for water + cyclic amides (2-pyrrolidinone, 1-methyl-2-pyrrolidinone and 1-vinyl-2-pyrrolidinone at different temperatures. J Chem Eng Data. 2004;49:235–42.
Bermudez-Salguero C, Gracia-Fadrique J, Calvo E, Amigo A. Densities, refractive indices, speeds of sound, and surface tensions for dilute aqueous solutions of 2-methyl-1-propanol, cyclopentanone, cyclohexanone, cyclohexanol, and ethyl acetoacetate at 298.15 K. J Chem Eng Data. 2011;56:3823–9.
Tsierkezos NG, Molinou IE, Filippou AC. Thermodynamic properties of binary mixtures of cyclohexanone with n-alkanols (C1–C5) at 293.15 K. J Solution Chem. 2005;34:1371–86.
Sanmamed YA, Navia P, Salgado DG, Troncoso J, Romaní L. Pressure and temperature dependence of isobaric heat capacity for [Emim][BF4], [Bmim][BF4], [Hmim][BF4] and [Omim][BF4]. J Chem Eng Data. 2010;55:600–4.
Nishikawa K, Ohomura K, Tamura K, Murakami S. Excess thermodynamic properties of mixtures of cyclohexanone and benzene at 298.15 and 308.15 K and the effect of excess expansion factor. Thermochim Acta. 1995;267:323–32.
Saini N, Yadav JS, Jangra SK, Sharma D, Sharma VK. Thermodynamic studies of molecular interactions in mixtures of o-toluidine with pyridine and picolines: excess molar volumes, excess molar enthalpies and excess isentropic compressibilities. J Chem Thermodyn. 2011;43:782–95.
Dubey GP, Sharma M. Temperature and composition dependence of the densities, viscosities, and speeds of sound of binary liquid mixtures of 1-butanol with hexadecane and squalane. J Chem Eng Data. 2008;53:1032–8.
Sharma VK, Rohilla A. Excess heat capacities of 1-methyl pyrrolidin-2-one and pyridine or picolines mixtures. Thermochim Acta. 2013;568:140–7.
Sabbah R. Xu-wu An, Chickos JS, Leitão MLP, Roux MV, Torres LA. Reference materials for calorimetry and differential thermal analysis. Thermochim Acta. 1999;331:137.
Redlich O, Kister AT. Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem. 1948;40:345–8.
Sharma VK, Kataria J. Topological investigations of excess heat capacities of binary liquid mixtures containing lactams and cycloalkanone. J Mol Liq. 2013;188:210–21.
Sharma VK, Bhagour S, Solanki S, Sharma D. Excess heat capacities of binary and ternary mixtures containing [emim][BF4] and organic liquids, J Chem Thermodyn. 2014;79:19–32.
Sharma VK, Kataria J, Bhagour S. Thermodynamic investigations of 1-ethyl-3-methylimidazolium tetrafluoroborate and cycloalkanone mixtures: excess molar volumes, excess molar isentropic compressibilities, excess molar enthalpies and excess heat capacities. J. Thermal Anal Calorim. 2014;118:431–47.
Bonchev D, Mekenyan O, Balaban AT. Algorithms for coding chemical compounds. In: Trinajstic N, editor. Mathematical and computational concepts in chemistry. Chichester, U. K.: Ellis Horwood; 1986. p. 34–47.
Sharma VK, Kataria J, Solanki S. Molecular interactions in binary mixtures of lactams with cyclic alkanones. J Solution Chem. 2014;43:486–524.
Huggins ML. The thermodynamic properties of liquids included solutions: Part 1. Intermolecular energies in mono atomic liquids and their mixtures. J Phys Chem. 1970;74:371–8.
Huggins ML. The thermodynamic properties of liquids included solutions: Part 2. Polymer solutions considered as diatomic system. Polymer. 1971;12:389–99.
Yadav JS, Sharma D, Sharma VK. Topological investigations of thermodynamic properties of binary mixtures containing 2-pyrrolidinone. Thermochim Acta. 2009;489:45–52.
Singh PP, Nigam RK, Singh KC, Sharma VK. Topological aspects of the thermodynamics of binary mixtures of non-electrolytes. Thermochim Acta. 1981;46:175–90.
Sharma VK, Solanki S. Topological investigations of binary mixtures containing 1-ethyl-3-methylimidazolium tetrafluoroborate and anilines. J Mol Liq. 2013;177:133–44.
Sharma VK, Siwach RK. Dimple. Excess molar volumes, excess molar enthalpies, and excess isentropic compressibilities of tetrahydropyran with aromatic hydrocarbons tetrahydropyran with aromatic hydrocarbons. J Chem Thermodyn. 2011;43:39–46.
Singh PP. Topological aspects of the effect of temperature and pressure on the thermodynamics of binary mixtures of non-electrolytes. Thermochim Acta. 1983;66:37–73.
Kier LB, Yalkowasky SH, Sinkula AA, Valvani SC. Physico-chemical properties of drugs, Chapter 9. New York: Mercel Dekker; 1980. p. 282–95.
Flory PJ. The statistical thermodynamic of liquid mixtures. J Am Chem Soc. 1965;87:1833–8.
Flory PJ. The thermodynamic properties of mixture of small non-polar molecules. J Am Chem Soc. 1965;87:1838–46.
Benson GC, Arcy PJD, Kumaran MK. Heat capacities of binary mixtures of n-heptane with hexane isomers. Thermochim Acta. 1984;75:353–60.
Acknowledgements
Jyoti Kataria is grateful to UGC, New Delhi, India, for the award of SRF. The authors are also grateful to the Head of Chemistry Department and authorities of M. D. University, Rohtak, for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, V.K., Kataria, J. & Sharma, D. Excess heat capacities of mixtures containing 1-ethyl-3-methylimidazolium tetrafluoroborate, lactams and cyclic alkanones. J Therm Anal Calorim 121, 777–796 (2015). https://doi.org/10.1007/s10973-015-4412-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4412-8