Abstract
The amino acid ionic liquids (AAILs) [Cnmim][Thr](n = 3, 5) were prepared, and their structures were confirmed by NMR spectroscopy and element analysis. The evaporation enthalpy of AAILs [Cnmim][Thr](n = 3, 5) and the sublimation enthalpy of benzoic acid were determined by the isothermogravimetrical analysis at their average temperature, respectively. Using Verevkin’s method, the difference of heat capacities between the vapor phase and the liquid phase, \(\Delta^{\text{g}} _{\text{l}} C_{\text{p}} ^{\text{o}} {}_{\text{m}}\), for AAILs [Cnmim][Thr](n = 3, 5) was calculated and in terms of \(\Delta^{\text{g}} _{\text{l}} C_{\text{p}}^{\text{o}} {}_{\text{m}}\), \(\Delta^{\text{g}} _{\text{l}} H^{\text{o}} _{\text{m}} \left({T_{\text{av}}} \right)\) can be transformed into \(\Delta^{\text{g}} _{\text{l}} H^{\text{o}} _{\text{m}} \left(T \right)\) at different temperatures, T, where the evaporation enthalpy, \(\Delta^{\text{g}} _{\text{l}} H^{\text{o}} _{\text{m}}\)(298.15), at 298.15 K was included. The difference between the value of \(\Delta^{\text{g}} _{\text{l}} H^{\text{o}} _{\text{m}}\)(298.15) and the corresponding one predicted by Tong’s model is less than the experimental error of 3.0 kJ mol−1, so that it is shown that the model has some reasonableness. According to Rebelo et al., the hypothetical normal boiling point, Tb, was estimated so that the vaporization entropy, \(\Delta^{\text{g}} _{\text{l}} S\)(Tb) was estimated also. In terms of \(\Delta^{\text{g}} _{\text{l}} C_{\text{p}}^{\text{o}} {}_{\text{m}}\), the vaporization entropy, \(\Delta^{\text{g}} _{\text{l}} S\left(T \right)\) and the evaporation Gibbs free energy, \(\Delta^{\text{g}} _{\text{l}} G^{\text{o}} _{\text{m}} \left(T \right)\), of the AAILs were determined at different temperatures. The results show that \(\Delta^{\text{g}} _{\text{l}} G^{\text{o}} _{\text{m}} \left(T \right)\) decreases with the temperature rise until the boiling point temperature is zero, and \(\Delta^{\text{g}} _{\text{l}} S\left(T \right)\) increases with the temperature rise until Tb is the maximum so that this indicted that the vaporization entropy is the driving force of the evaporation process of AAIL [Cnmim][Thr](n = 3, 5).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ionic liquids (ILs) are called “green solvent in the future”, because their special physical and chemical properties have great application potential in many fields [1,2,3,4,5,6,7,8,9,10]. In 2005 and 2006, the amino acid ionic liquids (AAILs) were synthesized by Kou et al. [11] and Fukumoto et al. [12], respectively. Since AAILs are derived from natural ions and heralded a new “natural ILs” or “bio-ILs”, both academia and industry have a widespread interest in AAILs as a new, easily biodegradable and environmentally friendly ILs [13,14,15].
One of the most attractive features of ILs is that the vapor pressure is negligible at room temperature. However, in many practical applications, ILs must be used at elevated temperatures, when their vapor pressure is close to a few Pa, and cannot be ignored in the long-term use [16, 17], so that stimulates interest in study on evaporation process of ILs. In addition, evaporation enthalpy and vapor pressure are not only the basic data for the practical application of ILs, but also the important parameters for the development of liquid theory and quantum chemistry calculation. However, experimental determination of the vapor pressure and vaporization enthalpy for ILs is a very difficult and challenging work, because the vapor pressure of ILs is very low at room temperature resulting in most of the traditional measurement techniques are not applicable. In order to meet the challenge, a new method for the determination of the enthalpy of vaporization of ionic liquids has been developed in recent years [18,19,20,21,22]. Among these new methods, isothermal thermogravimetry is an effective method for the rapid determination of vaporization enthalpy. This method is not only suitable for ionic liquids, but also for other substances with low vapor pressure which is great significance for chemistry and chemical engineering and for the research and design of radio, electronics, metallurgy, medicine, environmental protection and other fields of science and technology.
Using isothermal thermogravimetry, Tong et al. [23, 24] measured the vaporization enthalpy for ILs 1-alkyl-3-methylimidazolium propionate [Cnmim][Pro](n = 4, 5, 6) and put forward the evaporation enthalpy model of ILs and a new thermodynamic function—the molar surface free energy. Using the model, the empirical equation proposed by Kabo et al. [19] was proved. As a continuation of our research work, AAILs 1-alkyl-3-methylimidazolium threonine [Cnmim][Thr](n = 3, 5) were prepared and characterized by NMR spectroscopy and element analysis. The vaporization enthalpy for AAILs [Cnmim][Thr](n = 3, 5) and benzoic acid were determined by isothermal thermogravimetry, and the thermodynamic quantities of the evaporation process for the AAILs are discussed.
Experimental
Chemicals
Anion-exchange resin (type 717) was activated by the regular method [25]. The source and purity of the materials are listed in Table S1 of the Supporting Information.
Preparation of the ILs
The ILs, [Cnmim][Thr](n = 3, 5), were prepared by a neutralization method according to Fukumoto et al. [12]. The structures of the resulting materials were confirmed by 1H NMR and 13C NMR spectroscopy (see Section A and B in the Supporting Information). Element analysis (Flash EA1112 type produced by THERMO) showed that the purity of the synthesized ionic liquids is more than 0.99 (mass fraction) (see Section C in the Supporting Information). Thermogravimetry analysis (Switzerland METTLER TOLEDO Instruments TGA/SDTA851e) showed that the initial decomposition temperature of the synthesized ILs is about 220 °C that is 493.15 K (see Section D in the Supporting Information). The mass fractions of water in the ILs determined by a Karl Fischer moisture titrator (ZSD-2 type, Shanghai anting electronic instrument factory) were (0.00650, 0.00660) ± 0.0001, respectively.
Isothermal gravimetric analysis for the ILs and benzoic acid
In this work, a METTLER TOLEDO Instruments TGA/SDTA851e was used and calibrated for temperature according to Stewart’s method [26] using indium, tin, bismuth and lead. The accuracy of the temperature measurements was adjusted to be better than ± 0.2 K, the magnitude and linearity of the balance response was checked with standard milligram masses. Firstly, the conventional TGA curves of [Cnmim][Thr](n = 3, 5) were obtained by using METTLER TOLEDO Instruments TGA/SDTA851e (see Figs. D.1 and D.2 in the Supporting Information). According to these conventional TGA curves, the initial decomposition temperature of [Cnmim][Thr](n = 3, 5) is 490.15 K and 495.15 K, respectively. And then, the range of the isothermal gravimetric analysis was determined. Then, the isothermal gravimetric analysis curve for the ILs was measured according to optimal conditions recommended by Verevkin et al. [22] and our preliminary work [23, 24], some suitable experimental parameters are selected: a sample of about 50 mg was placed in a cylindrical platinum crucible, and argon was used as purge gas with a flow rate of 100 mL min−1. For each section of the constant temperature, the loss of the sample is controlled between 1.2 and 0.3 mg. The temperature jump rate between the constant temperature sections is 10 K min−1. According to the literature value of the vapor pressure of benzoic acid, the constant temperature range of benzoic acid was determined in the isothermal gravimetric analysis. Other contents of thermogravimetry program for the benzoic acid are similar to the AAILs.
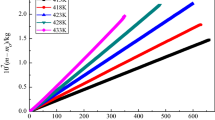
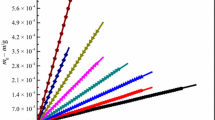
According to the isothermal gravimetric experiments, plotting (m0 − m)/kg versus (t − t0)/s (m is the sample mass, t is the time, subscript 0 means the initial state) for the ILs and the benzoic acid at each constant temperature, a series of good straight lines were obtained (see Figs. 1, 2). These straight lines are typical time-course isothermal TGA mass loss curves, and the values of their slopes of the IL and the benzoic acid, − dm/dt, are listed in Table 1, respectively. As can be seen from Figs. 1 and 2, the mass loss curves are all rigorously linear with correlation coefficients exceeding 0.999. The high linearity associated with the isothermal TGA curves reveals zero-order mass loss kinetics, providing strong evidence that the observed decrease in mass over time at constant temperature results from vaporization of the IL and does not originate from evolution of thermal degradation product or from impurity [27].
Plot of 106 × (m0 − m)/kg versus (t − t0)/s for [Cnmim][Thr](n = 3, 5) from the bottom to top: 413.15 K, 423.15 K, 433.15 K, 443.15 K, 453.15 K, 463.15 K, respectively. a [C3mim][Thr] (filled square 413.15 K: y = 2.18 × 10−3 + 1.38 × 10−4x, r2 = 0.9999, sd = 3.31 × 10−4; filled circle 423.15 K: y = − 9.06 × 10−4 + 2.75 × 10−4x, r2 = 0.9999, sd = 1.26 × 10−4; filled triangle 433.15 K: y = − 1.83 × 10−3 + 6.03 × 10−4x, r2 = 0.9999, sd = 2.92 × 10−4; filled inverted triangle 443.15 K: y = 3.44 × 10−15 + 1.33 × 10−3x, r2 = 0.9999, sd = 3.82 × 10−15; filled diamond 453.15 K: y = − 2.50 × 10−5 + 2.60 × 10−3x, r2 = 0.9999, sd = 2.94 × 10−5; filled leftpoint triangle 463.15 K: y = − 6.67 × 10−3 + 4.69 × 10−3x, r2 = 0.9998, sd = 9.36 × 10−3) and b [C5mim][Thr] (filled square 413.15 K: y = − 1.04 × 10−3 + 1.21 × 10−4x, r2 = 0.9995, sd = 9.62 × 10−4; filled circle 423.15 K: y = − 2.44 × 10−3 + 2.30 × 10−4x, r2 = 0.9994, sd = 2.02 × 10−3; filled triangle 433.15 K: y = 3.36 × 10−3 + 4.87 × 10−4x, r2 = 0.9999, sd = 1.03 × 10−3; filled inverted triangle 443.15 K: y = 7.77 × 10−3 + 1.15 × 10−3x, r2 = 0.9998, sd = 4.30 × 10−3; filled diamond 453.15 K: y = − 3.54 × 10−2 + 2.64 × 10−3x, r2 = 0.9998, sd = 7.96 × 10−3; filled leftpoint triangle 463.15 K: y = 6.30 × 10−3 + 5.13 × 10−3x, r2 = 0.9997, sd = 8.49 × 10−3, x: (t – t0)/s; y: 106 × (m0 − m)/kg
Plot of 106 × (m0 − m)/kg versus (t − t0)/s for benzoic acid from the bottom to top: 353.15 K, 358.15 K, 363.15 K, 368.15 K, 373.15 K, 378.15 K, 383.15 K, respectively (filled square 353.15 K: y = − 1.86 × 10−3 + 8.47 × 10−5x, r2 = 0.9998, sd = 6.08 × 10−4; filled circle 358.15 K: y = − 2.59 × 10−3 + 1.14 × 10−4x, r2 = 0.9998, sd = 8.23 × 10−4; filled triangle 363.15 K: y = − 3.92 × 10−3 + 1.81 × 10−4x, r2 = 0.9998, sd = 1.09 × 10−3; filled inverted triangle 368.15 K: y = − 5.56 × 10−3 + 2.75 × 10−4x, r2 = 0.9998, sd = 1.56 × 10−3; filled diamond 373.15 K: y = − 1.09 × 10−2 + 4.04 × 10−4x, r2 = 0.9995, sd = 3.38 × 10−3; filled leftpoint triangle 378.15 K: y = − 1.28 × 10−2 + 5.74 × 10−4x, r2 = 0.9992, sd = 5.75 × 10−3; filled rightpoint triangle 383.15 K: y = − 2.07 × 10−2 + 8.44 × 10−4x, r2 = 0.9996, sd = 5.90 × 10−3, x: (t – t0)/s; y: 106 × (m0 – m)/kg)
Results and discussion
The vapor pressure of the ILs
Price [28] has shown that it is possible to use thermogravimetry to determine the vapor pressure using the Langmuir equation for free evaporation in vacuo:
where − dm/dt is the rate of mass loss per unit area, p is the vapor pressure, M is the molecular weight of the effusing vapor, R is the gas constant, T is the absolute temperature, and α is the vaporization coefficient (usually assumed to be 1).
In the case of the isothermal thermogravimetric experiment, the ILs volatilized into a flowing gas stream at one atmosphere rather than in vacuo, it can no longer be assumed to be unity. Rearranging Langmuir equation yields:
where k = (2πR)1/2/α and ν = – dm/dt(T/M)1/2.
The literature values of the vapor pressure for benzoic acid are known and listed in Table 1. According to Eq. (2), the average value of k = (8.67 ± 0.05) × 109 was obtained using the values of p and ν of benzoic acid. So the values of the vapor pressure of [Cnmim][Thr](n = 3, 5) using the values of ν obtained by the isothermal thermogravimetric experiment were calculated in the temperature range from 413.15 to 463.15 K and are listed in Table 1. However, as can be seen from Tables 1 and S3, the vapor pressure values for the ILs are unrealistically high. This may indicate that benzoic acid may not be suitable for use as a calibration material for the TGA experiments of ILs for two reasons: (1) benzoic acid is crystalline and the ILs are liquid so that the mechanism of their vaporization may be different; and (2) the temperature range of the calibration and measurements is not overlap.
The vaporization enthalpies of the ILs
Dai et al. [20] first applied isothermal thermogravimetry to experimentally measure the evaporation enthalpy of ILs and derived a working equation for the calculation of vaporization enthalpies for the ILs combining Langmuir equation with Clausius–Clapeyron equation:
where c is an empirical parameter, \(\Delta^{\text{g}} _{\text{l}} H^{\text{o}} _{\text{m}}\) is the vaporization enthalpy of the ILs at the average temperature Tav (Tav = (Σ ni Ti)/n).
According to Eq. (1), the values of ln[(− dm/dt)T1/2] of [Cnmim][Thr](n = 3, 5) at different constant temperatures were calculated and are listed in Table 1. Plotting ln[(− dm/dt)T1/2] against 1/T for [Cnmim][Thr](n = 3, 5) and benzoic acid, three good straight lines with correlation coefficient square above 0.99 and standard deviation within the experimental error were obtained (see Fig. 3). The slopes of the straight lines for ILs were used to calculate the evaporation enthalpy of the ILs at the average temperature Tav:
where SL is the slope. The values of SL for [Cnmim][Thr](n = 3, 5) are –1.398 × 104 and –1.493 × 104, and the values of SL for benzoic acid are − 1.075 × 104. According to Eq. (3), the calculated values of \(\Delta^{\text{g}} _{\text{l}} H^{\text{o}} _{\text{m}} \left({T_{\text{av}}} \right)\) for the ILs at Tav = 438.15 K and for benzoic acid at Tav = 368.15 K are listed in Table S2.
Plot of ln[(− dm/dt) T1/2] versus 1/T for [Cnmim][Thr](n = 3, 5) and benzoic acid (filled square [C3mim][Thr]: y = 14.11 − 1.398 × 104x, r2 = 0.9986, sd =5.099 × 10−2; filled circle [C5mim][Thr]: y = 16.18 − 1.493 × 104x, r2 = 0.9949, sd =1.042 × 10−1; filled triangle benzoic acid: y = 10.12 − 1.075 × 104x, r2 = 0.9979, sd =3.936 × 10−2, x: K/T; y: ln[(− dm/dt)T1/2/(kg s−1 K1/2)])
In order to contrast the values of vaporization enthalpy obtained from different experimental methods, \(\Delta^{\text{g}} _{\text{l}} H^{\text{o}} _{\text{m}} \left({T_{\text{av}}} \right)\) should be converted into \(\Delta^{\text{g}} _{\text{l}} H^{\text{o}} _{\text{m}}\)(298.15) at the reference temperature, 298.15 K, using following equation:
where \(\Delta^{\text{g}}_{\text{l}} C_{\text{p}} ^{\text{o}}{}_{\text{m}}\) is the difference in heat capacity of the gaseous and liquid state of the IL at constant pressure (\(\Delta^{\text{g}}_{\text{l}} C_{\text{p}} ^{\text{o}}{}_{\text{m}} = C_{\text{P}} ^{\text{o}}{}_{\text{g}} {-}C_{\text{P}} ^{\text{o}}{}_{\text{l}}\)).
Recently, Verevkin et al. [29, 30] have suggested a new approach for estimating \(\Delta^{\text{g}}_{\text{l}} C_{\text{p}} ^{\text{o}}{}_{\text{m}}\) based on statistical thermodynamics and some auxiliary experimental data:
where αp is the thermal expansion coefficient, K−1, κT is the isothermal compressibility in Pa−1, and Vm is the molar volume in m3·mol−1. The molar volume, as well as thermal expansion coefficient, is usually derived from the density of ILs and the temperature dependence. The values of κT can be calculated from the speed of the sound W [31].
where ρ/kg m−3 is the density [32], \(C_{\text{p}}^{\text{o}}{}_{\text{m}}\) is the heat capacity of the liquid state of the IL at constant pressure, and the value of W was calculated by following equation:
where γ/J cm−2 is surface tension of the IL. The data needed in the calculation of the values of \(\Delta^{\text{g}}_{\text{l}} C_{\text{p}} ^{\text{o}}{}_{\text{m}}\) are listed in Table S2 of the Supporting Information.
Using the values of \(\Delta^{\text{g}}_{\text{l}} C_{\text{p}} ^{\text{o}}{}_{\text{m}}\), the experimental vaporization enthalpy at Tav can be adjusted to the one at 298.15 K: \(\Delta^{\text{g}}_{\text{l}} H^{\text{o}}_{\text{m}}\)(298.15)/kJ mol−1 = 126.0 for [C3mim][Thr] and \(\Delta^{\text{g}}_{\text{l}} H^{\text{o}}_{\text{m}}\)(298.15)/kJ mol−1 = 134.0 for [C5mim][Thr]. Thus, it can be seen that the contribution of each methylene (–CH2–) group in the alkyl chains of the imidazolium-based ILs, \(\Delta^{\text{g}}_{\text{l}} H^{\text{o}}_{\text{m}}\)(–CH2–) = 4.0 kJ mol−1, which is between \(\Delta^{\text{g}}_{\text{l}} H^{\text{o}}_{\text{m}}\)(–CH2–) = 4.85 ± 0.3 kJ mol−1 recommended by Archer et al. [33] and \(\Delta^{\text{g}}_{\text{l}} H^{\text{o}}_{\text{m}}\)(–CH2–) = 3.89 kJ mol−1 obtained by Zaitsau et al. [30].
Using Chickos’s empirical equation [34] which is widely used in sublimation enthalpy, \(\Delta^{\text{g}}_{\text{s}} H^{\text{o}}_{\text{m}}\):
the sublimation enthalpy of benzoic acid, \(\Delta^{\text{g}}_{\text{s}} H^{\text{o}}_{\text{m}}\)(298.15) = 91.45 kJ mol−1, at 298.15 K was calculated and the calculated value is in good agreement with (90.8 ± 0.6) kJ mol−1 measured by Price [28]. This fact further indicates the reliability of thermogravimetric method for determining the vaporization enthalpy of ILs.
The evaporation enthalpy model and molar surface Gibbs free energy for ILs
Tong et al. [23] proposed the evaporation enthalpy model for aprotic ILs, and the working equation is:
where γ is the surface tension, Vm is the molar volume, \(\Delta^{\text{g}}_{\text{l}} H^{\text{o}}_{\text{m}}\) is the vaporization enthalpy, N is Avogadro constant, Δε(kin) is the difference in kinetic energy of the gaseous and liquid state of a pair of positive and negative ions, x is the ratio of the coordination number between on the surface and in bulk phase. This model successfully proved the rationality of Kabo empirical equation [19].
Multiplied Eq. (10) by N1/3 yields:
where g = (γV 2/3m N1/3) is molar surface Gibbs free energy, NΔε(kin) is the difference in molar kinetic energy of the gaseous and liquid state of ILs so that Eq. (11) changed to:
where A and B are the empirical constants. Using Eq. (12), the predicted values: \(\Delta^{\text{g}}_{\text{l}} H^{\text{o}}_{\text{m}}\) = 125.7 kJ mol−1 for [C3mim][Thr] and \(\Delta^{\text{g}}_{\text{l}} H^{\text{o}}_{\text{m}}\) = 133.1 kJ mol−1 for [C5mim][Thr], were obtained in our previous article [35] and are in good agreement with the corresponding experimental values in this work. This fact shows that Tong’s model is reasonable.
The vaporization entropy of the ILs
In the previous article [32], the critical temperature, Tc = 1102 K for IL [C3mim][Thr] and Tc = 1044 K for IL [C5mim][Thr], was estimated. Rebelo et al. [36] pointed out that the hypothetical normal boiling point, Tb, of an IL is approximately equal to 0.60 of the critical temperature, that is, Tb ≈ 0.6Tc, so that Tb = 661 K for IL [C3mim][Thr] and Tb = 626 K for IL [C5mim][Thr]. These two ionic liquids exist in the form of hydrogen bonds acting on each other. Therefore, both of them have high boiling points. And the steric hindrance of [C5mim][Thr] is rather high, so the hydrogen bonds weaken and the boiling point drops. Therefore, the boiling point of [C3mim][Thr] is slightly higher than the boiling point of [C5mim][Thr].
At the hypothetical normal boiling point of an IL, the vapor pressure of the IL pb = 101.325 kPa, so that the vapor pressure of the IL, p1, at any temperature, T1, can be estimated according to Clausius–Clapeyron equation:
where \(\Delta^{\text{g}}_{\text{l}} H^{\text{o}}_{\text{m}} \left(T \right)\) is the average vaporization enthalpy between T1 and Tb. The values of p1 are listed in Table S3 of the Supporting Information. Compared with the data in Table 1, the vapor pressure in Table S3 is much smaller at the similar temperature. This fact also shows that benzoic acid is not suitable as a reference substance for the vapor pressure of ILs.
At the hypothetical normal boiling point, the Gibbs free energy of ionic liquid evaporation is zero, \(\Delta^{\text{g}}_{\text{l}} G^{\text{o}}_{\text{m}} \left({T_{\text{b}}} \right) = 0\), so that the vaporization entropy \(\Delta^{\text{g}}_{\text{l}} S\left({T_{\text{b}}} \right)\) is:
The calculated values of \(\Delta^{\text{g}}_{\text{l}} S^{\text{o}}_{\text{m}}\)(Tb) and \(\Delta^{\text{g}}_{\text{l}} H^{\text{o}}_{\text{m}} \left({T_{\text{b}}} \right)\) are listed in Table S3 of the Supporting Information. In terms of the difference in heat capacity of the gaseous and liquid state of the IL at constant pressure, \(\Delta^{\text{g}}_{\text{l}} C_{\text{p}}^{\text{o}}{}_{\text{m}}\), \(\Delta^{\text{g}}_{\text{l}} S^{\text{o}}_{\text{m}} \left({T_{\text{b}}} \right)\) can be converted to \(\Delta^{\text{g}}_{\text{l}} S\)(298.15) which is the vaporization entropy at 298.15 K:
At 298.15 K, the Gibbs free energy of ionic liquid evaporation, ΔvapG(298.15) is:
By using the similar method, the evaporation Gibbs free energy, \(\Delta^{\text{g}}_{\text{l}} G^{\text{o}}_{\text{m}} \left(T \right)\), and the evaporation entropy, \(\Delta^{\text{g}}_{\text{l}} S^{\text{o}}_{\text{m}} \left(T \right)\), for [Cnmim][Thr](n = 3, 5) can be calculated at any temperature. The values of \(\Delta^{\text{g}}_{\text{l}} G^{\text{o}}_{\text{m}} \left(T \right)\), \(\Delta^{\text{g}}_{\text{l}} S^{\text{o}}_{\text{m}} \left(T \right)\), \(T\Delta^{\text{g}}_{\text{l}} S^{\text{o}}_{\text{m}} \left(T \right)\) and \(\Delta^{\text{g}}_{\text{l}} H^{\text{o}}_{\text{m}} \left(T \right)\) are listed in Table S3 in the Supporting Information.
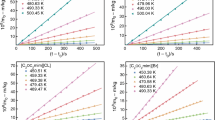
Using the data in Table S3, the curves of \(\Delta^{\text{g}}_{\text{l}} H^{\text{o}}_{\text{m}} \left(T \right)\) versus T, \(T\Delta^{\text{g}}_{\text{l}} S^{\text{o}}_{\text{m}} \left(T \right)\) versus T, \(\Delta^{\text{g}}_{\text{l}} G^{\text{o}}_{\text{m}} \left(T \right)\) versus T and p versus T are plotted in Fig. 4. Table S3 and Fig. 4 show that the evaporation Gibbs free energy of ILs [Cnmim][Thr](n = 3, 5) decreases with increasing temperature, reaching the hypothetical boiling point is zero, while the evaporation entropy increased with the temperature increasing. This fact shows that the evaporation entropy is the driving force of the evaporation process of the ILs.
Plot of \(\Delta^{\text{g}}{}_{\text{l}} H^{\text{o}}{}_{\text{m}}\)(T), T \(\Delta^{\text{g}}{}_{\text{l}} S^{\text{o}}{}_{\text{m}}\)(T), \(\Delta^{\text{g}}{}_{\text{l}} G^{\text{o}}{}_{\text{m}}\)(T) and p versus T for [Cnmim][Thr](n = 3, 5). a [C3mim][Thr] (filled square \(\Delta^{\text{g}}{}_{\text{l}} H^{\text{o}}{}_{\text{m}}\)(T) versus T: y = 146.74 − 0.0697x, r2 = 1.000, sd = 5.799 × 10−14; filled circle T \(\Delta^{\text{g}}{}_{\text{l}} S^{\text{o}}{}_{\text{m}}\)(T) versus T: y = 27.283 + 0.118x, r2 = 0.9980, sd = 3.513 × 10−1; filled triangle \(\Delta^{\text{g}}{}_{\text{l}} G^{\text{o}}{}_{\text{m}}\)(T) versus T: y = 119.46 − 0.188x, r2 = 0.9992, sd = 3.513 × 10−1, ○ p versus T: x: T; y: p) and b [C5mim][Thr] (filled square \(\Delta^{\text{g}}{}_{\text{l}} H^{\text{o}}{}_{\text{m}}\)(T) versus T: y = 154.99 − 0.0705x, r2 = 1.000, sd = 3.259 × 10−13; filled circle T \(\Delta^{\text{g}}{}_{\text{l}} S^{\text{o}}{}_{\text{m}}\)(T) versus T: y = 27.593 + 0.139x, r2 = 0.9985, sd = 3.552 × 10−1; filled triangle \(\Delta^{\text{g}}{}_{\text{l}} G^{\text{o}}{}_{\text{m}}\)(T) versus T: y = 127.40 − 0.209x, r2 = 0.9994, sd = 3.552 × 10−1; open circle p versus T: x: T; y: p)
Conclusions
With the use of the isothermogravimetry, the vaporization enthalpies, \(\Delta^{\text{g}}_{\text{l}} H^{\text{o}}_{\text{m}}\)(298.15), of AAILs [Cnmim][Thr](n = 3, 5) and the sublimation enthalpy were determined. However, using benzoic acid as reference material, the determined vapor pressure and vaporization enthalpy for the AAILs were unrealistically high. This means that benzoic acid may not be suitable for use as a reference material for the TGA experiments of ILs. But the determined sublimation enthalpy is in good agreement with that measured by Price [28]. According to Rebelo et al. [36], the hypothetical normal boiling point, Tb, and the vaporization entropy, \(\Delta^{\text{g}}{}_{\text{l}} S\left({T_{\text{b}}} \right)\), of the AAILs were determined. Then, their evaporation enthalpy, evaporation entropy, evaporation free energy and vapor pressure were calculated at different temperatures. The results of this work show that thermostatic thermogravimetry is based on modern high-precision, highly automated thermogravimetric analyzer, which is an effective method for the rapid determination of enthalpy of ILs.
References
Wei J, Li Z, Gu C, Pan Y, Xing NN, Tong J, Guan W. Determination of vaporization enthalpy for ionic liquids [Cnmim][Lact](n = 2, 3, 5) and applications of the molar Gibbs free energy. J Therm Anal Calorim. 2016;125:547–56.
Zhang MQ, Groves R, Counce RM, Watson JS, Zawodzinski TA. Melting/freezing points of high concentrations of AlCl3 in a saturated chloroaluminate ionic liquid. J Therm Anal Calorim. 2016;124:395–8.
Zheng L, Bu XX, Fan BH, Wei J, Xing NN, Guan W. Study on thermodynamic property for ionic liquid [C4mim][Lact](1-butyl-3-methylimidazolium lactic acid). J Therm Anal Calorim. 2016;123:1619–25.
Zhang ZH, Tan ZC, Li YS, Sun LX. Thermodynamic investigation of room temperature ionic liquid: heat capacity and thermodynamic functions of BMIBF4. J Therm Anal Calorim. 2006;85:551–7.
Li Y, Fang HG, Zhang D, Bahader A, Zhen B, Xu P, Ding YS. Synergetic effects of PEG arm and ionic liquid moiety contained in the tri-arm star-shaped oligomer on the crystallization behaviors of poly(lactic acid). J Therm Anal Calorim. 2016;125:849–60.
Usula M, Plechkova NV, Piras A, Porcedda S. Ethylammonium alkanoate-based ionic liquid + water mixtures A calorimetric and volumetric study at 298.15 K. J Therm Anal Calorim. 2015;121:1129–37.
Navarro P, Larriba M, Beigbeder JB, Garcia J, Rodriguez F. Thermal stability and specific heats of [bpy][BF4] + [bpy][Tf2 N] and [bpy][BF4] + [4bmpy][Tf2 N] mixed ionic liquid solvents. J Therm Anal Calorim. 2015;119:1235–43.
Amarasekara AS, Owereh OS. Thermal properties of sulfonic acid group functionalized Bronsted acidic ionic liquids. J Therm Anal Calorim. 2011;103:1027–243.
Keating MY, Gao F, Ramsey JB. TGA-MS study of the decomposition of phosphorus-containing ionic liquids trihexyl(tetradecyl)phosphonium decanoate and trihexyltetradecylphosphonium bis[(trifluoromethyl)sulfonyl] amide. J Therm Anal Calorim. 2011;106:207–11.
Feng WQ, Lu YH, Chen Y, Lu YW, Yang T. Thermal stability of imidazolium-based ionic liquids investigated by TG and FTIR techniques. J Therm Anal Calorim. 2016;125:143–54.
Tao GH, He L, Liu WS, Xu L, Xiong W, Wang T, Kou Y. Preparation, characterization and application of amino acid-based green ionic liquids. Green Chem. 2006;8:639–46.
Fukumoto K, Yoshizawa M, Ohno H. Room temperature ionic liquids from 20 natural amino acid. J Am Chem Soc. 2005;127:2398–9.
Zhang S, Wang J, Lu X, Zhou Q. Structures and interactions of ionic liquids. Heidelberg: Springer; 2014.
Fang DW, Tong J, Guan W, Wang H, Yang JZ. Predicting properties of amino acid ionic liquid homologue of 1-Alkyl-3-methylimidazolium glycine. J Phys Chem B. 2010;114:13808–14.
Tong J, Song B, Wang CX, Li L, Guan W, Fang DW, Yang JZ. Prediction of the physicochemical properties of valine ionic liquids [Cnmim][Val] (n = 2, 3, 4, 5, 6) by semiempirical methods. Ind Eng Chem Res. 2011;50:2418–23.
Earle MJ, Esperanc JMSS, Gilea MA, Lopes JNC, Rebelo LPN, Magee JW, Seddon KR, Widegren JA. The distillation and volatility of ionic liquids. Nature. 2006;439:831–4.
Deyko A, Lovelock KR, Corfield JA, Taylor AW, Gooden PN, Villar-Garcia IJ, Licence P, Jones RG, Krasovskiy VG, Chernikova EA, Kustov LM. Measuring and predicting Delta(vap)H298 values of ionic liquids. Phys Chem Chem Phys. 2009;11:8544–55.
Esperanc JMSS, Lopes JNC, Tariq M, Santos LMNBF, Magee JW, Rebelo LPN. Volatility of aprotic ionic liquids—a review. J Chem Eng Data. 2010;55:3–12.
Zaitsau DH, Kabo GJ, Strechan AA, Paulechka YU, Tschersich A, Verevkin SP, Heintz A. Experimental vapor pressures of 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imides and a correlation scheme for estimation of vaporization enthalpies of ionic liquids. J Chem Phys A. 2006;110:7303–6.
Luo H, Baker GA, Dai S. Isothermogravimetric determination of the enthalpies of vaporization of 1-alkyl-3-methylimidazolium ionic liquids. J Chem Phys B. 2008;112:10077–81.
Heym F, Etzold BJM, Kern C, Jess A. Analysis of evaporation and thermal decomposition of liquids by thermogravimetrical analysis at ambient pressure and high vacuum. Green Chem. 2011;13:1453–66.
Verevkin SP, Ralys RV, Zaitsau DH, Emel’yanenko VN, Schick C. Express thermo-gravimetric method for the vaporization enthalpies appraisal for very low volatile molecular and ionic compounds. Thermochim Acta. 2012;538:55–62.
Tong J, Yang HX, Liu RJ, Li C, Xia LX, Yang JZ. Determination of the enthalpy of vaporization and prediction of surface tension for ionic liquid 1-Alkyl-3-methylimidazolium propionate [Cnmim][Pro](n = 4, 5, 6). J Phys Chem B. 2014;118:12972–8.
Hong M, Liu RJ, Yang HX, Guan W, Tong J, Yang JZ. Determination of the vaporization enthalpies and estimation of the polarity for 1-alkyl-3-methylimidazolium propionate [Cnmim][Pro](n = 2, 3) ionic liquids. J Chem Thermodyn. 2014;70:214–8.
PRC National Standard Methods for pretreating ion exchange resins, GB 5476-85, 1985.
Stewart LN. In: McAdie HG, editors. Proceedings of the third Toronto symposium on thermal analysis. Toronto: Chemical Institute of Canada; February 25–26, 1969. p. 205.
Hinks D, Rafiq MI, Price DM, Montero GA, Smith B. A comparison of vapour pressure measurements of quinizarin and leucoquinizarin via transpiration and thermogravimetry. Color Technol. 2003;119:84–90.
Price DM. Vapor pressure determination by thermogravimetry. Thermochim Acta. 2001;367:253–62.
Verevkin SP, Zaitsau DH, Emel’yanenko VN, Yermalayeu AV. Making sense of enthalpy of vaporization trends for ionic liquids: new experimental and simulation data show a simple linear relationship and help reconcile previous data. J Phys Chem B. 2013;117:6473–86.
Zaitsau DH, Yernalayeu AV, Emel’yanenko VN, Verevkin SP, Welz-Biermann U, Schubert T. Structure-property relationships in ILs: A study of the alkyl chain length dependence in vaporisation enthalpies of pyridinium based ionic liquids. Sci China Chem. 2012;55:1526–31.
Paulechka YU, Zaitsau DH, Kabo GJ. On the difference between isobaric and isochoric heat capacities of liquid cyclohexyl esters. J Mol Liq. 2004;115:105–11.
Zhang D, Qu Y, Gong YY, Tong J, Fang DW, Tong J. Physicochemical properties of [Cnmim][Thr] (n = 3, 5, 6) amino acid ionic liquids. J Chem Thermodyn. 2017;115:47–51.
Archer DG, Widegren JA, Kirklin DR, Magee JW. Enthalpy of solution of 1-Octyl-3-methylimidazolium tetrafluoroborate in water and in aqueous sodium fluoride. J Chem Eng Data. 2005;50:1484–91.
Chickos JS, Hosseini S, Hesse DG, Liebman JF. Heat capacity corrections to a standard state: a comparison of new and some literature methods for organic liquids and solids. Struct Chem. 1993;4:271–8.
Tong J, Liu L, Li H, Guan W, Chen X. Measurement and estimation of the vaporization enthalpy for amino acid ionic liquids [Cnmim][Thr](n = 2, 4). J Chem Thermodyn. 2017;112:293–8.
Rebelo LPN, Lopes JNC, Esperancüa JMSS, Filipe E. On the critical temperature, normal boiling point, and vapor pressure of ionic liquids. J Phys Chem B. 2005;109:6040–3.
Acknowledgements
This project was supported by NSFC (21773100, 21673107 and 21373005).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, L., Jing, LQ., Liu, HC. et al. Measurement of vaporization enthalpy for amino acid ionic liquids [Cnmim][Thr](n = 3, 5) using the isothermogravimetrical analysis. J Therm Anal Calorim 134, 2247–2254 (2018). https://doi.org/10.1007/s10973-018-7607-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7607-y