Abstract
In this paper, isothermal thermogravimetric experiments were carried out on eight imidazolium-based ionic liquids. Their vapor pressure was determined using Langmuir equation, and their vapor pressure changed with temperature was discussed. It was found that their vapor pressure increased exponentially with temperature. Then, the evaporation enthalpy \(( \Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(}T_{{\text{av}}} {))}\) at average temperature \({(}T_{{\text{av}}} {)}\) of eight imidazolium-based ionic liquids was determined by combining Langmuir equation and Clausius–Clapeyron equation. For comparison purposes, we converted the evaporation enthalpy \({(}\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(}T_{{\text{av}}} {)) }\) to the evaporation enthalpy (\(\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(298}{\text{.15)}}\)) at ambient temperature (298.15 K), and discussed the differences in evaporation enthalpy of them and the possible reasons for these differences. Finally, the polarity of eight imidazolium-based ionic liquids was discussed using polarity scale \((\delta_{{\upmu }} )\) and polarity coefficient (P), and the universal applicability of the polarity coefficient (P) in ionic liquids and molecular liquids was demonstrated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ionic liquids (ILs) are widely used in catalysis [1], extraction [2], adsorption [3] and electrochemistry [4] due to their designability and excellent properties such as low vapor pressure, high thermal stability, high conductivity and wide electrochemical window. Although the vapor pressure of ILs is low at room temperature, considering that ILs must be used at higher temperatures in some practical applications, the vapor pressure of ILs at high temperatures is close to several pascals, so the loss of ILs caused by vapor pressure cannot be ignored [5, 6], it means that the researchers must know the evaporation process of ILs. Therefore, vapor pressure and evaporation enthalpy are indispensable basic data for the practical application of ILs. However, it is a very challenging and laborious task that to determine the vapor pressure and evaporation enthalpy of ILs through experiments, because the extremely low vapor pressure of ILs at room temperature makes most traditional measurement experimental techniques unsuitable [7]. To address this challenge, researchers have developed some new methods specifically for measuring the evaporation enthalpy of ILs [8,9,10,11,12]. Among these new methods, the isothermal thermogravimetric method is based on modern high-precision and highly automated thermogravimetric analyzer, which is an effective method for quickly measuring vapor pressure and evaporation enthalpy. It is not only suitable for ILs, but also for other substances with low vapor pressure. It is not only great significance in the fields of chemistry and chemical engineering, but also directly related to research and design in technology fields such as radio, electronics, metallurgy, medicine and environmental protection [13].
Polarity is also an extremely important property of ILs. In recent years, researchers have attempted to use the dielectric constant (e) to estimate the polarity of ILs [14, 15]. However, it is unreasonable to use dielectric constant to describe the polarity of ILs [16]. In addition to using dielectric constant to describe the polarity of ionic liquid, probe molecules can also be used to determine the polarity of solvents [17]. Welton et al. [18] summarized some effective rules for predicting solvents polarity based on the reaction rate theory of nucleophilic substitution reactions. However, these traditional polar scalars have some limitations in practical applications [19, 20]. To this end, we propose a new polarity scale \((\delta_{{\upmu }} )\) [21], and through further modifications, we propose a polarity coefficient (P) to estimate the polarity of ILs [22].
Imidazolium-based ILs are more stable than pyridine, phosphorus, ammonium, pyrrolidine and pyridine-based ILs [23]. Therefore, the vapor pressure and evaporation enthalpy of eight imidazolium-based ILs were determined by isothermal thermogravimetric experiments, and the law of vapor pressure variation with temperature during evaporation was discussed. The difference of evaporation enthalpy of eight imidazolium-based ILs was compared, and the possible reasons for such difference were discussed. In addition, we discussed the polarity of eight imidazolium-based ILs using polarity scale \((\delta_{{\upmu }} )\) and polarity coefficient (P), and demonstrated the widespread applicability of polarity coefficient (P) in both ILs and molecular liquids.
Experimental
Preparation of ILs
The preparation processes of eight imidazolium-based ILs have been introduced in our previous work [13, 22, 24, 25] and will not be further elaborated here. Their full names, abbreviations and structural formula are listed in Table 1.
Dynamic thermogravimetric experiments
The Mettler Toledo thermogravimetric analyzer was used to test the dynamic thermogravimetric experiments of eight imidazolium-based ILs. The specific experimental conditions are as follows: the temperature range: 298.15–673.15 K, heating rate: 10 K min-1, purging gas: nitrogen, gas flow rate: 50 mL min-1, crucible: 70 µL platinum crucible, specimen mass: about 10 mg.
Isothermal thermogravimetric experiments
The instrument used is the same as that used in dynamic thermogravimetric experiments, but the experimental conditions are different. The isothermal thermogravimetric experiments are as follows: the temperature range: 423.15–503.15 K, the temperature interval is 10 K, nitrogen is used as the purging gas, the gas flow rate is 100 mL min-1, crucible: 70 µL platinum crucible, specimen mass: about 50 mg.
Result and discussion
Decomposition temperature
The TG/DTG curves of eight imidazolium-based ILs were placed in the Supporting Information, in the TG/DTG curve, tangent lines were made, respectively, for the temperature at which decomposition began and the temperature at which the decomposition rate reached its maximum (Tp). The temperature corresponding to the intersection of the two tangent lines was the decomposition temperature (Td) of eight imidazolium-based ILs. And their Td and Tp can be seen, respectively, as shown in Table 2.
From the data in Table 2, it can be seen that there are significant differences in decomposition temperature (Td) among eight imidazolium-based ILs. It can be seen that the decomposition temperature (Td) of intermediate imidazolium-based ILs (such as [C2OC2mim][Cl], [C2OC2mim][Br], [C5mim][Br] and [C6mim][Br]) is significantly higher than that of amino acid ILs ([COC4mim][Ala], [COC4mim][Thr], [HEmim][Ala] and [HEmim][Thr]). Because the decomposition temperature (Td) is slightly different, the isothermal thermogravimetric temperature range of eight imidazolium-based ILs are also slightly different.
Instantaneous mass
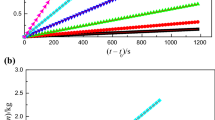
The maximum temperatures of the isothermal thermogravimetric experiments of eight imidazolium-based ILs are less than their Td, in order to prevent their decomposition during the experiment. Isothermal thermogravimetric experiments have recorded the mass changes of eight imidazolium-based ILs over time, and the trend diagram of the changes is listed in Figs. 1, 2.
As shown in Figs. 1, 2, the mass curves of eight imidazolium-based ILs at each temperature show a good linear relationship with time, with r2 greater than 0.99. The high linear correlation indicates that the mass loss of isothermal thermogravimetry is a strictly zero order kinetic process, that is, the evaporation of ILs is zero order kinetic process [26]. The relevant data are listed in Table 3.
Vapor pressure
It is well known that the vapor pressure curve can be described by the Antoine equation, as shown in Eq. (1):
where p is the vapor pressure, T is the absolute temperature, and A, B and C are Antoine constants within the studied temperature range.
However, the data obtained from the Antoine equation are limited either in the number of available compounds or in the temperature range studied. Therefore, the experimental principle of thermogravimetric analysis adopted in this study is based on Langmuir equation [27], as shown in Eq. (2):
where - [(dm/dt)]/a is the mass loss rate per unit area, M is the relative molecular mass of the ILs, and a is the evaporation coefficient. By rearranging Langmuir equation, Eq. (3) can be obtained:
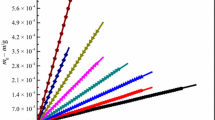
where k = (2pR)1/2/αυ = - (1/a)(dm/dt)(T/M)1/2. The value of k can be obtained by referring to our previous work [28], and the value of υ can be obtained from isothermal thermogravimetric experimental data. In this way, we can obtain the vapor pressure of eight imidazolium-based ILs at various temperatures, and the relevant data are listed in Table 4. The trends of vapor pressure variation with temperature of eight imidazolium-based ILs are shown in Fig. 3.
It can be seen from Fig. 3 that the vapor pressure of the eight imidazolium-based ILs increases exponentially with temperature, and high temperature is more conducive to the growth of their vapor pressure than low temperature. It is noteworthy that the vapor pressure of the four intermediate ILs is lower than that of the four amino acid ILs at the same temperature. For example, when the temperature is 450 K, the vapor pressure of the four intermediate ILs is less than 50 Pa, while the vapor pressure of the four amino acid ILs has reached several hundred Pa, which is much higher than the intermediate ILs. This trend is contrary to the trend of their decomposition temperature (Td), which accords with the characteristics of high thermal stability and low vapor pressure of ILs.
Evaporation enthalpy
According to the Clausius–Clapeyron equation, the relationship between vapor pressure (p) and evaporation enthalpy \({(}\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} )\) is as follows:
where c is the empirical constant and R is the gas constant, bringing Eq. (3) into Eq. (4), we can get:
Equation (5) can be written as:
It can be clearly seen from Eq. (6) that ln[- (dm/dt) T1/2] plots T-1 to get a straight line with the slope of which is \(- \Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} /R\) (Fig. S9 of Supporting Information), and then multiplied by the negative value of Gas constant R to get the values of the vaporization enthalpy of eight imidazolium-based ILs at their respective average temperatures. Their values are listed in Table S1 of Supporting Information.
However, the evaporation enthalpy obtained by fitting experimental data above is the value at the average temperature within the experimental temperature range, which is not commonly used. However, not all experimental temperature ranges for ILs are the same, but are chosen based on the actual situation of different ILs. In addition, different experimenters may not necessarily choose the same temperature range for the same ILs. Therefore, the evaporation enthalpy \((\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(}T_{{\text{av}}} {))}\) at average temperature \({(}T_{{\text{av}}} {)}\) cannot be well compared with the values obtained by other experimental methods. So we convert it to the the evaporation enthalpy (\(\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(298}{\text{.15)}}\)) at ambient temperature (298.15 K) [28].
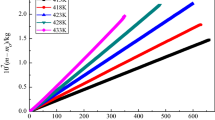
The values of evaporation enthalpy \((\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(}T_{{\text{av}}} {))}\) at average temperature \({(}T_{{\text{av}}} {)}\) of eight imidazolium-based ILs are also listed in Table 5. In order to more intuitively see the differences between them, we have plotted their results in Fig. 4.
We divided eight imidazolium-based ILs into two categories from different perspectives of anions and cations: 1) The anions are the same, but the cations are different, such as [C5mim][Br] and [C6mim][Br]. The \({ }\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(298}{\text{.15)}}\) of [C5mim][Br] is 143.67 kJ mol-1, the \(\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(298}{\text{.15)}}\) of [C6mim][Br] is 139.11 kJ mol-1, it can be seen that the contribution of each methylene (–CH2–) group in the alkyl chains of the imidazolium-based ILs, \(\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(298}{\text{.15)}}\) = 4.56 kJ mol-1, which is between \({ }\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(}T_{{\text{av}}} {)}\) = 4.85 kJ mol-1 recommended by Archer et al. [29] and \({ }\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(}T_{{\text{av}}} {)}\) = 3.89 kJ mol-1 obtained by Zaitsau et al. [30], indicating that the results in this paper are reasonable to a certain extent.
2) The cations are the same, but the anions are different, such as [C2OC2mim][Br] and [C2OC2mim][Cl], [COC4mim][Ala] and [COC4mim][Thr], [HEmim][Ala] and [HEmim][Thr]. It was found that the evaporation enthalpy was not significantly related to the molecular mass of anions or cations. We speculate that this may be related to the interaction force between the anions and cations in the ILs.
We compared some other imidazolium-based ILs (Table 6) and found that eight imidazolium-based ILs we studied had the same order of magnitude, which further proves the reliability of our experimental results.
Polarity
Polarity is one of the important properties of ILs, and it is also a physical property that cannot be ignored in some applications. The dielectric constant (e) is often used to represent the polarity of liquid solvents. However, in previous studies, it has been found that using the dielectric constant to describe the polarity of ILs is not very accurate. For example, the dielectric constant (e) of hydrophobic ILs [C4mim][NTf2] is 11.7 [37], while the dielectric constant (e) of hydrophilic ILs [C4mim][BF4] is also 11.7 [38], which is obviously unreasonable. This means that the dielectric constant (e) cannot be used to describe the polarity of ILs. Therefore, in our previous work, we proposed the polarity scale (dµ) of ILs [33].
The evaporation enthalpy \({ (}\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(298}{\text{.15))}}\) of ILs consists of the non-polarity portion of the induced dipole moment \({ (}\Delta_{\text{l}}^{\text{g}} H_{\text{mn}}^{{\uptheta }} )\) and the polarity portion of the average permanent dipole moment contribution of ion pairs \({(}\Delta_{\text{l}}^{\text{g}} H_{\text{m}{\upmu }}^{{\uptheta }} )\), namely:
where the non-polarity portion of the induced dipole moment \({ (}\Delta_{\text{l}}^{\text{g}} H_{\text{mn}}^{{\uptheta }} )\) can be calculated using the Lawson–Ingham equation [39]:
where C = 1.297 kJ cm-3, is the empirical constant, \(n_{\text{D}}\) is the refractive index, and V is the Molar volume, so the polarity portion of the average permanent dipole moment contribution of ion pairs \({ (}\Delta_{\text{l}}^{\text{g}} H_{\text{m}{\upmu }}^{{\uptheta }} )\) is the difference between the evaporation enthalpy \({ (}\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(298}{\text{.15)}}\)) of ILs and the polarity portion of the average permanent dipole moment contribution of ion pairs \({ (}\Delta_{\text{l}}^{\text{g}} H_{\text{m}{\upmu }}^{{\uptheta }} )\).
According to Hildebrand [40] theory, the solubility parameter dµ of the polar part is calculated:
where \(x{ = }\Delta_{\text{l}}^{\text{g}} H_{\text{mn}}^{{\uptheta }} /\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }}\), polarity portion solubility parameter dµ is the polarity scale. We have studied the polarity of many ILs using this polarity scale and obtained good results [41]. For example, dµ = 10.38 J1/2 cm-3/2 for [C4mim][NTf2] and dµ = 19.36 J1/2 cm-3/2 for [C4mim][BF4], the results showed that the polarity of [C4mim][BF4] was much greater than that of [C4mim][NTf2], which is consistent with the hydrophilicity and hydrophobicity of the two ILs. Polarity scale (dµ) of eight imidazolium-based ILs calculated using this method is listed in Table 7.
Although dµ can be regarded as the polarity scale of ILs, we find that using the scale (dµ) as the polarity scale of ILs has two disadvantages, one is that the scale (dµ) has a dimension, and J1/2 cm-3/2 as the dimension of liquid polarity is obviously inappropriate. Another is that the polarity of the solvent is the sum of all possible interactions between the solvent and solute, except for chemical interactions, and the scale (dµ) only the even contribution part was considered, without considering the nonpolar contribution part. Therefore, we have modified this polarity scale (dµ) and proposed the ILs polarity coefficient (P), which is defined as:
The larger the polarity coefficient (P) of ILs, the stronger the polarity of ILs. The values of polarity coefficients (P) of eight imidazolium-based ILs calculated by Eq. (10) are also listed in Table 7. In order to prove the wide applicability of the polarity coefficient (P), the P values of other imidazolium-based ILs in the literature were calculated, and the corresponding data are also listed in Table 7.
From the data in Table 7, it can be seen that the polarity order of the ILs obtained by using the polarity coefficient (P) is consistent with the polarity scale (dµ). In addition to ILs, we also apply them to common molecular liquids, the polarity coefficient (P) and polarity scale (dµ) of some molecular liquids are listed in Table 8, and since the polarity of the solvent is usually expressed by the dielectric constant (e), the dielectric constant (e) also is listed in Table 8. From Table 8, it can be seen that the dielectric constant (e) of pyridine is 12.5, and the dielectric constant (e) of acetone is 20.7, indicating that the polarity of acetone is stronger than that of pyridine. Using our proposed polarity coefficient (P), the polarity coefficient (P) of pyridine and acetone was calculated, which are 0.539 and 0.684, respectively. It can be seen that the polarity of acetone is stronger than that of pyridine, which is consistent with the results of dielectric constant. The change trend of the polarity coefficient (P) of other molecular liquids in Table 8 is positively correlated with the changing trend of the polarity scale (dµ) and the dielectric constant (e), which proves that the polarity coefficient (P) can be used not only as a scale of ILs polarity, but also as a scale of molecular liquid polarity, with wide applicability.
Conclusions
In this paper, eight imidazolium-based ILs were tested by isothermal thermogravimetric experiments, and the variation of their vapor pressure with temperature was discussed. It was found that the vapor pressure of eight imidazolium-based ILs increased exponential growth with the increase of temperature. In addition, the evaporation enthalpy \(( \Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(}T_{{\text{av}}} {))}\) at average temperature \({(}T_{{\text{av}}} {)}\) of eight imidazolium-based ILs was also determined and converted the evaporation enthalpy \({(}\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(}T_{{\text{av}}} {)) }\) to the evaporation enthalpy (\(\Delta_{\text{l}}^{\text{g}} H_{\text{m}}^{{\uptheta }} {(298}{\text{.15)}}\)) at ambient temperature (298.15 K), and the differences in evaporation enthalpy of them and the possible reasons for these differences were discussed. Finally, the polarity of eight imidazolium-based ILs was also discussed using the polarity scale (dµ) and polarity coefficient (P), and the universal applicability of polarity coefficient (P) in ILs and molecular liquids through other imidazolium-based ILs and common molecular liquids were demonstrated.
Supporting information available
Supporting Information Available: The TG/DTG curves of thermogravimetric analysis and partial experimental data. This information is available free of charge via the Internet at http://pubs.acs.org.
References
Seitkalieva MM, Samoylenko DE, Lotsman KA, Rodygin KS, Ananikov VP. Metal nanoparticles in ionic liquids: synthesis and catalytic applications. Coord Chem Rev. 2021;445:213982–4054.
Yue Z, Jing T. Investigation of the extraction ability and mechanism of environmentally friendly ionic liquids for phenol in the model oil. Fuel. 2023;341:127673–81.
Garip M, Gizli N. Ionic liquid containing amine-based silica aerogels for CO2 capture by fixed bed adsorption. J Mol Liq. 2020;310:113227–36.
Pham-Truong T-N, Randriamahazaka H, Ghilane J. Electrochemistry of bi-redox ionic liquid from solution to bi-functional carbon surface. Electrochim Acta. 2020;354:136689–95.
Earle MJ, Esperança JMSS, Gilea MA, Canongia Lopes JN, Rebelo LPN, Magee JW, et al. The distillation and volatility of ionic liquids. Nature. 2006;439:831–4.
Deyko A, Lovelock KR, Corfield JA, Taylor AW, Gooden PN, Villar-Garcia IJ, et al. Measuring and predicting Delta(vap)H298 values of ionic liquids. Phys Chem Chem Phys. 2009;11:8544–55.
Lu L, Yue Z, Jun SW, Mei H, Yuxia K, Jing T. A simple study on vaporization enthalpy of taurine anion-based ionic liquid. J Mol Liq. 2021;323:115007–14.
Esperanc JMSS, Canongia Lopes JN, Tariq M, Santos LMNBF, Magee JW, Rebelo LsPN. Volatility of aprotic ionic liquids—a review. J Chem Eng Data. 2010;55:3–12.
Zaitsau DH, Kabo GJ, Strechan AA, Paulechka YU. Experimental vapor pressures of 1-alkyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imides and a correlation scheme for estimation of vaporization enthalpies of ionic liquids. J Phys Chem A. 2006;110:7303–6.
Huimin L, Baker GA, Dai S. Isothermogravimetric determination of the enthalpies of vaporization of 1-alkyl-3-methylimidazolium ionic liquids. J Phys Chem B. 2008;112:10077–81.
Heym F, Etzold BJM, Kern C, Jess A. Analysis of evaporation and thermal decomposition of ionic liquids by thermogravimetrical analysis at ambient pressure and high vacuum. Green Chem. 2011;13:1453–66.
Verevkin SP, Ralys RV, Zaitsau DH, Emel’yanenko VN, Schick C. Express thermo-gravimetric method for the vaporization enthalpies appraisal for very low volatile molecular and ionic compounds. Thermochim Acta. 2012;538:55–62.
Jing T, Ye Q, Liqiang J, Lu L. Measurement of vapor pressure and vaporization enthalpy for ionic liquids 1-Hexyl-3-methylimidazolium threonine Salt [C6mim][Thr] by isothermogravimetric analysis. Acta Phys Chim Sin. 2018;34:194–200.
Weingärtner H. The static dielectric permittivity of ionic liquids. J Mol Liq. 2014;192:185–90.
Huang M-M, Jiang Y, Sasisanker P, Driver GW, Weingärtner H. Static relative dielectric permittivities of ionic liquids at 25 °C. J Chem Eng Data. 2011;56:1494–9.
Jing T, Lu L, Hui L, Wei G, Xia C. Measurement and estimation of the vaporization enthalpy for amino acid ionic liquids [Cnmim][Thr](n = 2, 4). J Chem Thermodyn. 2017;112:293–8.
Karmakar R, Samanta A. Steady-state and time-resolved fluorescence behavior of C153 and PRODAN in room-temperature ionic liquids. J Phys Chem A. 2002;106:6670–5.
Hallett JP, Welton T. Room-temperature ionic liquids: solvents for synthesis and catalysis. 2. Chem Rev. 2011;111:3508–76.
Weingartner H. Understanding ionic liquids at the molecular level: facts, problems, and controversies. Angew Chem Int Ed Engl. 2008;47:654–70.
Chiappe C, Pieraccini D. Ionic liquids: solvent properties and organic reactivity. J Phys Org Chem. 2005;18:275–97.
Hong M, Liu RJ, Yang HX, Guan W, Tong J, Yang JZ. Determination of the vaporisation enthalpies and estimation of the polarity for 1-alkyl-3-methylimidazolium propionate [Cnmim][Pro](n = 2, 3) ionic liquids. J Chem Thermodyn. 2014;70:214–8.
Duo Z, Wei J, Lu L, Ke Y, Mei H, Jing T. The molar surface Gibbs energy and polarity of ether-functionalized ionic liquids. J Chem Thermodyn. 2019;138:313–20.
Siedlecka EM, Czerwicka M, Stolte S, Stepnowski P. Stability of ionic liquids in application conditions. Curr Org Chem. 2011;15:1974–91.
Yue Z, Junfeng L, Haijiao L, Junshuang W, Jing T. Study on the polarity and molar surface Gibbs energy of ether-based amino acid ionic liquids [COC4mim][Gly], [COC4mim][Ala] and [COC4mim][Thr]. J Mol Liq. 2021;336:116094–101.
Yue Z, Chun G, Yuao L, Jian W, Jing T. Study on physicochemical and excess properties of [HEMIM][Ala] with DMSO/H2O binary mixtures. J Mol Liq. 2021;343:117491–8.
Hinks D, Rafiq MI, Price DM, Monteroa GA, Smitha B. A comparison of vapour pressure measurements of quinizarin and leucoquinizarin via transpiration and thermogravimetry. Color Technol. 2003;119:82–90.
Langmuir I. Phenomena, atoms and molecules. Soil Sci. 1950;69:417–8.
Junshuang W, Wenqing W, Lu L, Jing T. Measurement of evaporation entropy, evaporation enthalpy, and Gibbs free energy for the [C4Dmim]Gly and [C4Dmim]Ala. J Mol Liq. 2021;346:117142–51.
Archer DG, Widegren JA, Kirklin DR, Magee JW. Enthalpy of solution of 1-Octyl-3-methylimidazolium tetrafluoroborate in water and in aqueous sodium fluoride. J Chem Eng Data. 2005;50:1484–91.
Zaitsau DH, Yermalayeu AV, Emel’yanenko VN, Heintz A, Verevkin SP, Schick C, et al. Structure–property relationships in ILs: vaporization enthalpies of pyrrolidinium based ionic liquids. J Mol Liq. 2014;192:171–6.
Lu L, Yingping X, Xia C, Mei H, Jing T. Thermogravimetric analysis of enthalpy variation of 1-alkyl-3-methylimidazole chloride. Acta Phys Chim Sin. 2020;36:2004014–22.
Jie W, Xiaoxue B, Wei G, Nannan X, Dawei F, Yang W. Measurement of vaporization enthalpy by isothermogravimetrical method and prediction of the polarity for 1-alkyl-3-methylimidazolium acetate [Cnmim][OAc] (n = 4, 6) ionic liquids. RSC Adv. 2015;5:70333–8.
Jing T, Hongxu Y, Rujing L, Chi L, Linxing X, Jiazheng Y. Determination of the enthalpy of vaporization and prediction of surface tension for ionic liquid 1-alkyl-3-methylimidazolium propionate [C(n)mim][Pro](n = 4, 5, 6). J Phys Chem B. 2014;118:12972–8.
Lu L, Liqiang J, Haichun L, Dawei F, Jing T. Measurement of vaporization enthalpy for amino acid ionic liquids [Cnmim][Thr](n = 3, 5) using the isothermogravimetrical analysis. J Therm Anal Calorim. 2018;134:2247–54.
Lu L, Yuping X, Yue Z, Xia C, Jing T, Qingshan L. Using thermogravimetry analysis technology to appraise the vaporization enthalpy of 1-(2-Alkoxyethyl)-3-methylimidazolium + [Anion] ionic liquids. J Chem Eng Data. 2020;65:3933–40.
Yermalayeu AV, Zaitsau DH, Loor M, Schaumann J, Emel’yanenko VN, Schulz S, et al. Imidazolium-based ionic liquids: impact of the cation symmetry and alkyl chain length on the enthalpy of vaporization. Z Anorg Allg Chem. 2017;643:81–6.
Daguenet C, Dyson PJ, Krossing I, Oleinikova A, Slattery J, Wakai C, et al. Dielectric response of imidazolium-based room-temperature ionic liquids. J Phys Chem B. 2006;110:12682–8.
Wakai C, Oleinikova A, Ott M, Weingärtner H. How polar are ionic liquids? determination of the static dielectric constant of an imidazolium-based ionic liquid by microwave dielectric spectroscopy. J Phys Chem B. 2005;109:17028–30.
Lawson DD, Ingham JD. Estimation of solubility parameters from refractive index data. Nature. 1969;223:614–5.
Hildebrand JH. Factors determining solubility among non-electrolytes. Proc Natl Acad Sci USA. 1950;36:7–15.
Jian W, Junshuang W, Wenqing W, Jing T. Estimation of the polarity and prediction of the molar surface gibbs energy for amino acid ionic liquids — [C4Dmim][Gly] and [C4Dmim][Ala]. J Chem Thermodyn. 2021;158:106418–25.
Stoicescu C, Iulian O, Isopescu R. Liquid-liquid phase equilibria of 1-propanol + water + n-alcohol ternary systems at 298.15 K and atmospheric pressure. J Chem Eng Data. 2011;56:3214–21.
Neyband RS, Yousefi A, Zarei H. Experimental and computational thermodynamic properties of (benzyl alcohol + alkanols) mixtures. J Chem Eng Data. 2015;60:2291–300.
Benson GC, Murakami S, Jones DEG. The thermodynamic properties of acetone -I- cyclohexanol mixtures at 25 °C. J Chem Thermodynamics. 1971;3:719–31.
Zhankun J, Shoutao M, Lei W, Guoxin S, Yu C. Isobaric vapor-liquid equilibrium for the binary systems of sec-butyl acetate + methyl ethyl ketone, 2-methoxyethanol, or 1,2-dimethoxyethane at 101.3 kPa. J Chem Eng Data. 2016;61:336–41.
Pereiro AB, Tojo E, Rodriguez A, Canosa J, Tojo J. Properties of ionic liquid HMIMPF6 with carbonates, ketones and alkyl acetates. J Chem Thermodyn. 2006;38:651–61.
Resa J, Gonzales C, Concha R, Goenaga J. Experimental and predicted thermodynamic properties of mixtures containing corn oil with ketones and alkanes employed in their refine. Pol J Chem. 2006;80:129–41.
Dragoescu D. Refractive indices and their related properties for several binarymixtures containing cyclic ketones and chloroalkanes. J Mol Liq. 2015;209:713–22.
Abe J-I, Nakanishi K, Touhara H. Thermodynamic properties of aqueous solutions of hydrophilic compounds 1. Pyridine and methylpyridines J Chem Thermodyn. 1978;10:483–94.
Nayak JN, Aralaguppi MI, Aminabhavi TM. Density, viscosity, refractive index, and speed of sound for the binary mixtures of ethyl chloroacetate with n-alkanes (C6 to C12) at (298.15, 303.15, and 308.15) K. J Chem Eng Data. 2001;46:891–6.
Chen-Chieh W, Chein-Hsiun T. Densities, viscosities, refractive indexes, and surface tensions for binary and ternary mixtures of tetrahydofuran, 2-propanol, and 2,2,4-trimethylpentane. J Chem Eng Data. 2008;53:566–73.
Abboud’ J-LM, Notario R. The empirical treatment of solvent-solute interactions: 15 years of p*. J Phys Chem A. 1994;98:5807–16.
Tunc D, Gacal B, Yagcl Y. An amphipathic thioxanthone-anthracene photoinitiator for free-radical polymerization. Turk J Chem. 2013;37:525–37.
Galamba N, Paiva A, Barreiros S, Simões P. Solubility of polar and nonpolar aromatic molecules in subcritical water: the role of the dielectric constant. J Chem Theory Comput. 2019;15:6277–93.
Venkatramana L, Sivakumar K, Gardas RL, Reddy KD. Effect of chain length of alcohol on thermodynamic properties of their binary mixtures with benzylalcohol. Thermochim Acta. 2014;581:123–32.
Amp RJ, Supsup S, Sup S, Sup S, Vinita K. Static dielectric constants of the binary mixtures of N-methylformamide with water, ethyl alcohol, ethylene glycol, dimethylsulphoxide, acetone and 1,4-dioxane. Philos Mag Lett. 2010;90:463–70.
Kocsis LS, Kagalwala HN, Mutto S, Godugu B, Bernhard S, Tantillo DJ, et al. Mechanistic insight into the dehydro-Diels-Alder reaction of styrene-ynes. J Org Chem. 2015;80:11686–98.
Anno B, Delisi R, Goffredi M, Liveri VT. Effect of changes in solvent structure on proton migration, conductance and ion-pairing of hydrogen chloride in a methanol + methylcyclopentane mixture at 25°C. J Chem SOC, Faraday Trans. 1982;I(78):3101–8.
Kivilcim N, Seçkin T, Köytepe S. Porous pyridine based polyimide–silica nanocomposites with low dielectric constant. J Porous Mater. 2012;20:709–18.
Martin G, Mäki-Arvela P, Murzin DY, Salmi T. Solvent effects in the enantioselective hydrogenation of ethyl benzoylformate. Catal Lett. 2013;143:1051–60.
Tortai JH, Bonifaci N, Denat A. Insulating properties of some liquids after an electrical arc. EEE Trans Dielectr Electr Insul. 2002;9:3–9.
Ping W, Chenxin C. Solvent effects on the solid-state electrochemistry of samarium (III) hexacyanoferrate (II). J Electroanal Chem. 2005;576:49–56.
Acknowledgements
This project was supported by NSFC (21773100).
Author information
Authors and Affiliations
Contributions
Junshuang Wu was involved in conceptualization, methodology, writing—original draft, investigation, writing. Ning Wei helped in review & editing, visualization, Xiguang Chen helped in investigation, Rui Zhang contributed to validation. Xia Chen contributed to methodology. Jing Tong was involved in supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, J., Wei, N., Chen, X. et al. Determination of vapor pressure, evaporation enthalpy and polarity of imidazolium-based ionic liquids. J Therm Anal Calorim 149, 5511–5522 (2024). https://doi.org/10.1007/s10973-024-13107-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-024-13107-6