Abstract
Two-scale tests, microscale and bench scale, are conducted to analyze the flammability of a flexible polyurethane foam. Microscale tests include simultaneous thermal analysis coupled to Fourier transform infrared spectroscopy, and microscale combustion calorimeter (MCC). Evolved gas components, heat release rate per unit mass, total heat release, derived heat release capacity, and minimum ignition temperature are obtained. Bench scale tests are performed on cone calorimeter. Peak heat release rate per unit area, effective heat of combustion, minimum incident heat flux for ignition, and total heat release per unit area of different incident heat fluxes are obtained. FO-category of the PU foam is estimated by multiple discriminant function analysis based on the results of cone calorimeter test. The relationship between the two-scale tests is analyzed. The minimum ignition temperatures derived from multi heating rate MCC tests are used to predict the time to ignition and compared with the results from cone calorimeter tests. This PU foam is evaluated as a high fire hazard polymer having low heat release capacity, low ignition temperature, and short ignition time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyurethane (PU) foams have increasing applications in the world around us, including construction, insulation, furniture, and transportation industries [1]. But as Torvi et al. [1] mentioned, the fire safety and fire performance are key limited factors for its wide applications. During the previous decade, many serious building fires were related to PU. For example, one fire disaster occurred at Shanghai Jing’an Apartment caused unstandardized welded construction, resulting in 58 people dead and at least 70 people injured. For the residence fire, bedding and upholstered furniture are the first item ignited in roughly 19% of fatal fires [2]. In a white paper launched at the EU parliament in 2014, entitled “Europe is playing with fire”, Fire Safe Europe called on the European Commission to take actions to improve fire safety in buildings [3].
The flammability research on PU foams seems more important as the flame retardant is forbidden in PU foams used in upholstered furniture. In United States, Maine is the first state bans flame retardants in upholstered furniture, the new law will take effect on January 1, 2019, and prohibits the sale of residential upholstered furniture that contains more than 0.1% of flame-retardant chemical or more than 0.1% of a mixture that includes them [4]. This measure is due to the flame-retardant chemicals are linked to cancer, risk of birth defects, and learning disabilities in young children. Thus, fire behavior evaluation of PU foam will help fire protection engineers to improve fire prevention design and make reasonable fire control strategy.
Checchin [5] used cone calorimeter to study the fire behavior of seven types of polyurethane foams and pointed out that the cone calorimeter was effective enough for differentiating the fire behavior of materials with very low density, such as polyurethane foams. A special section of Fire Technology compiled three papers about research on the fire science and engineering of PU foams [2, 6, 7]. Cone calorimeter is one of the main facilities and data sources in all of these three papers used to describe burning behavior and establish fire safety engineering models of PU foams. It was also pointed out by Lefebvre et al. [8] that the evaluation of the flammability of flexible polyurethane foams using cone calorimeter was a suitable way to predict the fire behavior of foams when subjected to normalized fire tests. While Jerome Lefebvre et al. evaluated flammability of several flexible polyurethane foams by three standard tests, Federal Motor Vehicle Safety System (FMVSS No. 302), Cone calorimeter (ISO 5660), and Statutory Instrument 1324 (SI 1324) Schedule 1 Part test using the method of British Standard 5852, statistical study was also carried out to find correlations of test results and foam formulations by principle components analyses [8].
The microscale combustion calorimeter (MCC) was developed by Federal Aviation Administration (FAA) to offer industry a research tool to assist the FAA in its mandate to dramatically improve the fire safety of aircraft materials [9], as a quick screen method in the development of fire retardant materials. The tester has been validated by American Society of Testing Materials (ASTM), and MCC was established as an international standard test method ASTM D7309-13 [10]. MCC has been used as a handy tool in flammability analysis and screening fire retardant materials. Snegirev et al. [11] developed a comprehensive model, Pyropolis, to predict performance of polymer composite materials from either TGA or MCC measurements. Walters et al. [12] modified MCC to study the gas phase combustion of polymers and flame-retardant plastics. Stoliarov et al. [13] predicted burning rate of charring polymers in bench scale measurement based mainly on the data collected by TGA and MCC. Many researchers used MCC as one of the main facilities to develop and characterize of fire retardant materials [14,15,16].
In this study, two-scale experiments were conducted which leads to a comprehensive understanding of the flammability of the PU foam and provides reliable Material Flammability data for fire prediction and fire protection design. With the use of microscale TGA, DSC, and FTIR, the pyrolysis process and product of PU can be clearly understood, and MCC can obtain the information of the peak heat release rate, the peak heat release rate, the total heat release rate, and the heat release capacity of the PU in the non-flame combustion process. The ignition characteristics and flame burning characteristics of PU are obtained by bench scale tests, and the obtained specific parameters are the ignition time, the peak heat release rate, the peak heat release rate time, the effective combustion heat, and the total heat release. The relationship between the two-scale parameters is analyzed by empirical formula, and a comprehensive understanding of PU combustibility is achieved. The results of this study have implications about solid fuel burning behavior prediction [17, 18] and can help to get better understandings of relations between different scales of polymer fire hazards.
Experimental
Microscale tests
Simultaneous thermal analysis (STA, using a Netzsch STA 449C TG-DSC) was employed here, which applied TGA and DSC to PU samples under different gaseous atmosphere, nitrogen, air, and oxygen. A heating rate of 10, 20, 30, and 50 K min−1 to a maximum temperature of 600 °C was used with gas flow rates 75 mL min−1. Al2O3 crucibles without lid were used for measurements. Polyurethane sample was purchased from Yantai Wanhua Polyurethane Co., Ltd.
Coupled technique, STA-FTIR, that enables the identification of the gases evolved during PU pyrolysis was used in this research. The STA was coupled to Fourier transform infrared spectroscopy (FTIR), a type of 5700 instrument from Nicolet Instrument Company. For FTIR, the wavenumber range was set as 400–4000 cm−1. In this study, STA-FTIR instrument was employed to explore the degradation process of PU in air atmosphere.
MCC tests were conducted with a Govmark MCC-2 located at the VTT research center of Finland. Specifications of the Govmark MCC-2 instrument are as follows [9],
-
1.
Sample heating rate: 0–10 K s−1.
-
2.
Gas flow rate: 50–200 cm3 min−1, response time of < 0.1 s, sensitivity of 0.1% of full scale,
-
3.
Repeatability is ± 0.2% of full scale and an accuracy of ± 1% of full scale deflection.
-
4.
Sample size: 0.5–50 mg (milligrams).
-
5.
Detection limit: 5 mW.
-
6.
Repeatability: ± 2% (10 mg specimen).
Pyrolyzer heating temperature was from 75 to 600 °C, and combustor temperature was set at 900 °C.
All tests followed the “Method A” procedure of the MCC. In the “Method A” procedure, the specimen undergoes a controlled thermal decomposition [10] when subjected to controlled heating in an oxygen-free/anaerobic environment. The gases released by the specimen during operation are swept from the specimen chamber by nitrogen, subsequently mixed with excess oxygen, and then completely oxidized in a high-temperature combustion furnace. The volumetric flow rate and volumetric oxygen concentration of the gas stream exiting the combustion furnace are continuously measured during the test to calculate the rate of heat release by means of oxygen consumption. In Method A, the heat of combustion of the volatile component of the specimen (specimen gases) is measured but not the heat of combustion of any solid residue [10].
From the “Method A” procedure, the maximum HRR per unit mass pHRR, onset temperature Tonset, temperature at maximum HRR per unit mass TpHRR, total heat release THR, heat release capacity ηc, and oxygen concentration at maximum HRR △O2 can be determined.
The PU foam shows light yellow color and has a density of 44.0 kg m−3. Specimen of MCC test was taken from the foam panel. The specimens were prepared in three groups with nominal specimen masses of 1.50, 2.50, and 3.50 mg; the group of tests was labeled as 1.50, 2.50, and 3.50 mg. In the tests, nine heating rates, 0.1, 0.2, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, and 3.5 K s−1, were selected.
Bench scale tests
Bench scale tests of the PU foam were carried out on cone calorimeter in accordance with ISO 5660 [19]. For cone calorimeter tests, the specimens of PU foam were cut as 100 by 100 mm square samples, with a thickness of 20 mm. All test specimens were conditioned according to the standard, and during tests, were exposed in the horizontal orientation with the standard pilot operating. Specimens were tested with the use of an edge frame sample holder to retain the specimen as allowed in the standard. The edge frame holder reduces the test surface area to 0.0088 m2, and this is the area used in calculations. Specimens were packed to the correct test level height using ceramic fiber blanket. The specimen inside the holder was supported horizontally on a load cell and exposed to a set external heat flux with irradiance level of 17, 25, 35, 50, and 75 kW m−2. Ignition is promoted using a spark igniter. The nominal exhaust system flow rate for all tests was 0.024 m3 s−1.
Specimen mass was weighed by a Mettler AX205 Analytical Semi Micro Balance Delta Range with readability of 0.01 mg in the weighing range of 81 g.
Test results and analysis
TGA–DSC–FTIR
Within the experimental temperature range, the residual mass of the PU foam reaches 25% in N2 atmosphere, while no mass residue is observed in all experiments of air and O2 atmosphere. Almost all the PU foam was pyrolyzed in air and O2 atmosphere, which means that polyurethane reacted with oxygen which promoted its decomposition process. Four different heating rates, 10, 20, 30, and 50 K min−1 were performed for each atmosphere. However, different heating rates showed the same mass variation only with TGA curves shifting to higher temperature direction for high heating rate. In the following discussion, 10 K min−1 cases for different pyrolysis atmospheres are selected as examples for discussion and comparison.
From Fig. 1, it can be seen that the main mass loss peak of PU in nitrogen, is located at 310 °C, with two flat shoulders’ peak from 150 to 220 °C and 340 to 430 °C, and peak at 190 and 400 °C, respectively, and in accordance with three distinct endothermic peaks in DSC curve. It indicates that thermal degradation process of PU undergoes one principle and the other inconspicuous stage in nitrogen. As Fig. 2 shows, two obvious degradation steps can be observed, two evident DTG peaks is located at 335 and 535 °C, and two exothermic peaks are also in this temperature range as DSC curve shows in air. Moreover, the slight shoulder located at 190 °C exists. Figure 3 represents the TG–DTG–DSC curves of PU in oxygen atmosphere at the heating rate of 10 K min−1. At 325 and 494 °C, two primary peaks are showed in DTG curves, in accordance with the two exothermic peaks in DTG curve. The profile is almost the same as that in air, but the steps and peaks are slightly ahead of the latter. From [20,21,22,23], the pyrolysis of such flexible polyurethane shows similar variations with those of rigid polyurethane.
The results of FTIR experiment also indicate that the whole pyrolysis process is divided into two steps, and the main product is generated in these two steps, as shown in Figs. 4 and 5. There was no obvious difference for escaped gas content when the heating rate increased from 10 to 50 °C min−1. However, the infrared absorption increased almost three times, which means that the density of escaped gas increased. Under higher heating rate, the escaped gas would collect higher concentration.
In the absorption ranging from 4000 to 400 cm−1, two absorption peaks 667 and 2340 cm−1 are very obvious, which were caused by asymmetric stretching and bending vibrations of CO2. The absorption around 850–1050 cm−1 was caused by the stretching vibration of ether bond C–O–C, and the shoulder absorption peaks attributes to the stretching vibration of CO. In high absorption wavenumber, the vibration peak of 3600 cm−1 was caused by the generation of H2O and aliphatic alcohol. During the whole heating process, some small molecule ethers could volatilize during the low range temperature 100–175 °C. After the temperature reached 230 °C, the main escaped gas was CO2 accompanying with some CO and a little of –OH compound. During the temperature ranging from 200 to 644 °C, large amounts of CO2 produced, and its concentration reached maximum value when temperature reached 311 and 520 °C. At 311 °C, the main chain of PU broke and produced isocyanates, then isocyanates reacted dimerization and trimerization reactions, during which large parts of CO2 could be generated, meanwhile some other CO2 could be produced by the oxidative reaction of regenerated polyols [24, 25]. At 520 °C, the degradation reaction closed to the end, and the main production of residue oxidation was also CO2. During the temperature ranging from 273 to 650 °C, the absorption peak happened at 520 °C, which was mainly caused by the incomplete oxidation of aromatic hydrocarbons and amines.
MCC test
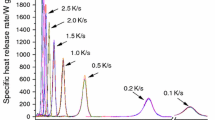
The HRR curves from MCC mainly show two main peaks with a small peak fluctuation between two main peaks, but there are two stages in general. The shape of HRR curve is closer to the DSC and DTG of PU in air and O2 atmospheres, but the peak value of most curves is the primary peak at low temperature stage (Fig. 6).
pHRRs increase with heating rates (as shown in Fig. 7), but the HRR is not as high as some other thermoplastic polymers in the literatures. For example, the pHRR of the PU foam at 1 K s−1 is 85.1 ± 4.7 W g−1, while the average pHRR of polymethylmethacrylate was 387 ± 22.2 W g−1 [26], around 900 and 520 W g−1 of two different extruded polystyrene foams [27], 693.1 W g−1 of an expanded polystyrene foam at 50 K min−1 [28], but it is close to a PVC with pHRR of 110 W g−1 [29]. Among 17 tested common polymers [30], only fluorinated ethylene propylene had a low pHRR of 82 W g−1, other 16 polymers’ pHRRs were above 150 W g−1.
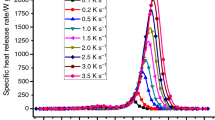
Compare with the seventeen polymers [30], this PU foam’s THR (from 16.5 to 17.6 kJ g−1 in average shown in Fig. 8) is in medium level, while the value of polymethylmethacrylate in [26] was 22.46 ± 0.42 kJ g−1, more than 22.9 for an expanded polystyrene foams in [28], 27.4 and 32.1 kJ g−1 for two kinds of extruded polystyrene foams in [27].
A shift of TpHRR to higher temperature as the heating rate β increases is observed in agreement with TGA tests. TpHRR versus β−1 can be fitted by Eq. 1. The fitting parameters are listed in Table 1. TpHRR was taken as ignition temperature in literature [31], thus minimum ignition temperatures can be calculated for PU foam by Eq. 2, as listed in Table 2. The average minimum TpHRR is 272.5 °C, and it is very low temperature for polymers [27, 30] and it would be ignited at a lower temperature (Fig. 9)
Cone calorimeter test
Figure 10 illustrates the HRR curves from cone calorimeter tests. Some typical parameters are given in Table 3.
As shown in Fig. 10, the PU foam can be ignited at lower incident heat flux, and it shows an obvious two-stage phenomenon described in the literatures [2, 7, 8]. The change of time to ignition is not significant for the tests of incident heat fluxes over 17 kW m−2.
The test results are summarized in Table 2. The incident heat flux was plotted versus the reciprocal ignition time (Fig. 11), which is known as Janssens’ method [32] and showed that the minimum heat flux required for ignition of the PU foam was 6.75 kW m−2. The time to ignition data of 50 and 70 kW m−2 are too close to each other and cannot be used in this method.
Material’s fire behavior in ISO 9705 room can be predicted based on the cone calorimeter tests of 50 kW m−2. The FO-category grouping classifies material belonging to which category according to the following set of rules [33]:
-
FO-category 1: products not reaching flashover during 1200 s of testing time.
-
FO-category 2: 600 s < tFO < 1200 s.
-
FO-category 3: 120 s < tFO < 600 s.
-
FO-category 4: tFO < 120 s.
Materials can be determined to which FO-category based on statistical information from cone calorimeter, known as Anne Steen Hansen’s multiple discriminant function analysis method (MDA) [33]. Using Fisher’s linear discriminant function for cases classification, the result of this analysis is a set of four linear functions, one for each of the four FO-categories. The parameters used by this method are:
-
z1 = mean density (kg m−3).
-
z2 = THR300 (MJ m−2) = total heat release during 300 s after apparent time to ignition.
-
z3 = ln(FIGRAcc) where FIGRAcc is the maximum value of the ratio between HRR and time when HRR was measured.
Hansen [33] gave four classification functions that are expressed as follows:
-
FFO1 = 0.01789z1 − 0.06057z2 + 0.971z3 − 7.910.
-
FFO2 = 0.01492z1 + 0.03354z2 + 1.877z3 − 7.418.
-
FFO3 = 0.008589z1 + 0.409z2 + 2.721z3 − 13.406.
-
FFO4 = 0.0000256z1 + 0.347z2 + 3.621z3 − 9.215.
For the PU foam in our tests the mean density is 44 kg m−3, from three 50 kW m−2 tests THR300 are 12.8, 13.4, and 13.1 MJ m−2; ln(FIGRAcc) are 1.94, 1.49, and 1.70. Substitute these data into above four classification functions, the results are listed in Table 3. For three 50 kW m−2 tests, all FFO4 give the largest value of all four Fisher’s liner discriminate functions. Thus, the PU foam can be determined as a member of FO-category 4, which would reach flashover in ISO room within 120 s, it is very dangerous.
The relation between MCC and cone calorimeter data
Equation 2 [34] is used to predict the temperature increase rate at sample surface
where hg is the total heat of gasification per unit mass of PU, and qnet is net heat flux at the sample surface in pilot ignition. The heat of gasification of PU is estimated with cone calorimeter test data [2, 35], dividing the measured heat of combustion by the slope of a plot of HRR versus nominal heat flux. Using the average EHC in Table 2, the resulting heat of gasification of the PU foam is estimated to be 41.3 MJ kg−1/2.18 = 18.95 MJ kg−1. Assuming qnet = incident heat fluxes, temperature increasing rates on sample surface are predicted with Eq. 2. The rates of surface temperature rise are listed in Table 4. The concept of virtual heating rate is introduced here, because it is difficult to achieve such a high heating rate in real fire scenario.
Using minimum Tmax listed in Table 1 as ignition temperature, the times to ignition are calculated and illustrated in Table 4. Times to ignition can be calculated directly by the following equation [35]:
where TRP is thermal response parameter, for PU foam is 105 kW s−1/2 m−2 [35], qr is incident heat flux (kW m−2), and qmin is the minimum heat flux for ignition which is 6.75 kW m−2.
The tig predicted by ignition temperature and heating rate is shorter than that predicted from Eq. 3, listed in Table 4, but the predicted tig at higher incident heat fluxes, 35, 50, and 75 kW m−2, are close to the results from cone calorimeter tests (Table 2).
Conclusions
Comparing the results of above two-scale tests, some conclusions can be drawn here.
First, in microscale experiments, results by MCC, STA, and FTIR showed that the pyrolysis process of the PU foam was mainly divided into two stages. However, there is no two-stage observed in bench scale experiment, which is quite different from those described in some literatures.
Second, the microscale MCC experiment shows that the peak heat release rate is not high, which is in agreement with the result obtained by the cone calorimeter experiment. However, the minimum ignition temperature speculated by MCC is much lower than those of other polymers obtained by the same method. It is combined with the short time to ignition obtained in the cone calorimeter experiment to determine that the PU foam is a kind of combustible material which is very easy to be ignited, but the rate of heat release is not high.
Third, it is found that the heat release capacity of the PU foam obtained by MCC still depends on the heating rates. This may lead the classification of flammability by heat release capacity inaccurately.
Comprehensive analysis of different scales tests and multiple parameters of flammability would be the basis of reliable fire safety evaluation.
Abbreviations
- EHC:
-
Effective heat of combustion
- h g :
-
Combustion heat
- MCC:
-
Microscale combustion calorimeter
- pHRR:
-
Maximum HRR per unit mass
- PU:
-
Polyurethane
- T onset :
-
Onset temperature
- T pHRR :
-
Temperature at maximum HRR per unit mass
- THR:
-
Total heat release
- t ig :
-
Time to ignition
- t pHRR :
-
Time to peak HRR
- TRP:
-
Thermal response parameter
- q r :
-
Incident heat flux
- q min :
-
Minimum heat flux for ignition
- q net :
-
Net heat flux
- β :
-
Heating rate
- h g :
-
The total heat of gasification per unit mass
- κ :
-
Heat conductivity
- ρ :
-
Density
- η c :
-
Heat release capacity
References
Torvi D, Weckman B. Guest editorial: special issue on polyurethane foam combustion. Fire Technol. 2014;50:633–4.
Pitts WM. Applied heat flux distribution and time response effects on cone calorimeter characterization of a commercial flexible polyurethane foam. Fire Technol. 2014;50:635–72. https://doi.org/10.1007/s10694-011-0235-8.
Fire Safe Europe-White paper, Dec 2014. www.firesafeeurope.eu.
Checchin M, Cecchini C, Cellarosi B, Sam FO. Use of cone calorimeter for evaluating fire performances of polyurethane foams. Polym Degrad Stab. 1999;64:573–6.
Hadden R, Alkatib A, Rein G, Torero JL. Radiant ignition of polyurethane foam: the effect of sample size. Fire Technol. 2014;50:673–91. https://doi.org/10.1007/s10694-012-0257-x.
Ezinwa JU, Robson LD, Obach MR, Torv DA. Evaluating models for predicting full-scale fire behaviour of polyurethane foam using cone calorimeter data. Fire Technol. 2014;50:693–719. https://doi.org/10.1007/s10694-011-0239-4.
Lefebvre J, Bastin B, Le Bras M, Duquesne S, Ritter C, Paleja R, Poutch F. Flame spread of flexible polyurethane foam: comprehensive study. Polym Test. 2004;23:281–90.
Govmark Datasheet of Micro-scale Combustion Calorimeter (MCC2), the Govmark Organization, Inc.
Standard Test Method for Determining Flammability Characteristics of Plastics and Other Solid Materials Using Microscale Combustion Calorimetry, ASTM D7309-13; 2013.
Snegirev AY, Talalov VA, Stepanov VV, Harris JN. A new model to predict pyrolysis, ignition and burning of flammable materials in fire tests. Fire Saf J. 2013;59:132–50.
Walters RN, Safronava N, Lyon RE. A microscale combustion calorimeter study of gas phase combustion of polymers. Combust Flame. 2015;162:855–63.
Stoliarov SI, Crowley S, Walters RN, Lyon RE. Prediction of the burning rates of charring polymers. Combust Flame. 2010;157:2024–34.
Liu Y, Zhang Y, Cao Z, Fang Z. Synthesis and performance of three flame retardant additives containing diethyl phosphite/phenyl phosphonic moieties. Fire Saf J. 2013;61:185–92.
Chen X, Zhuo J, Jiao C. Thermal degradation characteristics of flame retardant polylactide using TG-IR. Polym Degrad Stab. 2012;97:2143–7.
Wu K, Zhang YK, Zhang K, Shen MM, Hu Y. Effect of microencapsulation on thermal properties and flammability performance of epoxy composite. J Anal Appl Pyrolysis. 2012;94:196–201.
Jiang L, He JJ, Sun JH. Sample width and thickness effects on upward flame spread over PMMA surface. J Hazard Mater. 2018;342:114–20.
Jiang L, Miller C, Gollner M, Sun JH. Sample width and thickness effects on horizontal flame spread over a thin PMMA surface. Proc Combust Inst. 2017;36(2):2987–94.
ISO 5660-1, Reaction to fire tests- Heat release, smoke production and mass loss rate, Part 1: Heat release rate (cone calorimeter method); 2002.
Oprea S. Effect of structure on the thermal stability of crosslinked poly (ester-urethane). Polimery. 2009;54(2):120–5.
Zieleniewska M, Leszczyński MK, Szczepkowski L, et al. Development and applicational evaluation of the rigid polyurethane foam composites with egg shell waste. Polym Degrad Stab. 2016;132:78–86.
Salasinska K, Borucka M, Leszczyńska M, et al. Analysis of flammability and smoke emission of rigid polyurethane foams modified with nanoparticles and halogen-free fire retardants. J Therm Anal Calorim. 2017;130(1):131–41.
Jiang L, Zhang D, Li M, et al. Pyrolytic behavior of waste extruded polystyrene and rigid polyurethane by multi kinetics methods and Py-GC/MS. Fuel. 2018;222:11–20.
He JJ, Jiang L, Sun JH, et al. Thermal degradation study of pure rigid polyurethane in oxidative and non-oxidative atmospheres. J Anal Appl Pyrolysis. 2016;120:269–83.
Jiang L, Xiao HH, He JJ, et al. Application of genetic algorithm to pyrolysis of typical polymers. Fuel Process Technol. 2015;138:48–55.
Xu Q, Jin C, Griffin GJ, Matala A, Hostikka S. A PMMA flammability analysis using the MCC effect of specimen mass. J Therm Anal Calorim. 2016;126(3):1831–40. https://doi.org/10.1007/s10973-016-5688-z.
Xu Q, Jin C, Jiang Y. Compare the flammability of two extruded polystyrene foams with microscale combustion calorimeter and cone calorimeter tests. J Therm Anal Calorim. 2017;127(3):2359–66. https://doi.org/10.1007/s10973-016-5754-6.
Xu Q, Jin C, Jiang Y. Analysis of the relationship between MCC and thermal analysis results in evaluating flammability of EPS foam. J Therm Anal Calorim. 2014;118(2):687–93. https://doi.org/10.1007/s10973-014-3736-0.
Schartel B, Pawlowski KH, Lyon RE. Pyrolysis combustion flow calorimeter: a tool to assess flame retarded PC/ABS materials. Thermochim Acta. 2007;462:1–14.
Principles and Practice of Microscale Combustion Calorimetry, DOT/FAA/TC-12/53; 2013.
Lyon RE, Walters RN, Stoliarov SI. A new methodology for measuring flammability parameters of plastics. In: Proceedings of the 64th annual conference of the society of plastics engineers, May 7–11. Charlotte; 2006. p. 1626–30.
Janssens ML. Improved method of analysis for the LIFT apparatus, Part I: ignition. In: Proceedings of 2nd fire and materials conf. interscience communications, London, England; 1993. p. 37–46.
Hansen SH, Hovde PJ. Prediction of time to flashover in the ISO 9705 room corner test based on cone calorimeter test results. Fire Mater. 2002;26(2):77–86.
Lyon RE. Heat release kinetics. Fire Mater. 2000;24:179–86.
Tewarson A. Flammability Parameters of Materials: ignition, combustion, and fire propagation. J Fire Sci. 1994;12:329. https://doi.org/10.1177/073490419401200401.
Acknowledgements
This research is funded by the National Natural Science Foundation of China, No. 51776098, and supported by the Fundamental Research Funds for the Central Universities, no. 30918015101 and China-Slovak joint research project 8-8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Q., Jin, C., Majlingova, A. et al. Evaluate the flammability of a PU foam with double-scale analysis. J Therm Anal Calorim 135, 3329–3337 (2019). https://doi.org/10.1007/s10973-018-7494-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7494-2