Abstract
Flammability studies are conducted to evaluate the behavior of materials exposed to fire. In this study, microscale combustion calorimetry (MCC) and cone calorimetry methods were applied to acquire the flammability characteristics of red and grey extruded polystyrene (XPS) samples. To understand the effect of changes between parameters, Pearson’s correlation coefficient was used to examine their linear relationships. From the research, moderate and weak correlations were recorded between the total heat release rates from both methods for red and grey XPS, respectively. Plotting peak heat release rate against heat release temperature for MCC and ignition temperature for cone test showed that 25, 35 and 50 kW m−2 incident heat fluxes of the cone test fall within 0.2 K s−1 and 0.5 K s−1 heating rates of MCC. Also, all the MCC parameters except char yield and total heat release presented good correlations with the cone calorimetry flammability characteristics. Hence, MCC could be used in conjunction with cone calorimetry to accurately and reliably assess the flammability of materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microscale combustion and cone calorimetry experiments are thermal analysis methods that play active roles in effective flammability studies. They are carried out to assess the flammability of materials such as polymers. Among other polymers, polystyrene was used as a reference for the calibration of most of the flammability equipment. This material is widely used and has a high production rate around the world. Extruded polystyrene formed by the extrusion of polystyrene beads is normally used as insulation material for energy efficient buildings. Therefore, research on the flammability of extruded polystyrene using flammability test methods is highly beneficial.
Microscale combustion calorimetry (MCC) also known as the pyrolysis combustion flow calorimetry (PCFC) is a small-scale experiment used to test the flammability of milligram-sized samples [1]. The MCC experiment is conducted under an inert gas atmosphere, usually nitrogen or nitrogen mixed with oxygen, in the pyrolysis stage. The gases released from the pyrolysis are combusted in the presence of oxygen under controlled conditions [2]. It is worth noting that MCC has been extensively used in recent years to obtain precise flammability characteristics such as the total heat released, heat release capacity, peak heat release rate and temperature at peak heat release of materials to analyze their flammability [3]. Considering the type of materials that can be tested with this method, MCC has been successfully used to test not only polymeric materials but also wood [4]. Lyon et al. [5] applied MCC as a flammability screening method for flame retardants in plastic materials. On a similar note, Schartel et al. [6] assessed the use of MCC as a tool in evaluating the flammability of blends of polycarbonate (bisphenol A)/acrylonitrile–butadiene–styrene (PC/ABS) with additives and flame retardants. Also, the flammability of cotton and nylon fabrics treated with flame retardants was tested using MCC in the work of Yang et al. [7].
Aside MCC, another equally important and frequently used flammability test method is the cone calorimetry. This is a bench scale method which employs the use of a cone-shaped heater to heat samples of size 100 mm × 100 mm × 10–50 mm under controlled conditions. With this flammability experiment, the sample is positioned under a cone heater and heated within an incident heat flux of 10–75 kW m−2. The sample is ignited by an electric spark which remains lighted until the sample ignites. The gases released are collected in a hood from which the various parameters are measured [8, 9]. Cone calorimetry measures the peak heat release rate and time to ignition of the materials tested. However, unlike MCC, the parameters measured from the cone calorimetry experiment are highly dependent on the ignition source, orientation of sample and its thickness [10]. Cone calorimetry has been widely adopted by several scholars in their research to evaluate fire safety performance of polymeric materials. In the work of Qin et al. [11,12,13], cone calorimetry experiment was conducted to assess the thermal stability and flammability of polyethylene/clay nanocomposites, polypropylene/montmorillonite composites and polyamide 66/montmorillonite nanocomposites. Similarly, Delichatsios et al. [14] employed cone calorimetry in their research to analyze the flammability of charring materials.
Cone calorimetry could be used to represent specific fire scenarios as well as the prediction of specific parameters in small scale, large scale and real fire behavior [10]. It has been stated in [10] that results from cone calorimetry have the capability of simulating real fire behavior and other fire tests. It has also been proven experimentally in [15] that the result from cone calorimetry can be used to predict full-scale smoke production at 400 kW heat release rate.
In recent studies, both microscale combustion and cone calorimetry methods have been incorporated in research to make a comparison and evaluation of flammability characteristics. Combining the two methodologies gives an in-depth understanding on the flammability of the materials under consideration. Also, the joint assessment could serve as a tool for predicting bench scale flammability measurements from small-scale tests. This is demonstrated in the research of Xu et al. [16] where they compared the flammability of two different extruded polystyrene foams using MCC and cone calorimetry. They made an inference that results from MCC can be used to predict the ignition time of cone calorimeter tests. However, Xu et al. did not consider correlation analysis of the two experiments. It is important to note that correlations between parameters measured from both experiments also offer some advantages in flammability studies by giving details on the relationship between the various flammability characteristics. The methodology described has been used to analyze the flammability of numerous materials by several researchers. Cogen et al. [17] effectively run Pearson’s correlations between MCC and other conventional flammability tests on flame-retardant polyolefin compounds free of halogen. A quite similar method was used by Lyon et al. [18] to provide the relationship between heat release capacity from MCC and thermogravity analysis (TG) test for 14 different polymers. Also, Lin et al. [19] conducted flammability analysis of flame-retardant wire and cable compounds using MCC, cone calorimeter, limiting oxygen index and UL94. Subsequently, correlations between the parameters were calculated to show the strength of their relationships.
Despite the fact that cone calorimetry has achieved a high degree of accuracy in flammability studies, it’s use is limited due to high cost, equipment complexity and maintenance [17]. MCC has the capability of correlating fire behavior with material properties, but the results do not include ignition parameters. Owing to this, correlations are conducted between cone calorimetry and MCC to ascertain their linear relationships for prediction and joint assessment purposes. Therefore, this research seeks to perform correlation analysis on the flammability characteristics of MCC and cone calorimetry with grey and red extruded polystyrene as the reference materials. In this study, Pearson’s correlation coefficients between cone calorimetry and MCC parameters will be derived to evaluate their linear relationships. It attempts to show how MCC could be used in conjunction with cone calorimetry to accurately and reliably assess the flammability of materials.
Experimental
Material
The materials used for the experiments were red and grey extruded polystyrene (XPS) with 3% flame-retardant additives manufactured by Zheng bang Newly Building Material Co. Ltd. in China. The XPS was produced by extrusion of the polystyrene material used as a reference for calibration of most fire equipment. The properties of the XPS used are shown in Table 1.
The thermal properties of the materials were measured with a Hot Disk TPS 2500 s. The samples were taken from large XPS boards and weighed with a Mettler AX205 AX-205 Analytical Semi Micro Balance Delta Range with a readability of 0.01 mg and a weighing range of 81 g.
Microscale combustion calorimetry
The MCC experiment was conducted in accordance with the set guidelines in ASTM D7309-13 [2]. Milligram samples of red and grey extruded polystyrene were tested in an MCC-2 equipment from Govmark Limited at the VTT Technical Research Center of Finland. The masses of the samples of red XPS were in groups of 1 mg, 1.5 mg and 2 mg with standard errors of 0.043, 0.052, and 0.055 mg, respectively. Likewise, the grey XPS samples were prepared in three groups specifically 1.5 mg, 2.5 mg and 3.5 mg with 0.188, 0.101 and 0.159 mg as the standard errors. Twenty-seven samples were tested for each type of XPS.
The samples were heated at nine selected heating rates (β), thus, 0.1, 0.2, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, and 3.5 K s−1 under controlled conditions. The method A procedure for MCC experiments as described in [1, 2, 16, 20] was applied. Samples were pyrolyzed in nitrogen atmosphere and combusted in a high-temperature tubular furnace with a mixture of nitrogen and oxygen. The gases used for the experiment were Instrument Nitrogen 5.0, Chemical oxygen 3.5, and Instrument synthetic air 5.0 produced by AGA industrial gases, Finland. The temperature for pyrolysis and combustion was 75–600 °C and 900 °C, respectively. Oxygen consumption calorimetry was applied in calculating the heat release rate from the volumetric flow rate and oxygen concentration of the gases that flowed out of the combustor. During the experiment, three replicate prepared samples were tested for each polystyrene type and an average of the measured results was recorded. Samples were labeled according to their heating rates as xps_red_0.1_1 representing the first sample of red XPS tested under 0.1 K s−1. The time to peak heat release rate, heat release capacity, temperature at peak heat release, char yield and total heat released were measured and recorded.
Cone calorimetry
The cone calorimeter experiment was performed at the State Key Laboratory of Fire Science in China. Samples of 100 ± 1 mm × 100 ± 1 mm × 20 ± 1 mm were cut from large red and grey extruded polystyrene (XPS) boards and prepared for the experiment. The bottom and sides of the samples were wrapped in aluminum foils, carefully covered to prevent the exposure of excess foils and placed in a specimen holder horizontally. The samples were heated in an open-centered conical heater, while the volatile combustion products flowed into an exhaust tube [8,9,10, 16]. Three replicate samples of XPS were tested under 25, 35, and 50 kW m−2 incident heat flux according to ISO 5660-1 [9]. Samples were labeled according to the tested incident heat flux and color as xps_red_25_1 representing the first red sample tested under 25 kW m−2. To ensure accuracy of the experiment, each test was repeated three times and the average of the three results was recorded. Also, all required preliminary calibrations on the cone calorimeter were performed before conducting the experiments to ensure precision of measurements and readings. The test surface area for this experiment was 0.008848 m2 as well as a sampling rate of 1 sample s−1.
Pearson’s correlation coefficient
Pearson’s correlation coefficient is a statistical method used to measure the linearity of two variables [21]. The magnitude of the linear relationship is indicated by an R-value which ranges from − 1 to + 1. Positive linear relationships denote the variables increase or decrease simultaneously, whereas negative R-values signify a decrease in one parameter as the other increases [22]. The Pearson correlation coefficient \((r_{\text{xy}} )\) of two variables X and Y having a series of measurements \(x_{\text{i}}\) and \(y_{\text{i}}\) where \(i = 1,2, \ldots ,n\) is calculated by using Eq. (1) [23],
where n is the sample size, \(\left( {\sum x_{\text{i}} } \right)\) and \(\left( {\sum y_{\text{i}} } \right)\) are the sum of the X and Y variables, respectively. Pearson’s correlation was used in this research to assess the effect of a change in a parameter over the other flammability characteristics in both MCC and cone calorimetry. The X and Y variables were the flammability characteristics of both methods. In relation to MCC, \(x_{\text{i}}\) and \(y_{\text{i}}\) were the measurements obtained at different heating rates, i.e., \(i = 0.1,0.2, \ldots ,3.5\). Similarly, with cone calorimetry, variables measured at three heat fluxes were used. Furthermore, this correlation method was applied in matching the parameters of MCC against cone calorimetry. The classification of the range of R-values and their corresponding magnitudes as used in this paper are presented in Table 2. A statistical analysis tool was used in deriving the Pearson correlation coefficients. A two-tailed test of significance was applied with all correlations being significant at the 0.05 level.
Results and discussion
Analysis of MCC data
The parameters used for the MCC analysis in this research were the peak heat release rate (pHRR), heat release capacity (\(\eta_{\text{c}}\)), temperature at pHRR (pTemp), total heat release (THR) and char yield. Results interpretation of MCC data requires some parameters to be calculated from the basic ones that are measured from the experiment. According to method A from MCC standards in [2] the heat release capacity, char yield and total heat released were derived using the equations shown.
\(\eta_{\text{c}}\) describes the thermal stability of a material and it represents the average heat released in the combustion stage per change in temperature [2, 20, 21].
where Qmax = pHRR/W g−1 and \(\beta\) = average heating rate over the measurement range/K s−1.
Char yield represents the mass ratio of the sample which does not undergo combustion in the flame zone [2, 10]. It is defined as the mass of residue (mp/g) divided by initial mass of sample (mo/g).
THR signifies the heat output at a specific heat release rate and time (t) [10]. It is characterized by the area under the HRR versus time graph.
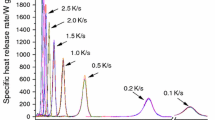
Generally, the flammability of materials is characterized by the amount of heat released when the material is exposed fire. Therefore, the most important characteristic that is measured from an MCC experiment is HRR [24]. Specific heat release rates at nine heating rates were plotted against their respective temperatures for red and grey XPS in Figs. 1 and 2, respectively. It is clearly seen in Figs. 1 and 2 that the peaks of the curves representing the peak heat release rate and their respective temperatures increase as heating rate increases. Comparing the two figures revealed that the temperature at peak heat release rate is almost similar for the two materials at the same heating rates. On the other hand, it was observed that red XPS achieved higher specific heat release rate than grey XPS at the same heating rate. This confirms what has been done in literature by Xu et al. [16]. The results obtained for testing 1.5 mg samples of red and grey XPS are presented in Tables 3 and 4.
Tables 5 and 6 show the R-values derived from the Pearson correlation between the MCC parameters obtained for red and grey XPS. The results from the tables clearly show that pHRR had a very strong negative linear relationship (− 0.95 and − 0.98) with heat release capacity. For most polymeric materials, pHRR increases, while \(\eta_{\text{c}}\) decreases with increasing heating rate [19, 20]. This explains the strong negative values presented. pHRR increases with an increase in pTemp and it is evidently shown by a strong positive relationship (0.96 for red and 0.97 for grey) for pHRR/pTemp.
It is worth noting that R-values observed for THR and char yield differed for both materials. pHRR had a strong relationship with THR for grey and a weak relationship for red XPS. Char yield for red XPS presented strong linear relationships with all the sets with the exception of THR (− 0.48). On the other hand, the char yield for grey XPS showed very weak linear relationships for pTemp, pHRR and \(\eta_{\text{c}}\). A moderate relationship (− 0.48) was recorded for char yield/THR in Table 6. From the analysis, similar R-values and significance were seen in the pairs of char yield/THR for both materials. The independence of char yield on THR could be due to the forced complete combustion of pyrolysis products from the MCC experiment.
Analysis of cone calorimetry data
In analyzing the cone calorimetry test, the parameters used were peak heat release rate (qmax), ignition temperature (Tig), time to ignition (tig), THR, fire growth rate (FIGRA) and fire performance index (FPI). FIGRA and FPI were derived from the basic parameters measured from the experiment. There was no residue left in this test so char yield was not included in the evaluation.
FIGRA is defined by pHRR divided by the time to peak heat release rate. In flammability analysis, a better flame resistance ability is signified by low FIGRA values [9, 16].
FPI is the time to peak heat release rate divided by peak heat release rate. Higher FPI values denote better flame resistance [16].
The values of fire performance index of red and grey XPS for the cone calorimetry experiment were plotted against time to ignition in Figs. 3 and 4. The figures show three samples for each incident heat flux tested. From the figures, fire performance index and time to ignition decreases as the incident heat flux increases. Thus, at high incident heat flux, low fire performance and shorter ignition times were recorded. Therefore, it can be deduced from the two plots that rapid ignition produces low fire performance and vice versa. Both materials exhibit good flame resistance and longer time to ignition at low incident heat flux as seen in most polymers.
Also, shown in Figs. 5 and 6 are plots of fire growth rate against the time to ignition at 25, 35 and 50 kW m−2 incident heat flux of red and grey XPS. From the graph illustrated, FIGRA increases with an increase in incident heat flux, while time to ignition decreases with increasing incident heat flux. Therefore, fire growth rate reduces at longer ignition times and vice versa.
To determine whether the foam used for the experiment was thermally thin or thick the Biot’s number for the material was calculated using Eq. (7) [25];
where Bi is the Biot’s number with a material having Bi > 0.1 being thermally thick as used in [25], Lc represents the characteristic length defined by the volume of a material per its area, h is the heat transfer coefficient and k, the thermal conductivity of the extruded polystyrene. With a Biot number of 0.6, the XPS material was found to be thermally thick; therefore, temperature varied throughout the material.
To compare temperature at peak heat release rate from MCC and cone calorimetry, it was imperative to consider the surface temperature gradients during pyrolysis for the samples tested. Thermally thick materials undergo convection, radiation and conduction processes [26,27,28]. Thus, all these processes must be accounted for to obtain the surface temperature. Also, the thermal inertia \(\left( {k\rho c} \right)\) of XPS play a key role in acquiring the surface temperature [29, 30]. The higher the thermal inertia of a material the lower the ignition temperature. Where \(k\) is the thermal conductivity of extruded polystyrene, \(k\) = 0.1316 W m−1 K−1 for red XPS and 0.1357 W m−1 K−1 for grey XPS, \(\rho\) is the density of the molten red and grey XPS (828 kg m−3 for red and 806 kg m−3 for grey) and \(c\) represents the specific heat capacity which is 1.34 kJ kg−1 K−1 for polystyrenes. To predict the ignition temperature for the experiment Eq. (8) from [29] and Eq. (9) are used.
Ignition of materials in flammability experiments occurs after pyrolysis. Hence, the ignition temperature is the pyrolysis temperature in addition to any increment made toward ignition.
where Tig is the ignition temperature, \(T_{\text{py}}\) represents the pyrolysis temperature (397 °C for red XPS and 398 °C for grey XPS [16]) and \(q_{\text{in}}\) is the incident heat flux applied. Tables 7 and 8 show the measured and calculated cone test data for red and grey XPS.
Correlations were conducted on parameters measured and derived from the cone calorimetry test of red and grey XPS in Tables 9 and 10. From the results, qmax had strong correlations with all parameters except THR of red XPS. Correlation between FPI and qmax was − 0.84 and − 0.83 for red and grey, respectively, since high peak heat release rates demonstrates low fire performance. It is interesting to note that FIGRA and FPI showed very strong negative correlations proving the fact that high FIGRA values also represents a reduced fire performance. Similarly, tig had very strong negative relationships with FIGRA and rather strong positive correlations with Tig and FPI. Furthermore, an increase in tig depicts a better fire performance index shown by a correlation coefficient of 0.97 and 0.99. All the pairs from the cone calorimetry experiment either had strong or very strong linear correlations with the exception of the set of THR correlations. The weakest correlations were recorded between pairs of THR/Tig and THR/FIGRA.
Correlations between MCC and cone calorimetry parameters
To establish relationships between the cone calorimeter and MCC experiments, correlations were conducted on the parameters from both methods for red and grey XPS. From the correlations presented in Tables 11 and 12, pHRR, pTemp and \(\eta_{\text{c}}\) had strong positive and negative linear relationships with all the cone calorimetry parameters analyzed except THR and Tig. THR and char yield from MCC did not show strong correlations with any of the parameters which could likely be the forced complete combustion in MCC. Also, considering correlations between THR from both methods, a weak correlation was recorded for grey XPS, while a moderate R was obtained for red XPS. The difference in correlations between the THR pair can be attributed to the different char formation ability in grey and red XPS which resulted in higher THR values for red XPS in the MCC experiment. The incomplete combustion in cone calorimetry being compared with the complete non-flaming combustion in MCC could possibly be the reason for the poor correlations.
From the ongoing discussion it can be deduced that almost all the parameters thus, pHRR, pTemp and \(\eta_{\text{c}}\) from MCC correlates very well with most of the parameters from cone calorimetry. In view of this MCC experiment and cone calorimetry in some degree can be used together to assess flammability of materials.
Additionally, an analysis between pHRR and pTemp as well as qmax and Tig were made for both grey and red XPS. Graphs are drawn in Figs. 7 and 8 between these parameters to show how the results from MCC differed from the cone test. From the plots, it was observed that the cone test achieved moderate temperatures and peak heat release rates at the various incident fluxes applied as compared to the MCC results at nine heating rates. The data from the cone test fell within 0.2 K s−1 and 0.5 K s−1 heating rates of MCC for both red and grey XPS.
With this analogy, a model for predicting qmax was derived using linear regression. A very strong correlation (0.84 for grey and 0.86 for red XPS) was observed between qmax and pHRR indicating a strong relationship between the two parameters. In view of this, a linear regression analysis was conducted between pHRR values of MCC at the heating rates of 0.2 and 0.5 K s−1 and the averages of the cone calorimeter test data for each heat flux. This was done to obtain the regression equation in the form \(y = ax + b\), where y is the dependent variable (qmax), a is the slope of the line, b is the intercept and x is the independent variable (pHRR) for both red and grey XPS (Fig. 9). The prediction equation obtained for red XPS was \(q_{ \hbox{max} } = 0.99{\text{pHRR}} - 159.7\) and the model for grey XPS was \(q_{ \hbox{max} } = 1.18{\text{pHRR}} - 25.04\).
Conclusions
Correlations between flammability experiments are conducted to evaluate the dependence and linear relationship between the parameters. In this research, correlations were made between the results obtained from cone calorimetry and MCC experiments of red and grey samples of extruded polystyrene. Strong correlations were observed by most of the flammability characteristics analyzed. Averagely, cone calorimetry parameters displayed the strongest correlations for both materials. The weakest correlation in MCC was recorded by THR and pHRR pair. Also, correlating MCC characteristics with cone calorimetry results produced moderate and weak correlations between the two THR parameters for red and grey XPS, respectively. Analyzing the qmax, Tig, pTemp and pHRR plots for the two methods showed that cone calorimetry data of both red and grey XPS at 25, 35 and 50 kW m−2 fell within 0.2 and 0.5 K s−1 heating rates of MCC.
Abbreviations
- \(\beta\) :
-
Heating rate in MCC test/K s−1
- \({\text{Bi}}\) :
-
Biot’s number
- \(c\) :
-
Specific heat/J g−1 K−1
- \(h\) :
-
Heat transfer coefficient/W m−2 K−1
- \(h_{\text{g}}\) :
-
Heat of gasification/MJ kg−1
- \(k\) :
-
Thermal conductivity/W m−1 K−1
- \(L_{\text{c}}\) :
-
Characteristic length/m
- \(m_{\text{o}}\) :
-
Initial mass of sample/g
- \(m_{\text{p}}\) :
-
Mass of residue/g
- \(\eta_{\text{c}}\) :
-
Heat release capacity/J g−1 K−1
- pHRR:
-
Peak heat release rate/W g−1
- pTemp:
-
Temperature at pHRR/°C
- \(q_{\text{in}}\) :
-
Incident heat flux/kW m−2
- \(q_{\hbox{max} }\) :
-
Maximum value of heat release rate per unit area/kW m−2
- THR:
-
Total heat release/kJ g−1
- T ig :
-
Ignition temperature/°C
- t ig :
-
Time to ignition/s
- \(\Delta T_{\text{ig}}\) :
-
Change in ignition temperature/°C
- T py :
-
Pyrolysis temperature/°C
- \(\rho\) :
-
Density/kg m−3
References
Lyon RE, Walters R. A microscale combustion calorimeter. Washington: Federal Aviation Administration; 2002.
Standard test method for determining flammability characteristics of plastics and other solid materials using microscale combustion calorimetry. ASTM D7309-13. 2013.
Walters RN, Lyon RE. Microscale combustion calorimeter for determining flammability parameters of materials. Evol Technol Compet Edge. 1997;42:1335–44.
Hostikka S, Matala A. Pyrolysis Model for Predicting the Heat Release Rate of Birch Wood. Combust Sci Technol. 2017;189(8):1373–93.
Lyon RE, Walters RN, Stoliarov SI. Screening flame retardants for plastics using microscale combustion calorimetry. Polym Eng Sci. 2007;47(10):1501–10.
Schartel B, Pawlowski KH, Lyon RE. Pyrolysis combustion flow calorimeter: a tool to assess flame retarded PC/ABS materials. Thermochim Acta. 2007;462(1–2):1–4.
Yang CQ, He Q, Lyon RE, Hu Y. Investigation of the flammability of different textile fabrics using micro-scale combustion calorimetry. Polym Degrad Stab. 2010;95(2):108–15.
Babrauskas V. The cone calorimeter. In: Hrtkey MJ, et al., editors. SFPE handbook of fire protection engineering. New York: Springer; 2016. p. 952–80.
ISO 5660-1:2002 Reaction-to-fire tests-heat release, smoke production and mass loss rate-part 1: heat release rate (cone calorimeter method).
Schartel B, Hull TR. Development of fire-retarded materials—interpretation of cone calorimeter data. Fire Mater. 2007;31(5):327–54.
Qin H, Zhang S, Zhao C, Feng M, Yang M, Shu Z, Yang S. Thermal stability and flammability of polypropylene/montmorillonite composites. Polym Degrad Stab. 2004;85(2):807–13.
Qin H, Su Q, Zhang S, Zhao B, Yang M. Thermal stability and flammability of polyamide 66/montmorillonite nanocomposites. Polymer. 2003;44(24):7533–8.
Zhao C, Qin H, Gong F, Feng M, Zhang S, Yang M. Mechanical, thermal and flammability properties of polyethylene/clay nanocomposites. Polym Degrad Stab. 2005;87(1):183–9.
Delichatsios M, Paroz B, Bhargava A. Flammability properties for charring materials. Fire Saf J. 2003;38(3):219–28.
Heskestad AW, Hovde PJ. Empirical prediction of smoke production in the ISO room corner fire test by use of ISO cone calorimeter fire test data. Fire Mater. 1999;23(4):193–9.
Xu Q, Jin C, Jiang Y. Compare the flammability of two extruded polystyrene foams with micro-scale combustion calorimeter and cone calorimeter tests. J Therm Anal Calorim. 2017;127(3):2359–66.
Cogen JM, Lin TS, Lyon RE. Correlations between pyrolysis combustion flow calorimetry and conventional flammability tests with halogen-free flame-retardant polyolefin compounds. Fire Mater. 2009;33(1):33–50.
Lyon RE, Walters RN, Stoliarov SI. Thermal analysis of flammability. J Therm Anal Calorim. 2007;89(2):441.
Lin TS, Cogen JM, Lyon RE. Correlations between microscale combustion calorimetry and conventional flammability tests for flame retardant wire and cable compounds. In: Proceedings of the 56th IWCS conference, Lake Buena Vista, FL; 2007.
Xu Q, Jin C, Majlingova A, Restas A. Discuss the heat release capacity of polymer derived from microscale combustion calorimeter. J Therm Anal Calorim. 2017;133:1–9.
Hall G. Pearson’s correlation coefficient. http://www.hep.ph.ic.ac.uk/~hallg/UG_2015/Pearsons.pdf. Accessed 20 Mar 2018.
Zou KH, Tuncali K, Silverman SG. Correlation and simple linear regression. Radiology. 2003;227(3):617–28.
Xu Q, Majlingova A, Zachar M, Jin C, Jiang Y. Correlation analysis of cone calorimetry test data assessment of the procedure with tests of different polymers. J Therm Anal Calorim. 2012;110(1):65–70.
Ayoola BO, Balachandran R, Frank JH, Mastorakos E, Kaminski CF. Spatially resolved heat release rate measurements in turbulent premixed flames. Combust Flame. 2006;144(1–2):1–6.
Di Blasi C. Kinetic and heat transfer control in the slow and flash pyrolysis of solids. Ind Eng Chem Res. 1996;35(1):37–46.
Xu Q, Jin C, Hristov J, Griffin G, Jiang Y. The melt\shrink effect of low density thermoplastics insulates: cone calorimeter tests. Therm Sci. 2015;00:58.
Jiang L, He JJ, Sun JH. Sample width and thickness effects on upward flame spread over PMMA surface. J Hazard Mater. 2018;342:114–20.
An W, Jiang L, Sun J, Liew KM. Correlation analysis of sample thickness, heat flux, and cone calorimetry test data of polystyrene foam. J Therm Anal Calorim. 2015;119(1):229–38.
Mowrer FW. An analysis of effective thermal properties of thermally thick materials. Fire Saf J. 2005;40(5):395–410.
Delichatsios MA. Ignition times for thermally thick and intermediate conditions in flat and cylindrical geometries. Fire Saf Sci. 2000;6:233–44.
Acknowledgements
This research is supported by the National Natural Science Fund of China, No. 51776098 and the Fundamental Research Funds for the Central Universities, No. 30918015101.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mensah, R.A., Xu, Q., Asante-Okyere, S. et al. Correlation analysis of cone calorimetry and microscale combustion calorimetry experiments. J Therm Anal Calorim 136, 589–599 (2019). https://doi.org/10.1007/s10973-018-7661-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7661-5