Abstract
The kinetics of the epoxy–amine polycondensation and the epoxy homopolymerization in off-stoichiometric epoxy/amine formulations with excess of epoxy groups, and in the presence of an anionic initiator have been investigated. Diglycidyl ether of bisphenol A (DGEBA) and diethylenetriamine (DETA) have been used as epoxy and amine reagents, respectively, and 2-methylimidazole (2MI) has been used as anionic initiator. This study has been carried out using a differential scanning calorimeter (DSC). The thermal–mechanical properties of the partially cured and fully cured materials with and without initiator have been determined by DSC and dynamic-mechanical analysis. First, off-stoichiometric DGEBA/DETA mixtures with excess of DGEBA, with and without 2MI, have been reacted isothermally at low temperatures, where only the epoxy–amine condensation takes place, because the epoxy homopolymerization has a very low curing rate. Afterward, samples containing 2MI have been heated at different heating rates to study the homopolymerization process of the epoxy excess. The kinetics of both processes have been analyzed with an isoconversional method to determine the activation energy, and the Šesták–Berggren equation has been applied to determine the frequency factor and the orders of reaction. In the isothermal curing, amine–epoxy condensation, the activation energy and the frequency factor decrease with increasing degree of conversion, but in the homopolymerization process, both magnitudes increase with the degree of conversion. Results show that the dual-curing character of off-stoichiometric DGEBA/DETA thermosets with 2MI as anionic initiator renders them suitable for multistage curing processes in which the degree of cure and material properties in the intermediate stage can be controlled easily and final material properties can be enhanced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epoxy resins are used in a wide range of applications such as adhesives, coatings, electrical laminates, encapsulation of semiconductor devices, matrix material for composites, structural components [1,2,3,4,5,6,7,8,9,10,11,12], flame retardants [13, 14] and cryogenic engineering [15,16,17] because of their superior mechanical properties, adhesion and chemical resistance. Epoxy resins are also highly versatile because of the variety of curing agents or hardeners available [4, 18, 19]. Curing of an epoxy–amine mixture proceeds through different reaction mechanisms. In competition with the main stepwise polycondensation reaction between epoxy and amine groups [18], homopolymerization of epoxy groups can take place at higher temperatures and can be catalyzed by the tertiary amines formed by the polycondensation reaction or additional anionic initiators [20, 21].
Dual-curing methodology is a useful strategy to obtain thermosets by combining two different polymerization reactions. Sequential dual curing allows the preparation of storable and handleable materials after an initial curing stage, which upon application of a second stimulus, attain their ultimate properties [22]. Among dual-curing methodologies, the preparation of dual materials from off-stoichiometric formulations has recently gained interest, because it allows the combination of a step growth polymerization with a chain-growth polymerization [23,24,25]. In this case, the first stage of curing is a self-limiting click reaction between two monomers with an excess of one of them. The second curing stage is the homopolymerization, thermally or UV induced, of this excess of unreacted groups. The key point of this strategy is to ensure that the second stage does not start during the first curing stage.
The main objective of this work has been to develop a new dual-curing system based on sequential epoxy–amine click polycondensation and epoxy anionic homopolymerization in order to exploit the concept of dual-curing based on the combination of two thermal curing processes with different reaction temperatures.
For this objective, the kinetics of the epoxy–amine polycondensation and the epoxy homopolymerization of off-stoichiometric epoxy/amine formulations with excess of epoxy groups and in the presence of an anionic initiator have been investigated. This study has been carried out using a differential scanning calorimetry (DSC). We have also determined the thermal–mechanical properties of the partially cured and fully cured materials with and without initiator by DSC and DMA (dynamic-mechanical analysis). The results indicate that the dual-curing character of off-stoichiometric thermosets with an anionic initiator renders them suitable for multistage curing processes [26] in which the degree of cure and material properties in the intermediate stage can be controlled easily and final material properties can be enhanced.
Experimental
Materials
Diglycidyl ether of bisphenol A (DGEBA) with an epoxy equivalent of 182 g ee−1 (EPIKOTE 827, Shell Chemicals) was dried in vacuum before use. Diethylenetriamine (DETA, Sigma-Aldrich) was used as hardener, and 2-methylimidazole (2MI, Sigma-Aldrich) has been used as anionic initiator.
Preparation of curing formulations
Three systems have been studied: a stoichiometric DGEBA/DETA formulation (E827-DETA 100), a DGEBA/DETA formulation with a 50% of excess of epoxy groups without added initiator (E827-DETA 50) and with added 2% of 2MI (E827-DETA 50-2MI). The components of the systems have been mixed at room temperature and vigorously stirred.
Differential scanning calorimetry
The kinetics of the curing has been evaluated by DSC analysis (Mettler DSC-822e calorimeter). First, the three systems have been cured isothermally at different temperatures (30, 40, 50, 60 and 70 °C). After that, two dynamic scans have been performed from − 100 to 300 °C at 10 °C min−1. In the first one the glass transition temperature (Tg) has been determined, and in the second one the ultimate Tg (\(T_{\text{g}\infty}\)) has been obtained. In the case of the system E827-DETA 100 the Tg has been determined by alternate differential scanning calorimetry (ADSC) because the relaxation and residual peaks could not be separated in the DSC scan. In the ADSC experiments the samples were heated from 0 to 250 °C at a heating rate of 2 °C min−1, an amplitude of 0.5 °C and a modulation period of 1 min. The value of the Tg has been taken as the mid-point of the heat capacity step in the reversing heat flow curve. The total heat of curing has been determined from a dynamic scan from 0 to 300 °C at 10 °C min−1 in samples without isothermal treatment.
The system E827-DETA 50-2MI has also been dynamically cured at different heating rates (2.5, 5, 7.5, 10 and 15 °C min−1) from 0 to 300 °C after an isothermal curing at 40 °C for 4 h, to study the homopolymerization process.
Dynamic-mechanical analysis
To prepare the specimens, the mixtures have cured in a rectangular mold, with different thermal treatments. The dimensions of the specimens were 20 × 10 × 2 mm3, approximately. The samples have been tested in a dynamic-mechanical analysis (DMA, TA instruments Q800) at 3 °C min−1 using the single cantilever mode, with an amplitude of 10 μm and a frequency of 1 Hz.
Theory
In the isothermal curing studied, the relative degree of conversion is defined as:
where Δh t is the heat released up to a time t and Δhiso is the total reaction heat released during the isothermal cure. The kinetics of this curing has been analyzed by means of an integral isoconversional method, using the following equation [27]:
where R is the gas constant and at a determined value of relative degree of conversion, t α is the time attained, A α is the frequency factor, E α is the activation energy, and g(α) is the following integral obtained from a function of the degree of conversion f(α):
The activation energy at a given conversion can be obtained from the slope of the representation of ln t α versus the reciprocal of the temperature [see Eq. (2)]. Knowing the kinetic model, the frequency factor at each relative degree of conversion can also be determined from the intercept at the origin.
In the dynamic post-curing studied the relative degree of conversion is defined as:
where ∆hT is the heat released up to a temperature T and ∆hdyn is the total reaction heat released during the post-curing.
The kinetics of non-isothermal post-curing has been analyzed by means of an integral isoconversional method, using the Kissinger–Akahira–Sunose equation [27]:
where T α is the temperature attained at a determined value of relative degree of conversion. The activation energy at a given conversion can be obtained from the slope of the representation of ln(β/T 2 α ) versus the reciprocal of the temperature [see Eq. (5)]. As in the isothermal curing, the frequency factor can be obtained from the intercept at the origin assuming a certain kinetic model.
Results and discussion
Differential scanning calorimetry
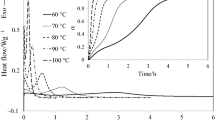
Figure 1 shows the dynamic scans at 10 °C min−1 of the three studied systems without previous thermal treatment. It can be seen that the systems without anionic initiator (E827-DETA 50 and E827-DETA 100) only present a peak, corresponding to the reaction between the epoxy and amine groups. The size of the peak corresponding to the system E827-DETA 100 is greater than in E827-DETA 50 because in the first one there are more amine groups and then the extent of the reaction and the corresponding curing heat are greater. In the system E827-DETA 50-2MI there are two peaks, the first one also corresponds to the reaction between the epoxy and amine groups and the second one, at higher temperatures, to the homopolymerization of the excess epoxy groups initiated by 2MI. Although the separation between the peaks in the dynamic experiment is not perfect, these results indicate the E827-DETA 50-2MI can be potentially used as a dual-curable system with controlled curing sequence.
Table 1 shows the dynamic curing heat and the \(T_{\text{g}\infty}\) values of the three systems. In E827-DETA 50 the dynamic curing heat is smaller (50% of the theoretical value) [28, 29] because 50% of the epoxy groups have not reacted. E827-DETA 50 exhibits two glass transition temperatures, probably the higher Tg related with the epoxy–amine network formed and the lower Tg with regions where the epoxy excess exerts a plasticizing effect, but they are lower than those of the other two systems due to the lower quantity of reacted epoxy groups and to the plasticizing effect of the epoxy excess. The other systems, where the epoxy groups are completely reacted, have values of \(T_{\text{g}\infty}\) and the dynamic curing heat very similar. The \(T_{\text{g}\infty}\) of the E827-DETA-100 system is similar to that reported previously by us [20], but that of the E827-DETA 50-2MI system is somewhat lower, suggesting that either the dynamic curing might not have led to the same network structure, either by incomplete reaction of epoxy groups or thermal degradation. In that work, the post-curing of the sample was set to 2 h at 180 °C [20] in order to ensure complete reaction and prevent side reactions or degradation.
Given the potential of the E827-DETA 50-2MI as dual-curable system, the curing kinetics and the properties of partially and totally cured materials were studied in more detail and compared with the other systems.
Figure 2 shows the isothermal curing at different temperatures of the system E827-DETA 50-2MI. In the inset of this Figure the relative degree of conversion [Eq. (1)] has been represented as a function of the time. It can be seen, as expected, that the rate of the reaction increases with temperature. Similar results have also been found for the other studied systems (E827-DETA 50 and E827-DETA 100).
Figure 3 compares the isothermal curing at 40 °C of the three systems. In the inset of this figure, the relative degree of conversion found using Eq. (1) has also been represented versus the time. In the system E827-DETA 50-2MI, the anionic initiator slightly increases the rate of reaction with respect to E827-DETA 50 system, because its amine groups increase the reactivity of DGEBA. In the system E827-DETA 100 the reaction is faster due to the higher concentration of reactive species and the tertiary amine formed. The epoxy excess in off-stoichiometric formulations exerts a significantly dilution effect, and consequently, the curing is decelerated.
Figure 4 shows the dynamic post-curing of E827-DETA 50-2MI after isothermal curing at 40 °C for 4 h at different heating rates. In the inset, the relative degree of conversion found using Eq. (4) has been represented versus the temperature. As it was expected, the scans move to higher temperatures on increasing the heating rate. The dynamic post-curing observed at temperatures above isothermal curing temperature corresponds to the homopolymerization process. This process presents a peak without any shoulder at all heating rates. No such post-curing was observed in samples E827-DETA 50 without added initiator, because of the lower activity of the formed aliphatic tertiary amines as initiators for the epoxy homopolymerization. Partially cured samples of E827-DETA 100 experienced a post-curing that started immediately after relaxation of the vitrified network (results not shown).
Table 2 shows the activation energy and the frequency factor of the isothermal curing of the three studied systems at different relative degrees of conversion, found using Eq. (2). The average regression coefficient on applying this equation is 0.999. The average error bar of the activation energy values listed in Table 2 is 3.6 kJ mol−1. To determine the orders of the reaction, a model-fitting method and the Šesták–Berggren equation f(α) = αm (1 − α)n [27] has been used with m + n = 2 [30]. Under this condition, the integral function of the degree of conversion g(α) can be expressed as:
At the beginning of the process, the values of the activation energy and the frequency factor are similar for the three systems. At the end of the process, the activation energy and the frequency factor decrease in the off-stoichiometric systems, the decrease being slightly more pronounced in the system with anionic initiator. On the other hand, the stoichiometric system exhibits a smaller decrease in these magnitudes, the kinetic parameters remaining almost constant in all ranges of curing. The values of the parameters m and n are indicated in Table 1. They are similar for the three systems. In general, it can be concluded that the kinetics of curing are affected barely by 2-MI but more by the dilution effect of the epoxy excess.
Table 3 shows the Tg values after isothermal curing at different temperatures. The stoichiometric system always exhibits greater values of Tg compared to the other systems, because, at any given temperature below its ultimate Tg, it cures until it vitrifies, eventually reaching a Tg that somewhat higher than the isothermal curing temperature [31]. Because in the E827-DETA 100 formulation the curing resumes as soon as the system devitrifies, for this system the Tg had to be determined using ADSC. In contrast, in the curing of off-stoichiometric systems, the curing first proceeds until the reaction of all the amine groups from DETA is over. In the absence of added initiator, as in E827-DETA 50 system, no more reaction takes place, so that the Tg of the partially cured sample remains practically constant regardless of the curing temperature. The slight increase in the Tg of E827-DETA 50 formulation with increasing curing temperature may be caused by the onset of vitrification and a too early stop of the DSC measurement. At lower curing temperatures, both off-stoichiometric systems have similar Tg, but at higher temperatures, the system with 2MI exhibits greater values. DGEBA homopolymerized with 2 phr of 1-methylimidazole has a Tg of 180 °C [20], while this value goes down to 156 °C using 5 phr of initiator [32]. Although the neat DGEBA formulation with 2MI was not studied, it can be hypothesized that the higher Tg of 2MI formulation is due to a contribution of the epoxy homopolymerization, that is already being activated at moderate temperatures, as low as 60 or 70 °C. Vitrification may also take place in formulation E827-DETA 50-2MI at 60 and 70 °C, reaching Tg values higher than the isothermal curing temperature. E827-DETA 50 formulation cured at 70 °C shows two glass transitions, in the similar way than the same formulation after the complete dynamic cure.
Table 4 shows the values of the degree of conversion attained at the end of the isothermal curing (αiso) with respect to the total reaction heat. This degree of conversion has been calculated as:
αiso increases with temperature for the three systems studied, in agreement with the increasing Tg with increasing temperature shown in Table 3. In the systems E827-DETA 50 and E827-DETA 100, where the anionic initiator has not been added, the reaction is nearly complete at 70 °C, because the values of αiso are closer to 1. However, in the system E827-DETA 50-2MI, the values of αiso are lower because the homopolymerization has only taken place to a limited extent or it has not still begun. Probably, the nucleophilic attack of the 2MI to the epoxy ring takes place and homopolymerization starts, but the temperature is not enough to allow quantitative conversion of all the epoxy groups because of vitrification.
Table 5 shows the activation energy and the frequency factor of the dynamic post-curing of the system E827-DETA 50-2MI performed after an isothermal curing at 40 °C for 4 h, found using Eq. (5). The average regression coefficient on applying Eq. (5) is 0.994. The average error bar of the activation energy values listed in Table 5 is 16.7 kJ mol−1. The values of the activation energy of Table 5 are greater than those in the case of isothermal curing for the same system. Although at the beginning of the process, these values stay relatively constant, they increase at the end. This trend is different from the case of isothermal curing, where the values decrease. We have also used the Šesták-Berggren equation with m + n=2 to find the orders of the reaction. These values are 0.196 and 1.804, for m and n, respectively, and they are remarkably different than those for isothermal curing, due to the differences between both processes. This result suggests that the kinetics of epoxy homopolymerization is quite different than epoxy–amine polycondensation. The values of activation energy showed in Table 5 are higher than those obtained for pure epoxy anionic homopolymerization [32] or for dual cure formulation based on sequential thiol–epoxy click polycondensation followed by epoxy anionic homopolymerization of the epoxy excess [23]. Probably, the densely cross-linked network formed during first curing stage, restricts the mobility of the active species, especially at higher conversions, making the homopolymerization more hindered and increasing the activation energy. In addition, one should also take into consideration that the activity of 2-MI may be different from the initiator employed in that work [23].
All these results indicate that the E827-DETA 50-2MI formulation is a dual-curable system with a fairly good control of the curing sequence, in a similar way to off-stoichiometric thiol-epoxy systems [23]. The initial isothermal temperature should be high enough in order to achieve complete epoxy–amine polycondensation and exhaustion of reactive amino groups from DETA, but not too much so as to avoid an undesired start of the epoxy homopolymerization process. Tentatively, a maximum isothermal temperature of 50 °C for this first process might be considered. The second homopolymerization process would only be activated at higher temperatures but, given that the Tg of this system is 30 °C, the intermediate material could be safely stored under vitrified conditions at room temperature or better with mild refrigeration conditions (e.g., 5 °C) to guarantee stability for a prolonged period of time before the final application. This curing system is also highly versatile in terms of tailoring the intermediate and final properties. For instance, if an amine with a functionality lower than DETA or more flexible backbone was used, the low Tg of the epoxy–amine network formed could be enhanced with a second curing stage based on the anionic homopolymerization of the epoxy excess. Conversely, the intermediate properties might be changed as well, leading to softer materials that might find a specific application before the second curing process was activated. Changing the ratio between DETA and E827 could also be an interesting way of tailoring the intermediate and final material properties and applicability of the curing system [23].
Dynamic-mechanical analysis
In Figs. 5–7 some DMA experiments are shown. Figure 5 shows the plastifying effect of the excess of DGEBA, the dual nature of the curing of off-stoichiometric amine–epoxy thermosets and the latency of these systems after the first curing stage. This latency is controlled by the self-limiting character of epoxy–amine condensation, vitrification if samples are stored at temperatures lower than the Tg of the partially cured system, and to the fact that epoxy cannot homopolymerize at low temperatures. Although all formulations devitrify when heated above their Tg and a second curing process is observed before reaching the ultimate Tg, some important differences between formulations must be noted. The temperature of the maximum of tan δ after first curing stage is much lower and the decrease on the module during Tg is much higher in off-stoichiometric formulations than in the stoichiometric formulation. This effect is more significant for E827-DETA 50 mixture because once the system devitrifies, the partially reacted network structure relaxes completely and very slow reaction, if any, takes place when the temperature is further increased leading to a very small change in modulus. In the case of E827-DETA 50-2MI, the full relaxation of the partially cross-linked network is observed, in a similar way to the E827-DETA 50 formulation. The shape and the size of the tan δ peak, and the drop in modulus during relaxation are almost identical, which suggests that in both cases the epoxy–amine reaction is complete and that the second homopolymerization process has not been activated in the case of the E827-DETA 50-2MI formulation. However, because of the presence of 2MI, when the temperature increases sufficiently the second reaction process is activated and cross-linking takes place, leading to a significant increase in modulus. This second process is complex from the network build-up point of view because other vitrification/devitrification processes are observed, as seen from the multiple peaks in the tan δ curve, the last one corresponding to the relaxation of the fully cured material. If we analyze the result of the E827-DETA 100 formulation, the complete relaxation of the partially cured network structure is not observed because the curing process resumes as soon as the system starts to devitrify; therefore, the intermediate modulus observed is much higher than in the case of E827-DETA 50-2MI formulation.
Figure 6 shows for E827-DETA 50-2MI formulation the DMA traces after isothermal curing at 40 °C with and without isothermal post-curing at 150 °C. It can be observed that post-curing results in an increase of the storage modulus and the apparition of the new maximum of tan δ (related with Tg) at higher temperatures that is in agreement with the last relaxation process observed during post-curing of the partially reacted sample in the DMA. The same final relaxed modulus is obtained accordingly. Figure 7 shows DMA curves for the stoichiometric system (E827-DETA 100) with the same curing treatment. Again, it can be seen in the sample cured at 40 °C (without post-cure) the devitrification and post-curing processes and the apparition of the ultimate Tg at a higher temperature. A comparison of Figs. 6 and 7 shows the effect of post-curing is significantly higher in E827-DETA 50-2MI than in E827-DETA 100, due to its lower degree of conversion after isothermal curing at 40 °C (see Table 4) and the high glass transition of the epoxy homopolymer network [20].
The above results also point at a fundamental difference between them in terms of multistage processing. Clearly, the E827-DETA 100 system allows for very little manipulation after devitrification of the material because of its high intermediate modulus and rapidly changing properties due to fast post-curing. In contrast, the E827-DETA 50-2MI formulations makes it possible to perform a number of operations or manipulations once it devitrifies, as the material is very soft (low intermediate modulus) and it takes longer for the second reaction to start. Therefore, the dual-curing E827-DETA 50-2MI system would be a suitable candidate for B-stage processing applications, for instance.
Conclusions
A new dual-curing system based on off-stoichiometric amine–epoxy formulations has been developed. Epoxy–amine condensation takes place at low temperatures in the first curing stage, while the excess of epoxy mostly homopolymerizes in the second curing stage at higher temperatures.
After first curing stage, off-stoichiometric amine–epoxy formulations show latency and can be safely stored at temperatures below their glass transition temperatures. This latency is double controlled by the vitrification and by the inability of the epoxy groups to homopolymerize at low temperature. The novel methodology allows the custom-tailoring of the material properties at the intermediate and final stages by changing the excess of epoxy groups in the formulation and therefore the relative extent of amine–epoxy condensation and epoxy homopolymerization.
Epoxy–amine polycondensation and epoxy homopolymerization present different kinetic parameters that put in evidence the different nature and reactivity of both processes. These differences allow the sequential curing at two different temperatures of off-stoichiometric amine–epoxy mixtures in the presence of a tertiary amine.
References
Riew CK, Siebert AR, Smith RW, Fernando M, Kinloch AJ. Toughened epoxy resins: performed particles as tougheners for adhesives and matrices. In: Riew CK, Kinloch AJ, editors. Toughened plastics II novel approaches in science and engineering. Advances in Chemical Series, vol. 252. Washington: American Chemical Society; 1996. p. 33–44.
Saiki N, Yamazaki O, Ebe K. UV/heat dual-curable adhesive tapes for fabricating stacked packages of semiconductors. J Appl Polym Sci. 2008;108:1178–83.
Kang B-U. Interfacial fracture behavior of epoxy adhesives for electronic components. J Korea Acad Ind Cooper Soc. 2011;12:1479–87.
May CA, Tanaka GY. Epoxy resins. In: May CA, editor. Chemistry and technology, Chap. 1. New York: Marcel Dekker; 1988.
Petrie EM. Epoxy adhesive formulations. New York: McGraw-Hill; 2006.
Pascault JP, Williams RJJ. Epoxy polymers: new materials and innovations. Weinheim: Wiley-VCH; 2010.
Kinloch AJ, Shaw SJ, Tod DA, Hunston DL. Deformation and fracture behavior of a rubber-toughened epoxy: 1. Microstructure and fracture studies. Polymer. 1983;24:1341–54.
Ho T-H, Wang C-S. Toughening of epoxy resins by modification with dispersed acrylate rubber for electronic packaging. J Appl Polym Sci. 1993;50:477–83.
Mezzenga R, Boogh L, Månson JAE. A review of dendritic hyperbranched polymer as modifiers in epoxy composites. Compos Sci Technol. 2001;61:787–95.
Guo QP, Habrard A, Park Y, Halley PJ, Simon GP. Phase separation, porous structure, and cure kinetics in aliphatic epoxy resin containing hyperbranched polyester. J Polym Sci B. 2006;44:889–99.
Ratna D, Varley R, Simon GP. Toughening of trifunctional epoxy using an epoxy-functionalized hyperbranched polymer. J Appl Polym Sci. 2003;89:2339–45.
He S, Shi K, Bai J, Zhang Z, Li L, Du Z, Zhang B. Studies on the properties of epoxy resins modified with chain-extended ureas. Polymer. 2001;42:9641–7.
Zhou L, Zhang G, Li J, Jing Z, Qin J, Feng Y. The flame retardancy and thermal stability properties of flame-retarded epoxy resins based on α-hydroxyphosphonate cyclotriphosphazene. J Therm Anal Calorim. 2017;129:1667–78.
Mao W, Li S, Yang X, Cao S, Li M, Huang K, Xia J. Preparation of a flame-retardant epoxy curing agent based on castor oil and study on the curing reaction kinetics. J Therm Anal Calorim. 2017;130:2113–21.
Evans D, Canfer SJ. Radiation stable, low viscosity impregnating resin systems for cryogenic applications. Adv Cryog Eng. 2000;46:361–8.
Ueki T, Nishijima S, Izumi Y. Designing of epoxy resin systems for cryogenic use. Cryogenics. 2005;45:141–8.
Nishijima S, Honda Y, Okada T. Application of the positron annihilation method for evaluation of organic materials for cryogenic use. Cryogenics. 1995;35:779–81.
Pascault JP, Sautereau H, Verdu J, Williams RJJ. Thermosetting polymers. 1st ed. New York: Marcel Dekker, Inc.; 2002.
Thanki JD, Parsania PH. Dynamic DSC curing kinetics and thermogravimetric study of epoxy resin of 9,9′-bis-(4-hydroxyphenyl)anthrone-10. J Therm Anal Calorim. 2017;130:2145–56.
Fernández-Francos X, Santiago D, Ferrando F, Ramis X, Salla JM, Serra A, Sangermano M. Network structure and thermomechanical properties of hybrid DGEBA networks cured with 1-methylimidazole and hyperbranched poly(ethyleneimine)s. J Polym Sci Part B Polym Phys. 2012;50:1489–503.
Leena K, Soumyamol PB, Baby M, Suraj S, Rajeev R, Mohan DS. Non-isothermal cure and decomposition kinetics of epoxy–imidazole systems. J Therm Anal Calorim. 2017;130:1053–61.
Ramis X, Fernández-Francos X, De La Flor S, Ferrando F, Serra À. Click-based dual-curing thermosets. In: Guo Q, editor. Thermosets: structure, properties and applications, Chapter 9. 2nd ed. Amsterdam: Elsevier; 2017.
Fernández-Francos X, Konuray AO, Belmonte A, De la Flor S, Serra A, Ramis X. Sequential curing of off-stoichiometric thiol–epoxy thermosets with a custom-tailored structure. Polym Chem. 2015;7:2280–90.
González G, Fernández-Francos X, Serra À, Sangermano M, Ramis R. Environmentally-friendly processing of thermosets by two-stage sequential aza-Michael addition and free-radical polymerization of amine–acrylate mixtures. Polym Chem. 2015;6:6987–97.
Nair DP, Cramer NB, Gaipa JC, McBride MK, Matherly EM, McLeod RR, Shandas R, Bowman CN. Adv Funct Mater. 2012;22:1502–10.
Tech Tip 20. B-stage epoxy. In: Epo-tek tech tips. Epoxy technology. 2012. http://www.epotek.com/site/files/Techtips/pdfs/tip20.pdf. Accessed 21 July 2014.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Ooi SK, Cook WD, Simon GP, Such CH. DSC studies of the curing mechanisms and kinetics of DGEBA using imidazole curing agents. Polymer. 2000;41:3639–49.
Fernández-Francos X, Cook WD, Serra À, Ramis X, Liang GG, Salla JM. Crosslinking of mixtures of DGEBA with 1,6-dioxaspiro[4,4]nonan-2,7-dione initiated by tertiary amines. Part IV. Effect of hydroxyl groups on initiation and curing kinetics. Polymer. 2010;51:26–34.
Flores M, Fernández-Francos X, Ramis X, Serra A. Novel epoxy-anhydride thermosets modified with a hyperbranched polyester as toughness enhancer. Thermochim Acta. 2012;544:17–26.
Santiago D, Fernández-Francos X, Ramis X, Salla JM, Sangermano M. Comparative curing kinetics and thermal-mechanical properties of DGEBA thermosets cured with a hyperbranched poly(ethylenimine) and an aliphatic triamine. Thermochim Acta. 2011;526:9–21.
Acebo C, Fernández-Francos X, Ferrando F, Serra À, Ramis X. New epoxy thermosets modified with multiarm star poly(lactide) with poly(ethyleneimine) as core of different molecular weight. Eur Polym J. 2013;49:2316–26.
Acknowledgements
The authors would like to thank MINECO (Ministerio de Economía y Competividad) and FEDER (Fondo Europeo de Desarrollo Regional) (MAT2014-53706-C03-01, MAT2014-53706-C03-02 and MAT2017-82849-C2-2-R) and to the Comissionat per a Universitats i Recerca del DIUE de la Generalitat de Catalunya (2014-SGR-67). Xavier F.-F. acknowledges the Serra Hunter programme of the Generalitat de Catalunya.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morancho, J.M., Ramis, X., Fernández-Francos, X. et al. Curing of off-stoichiometric amine–epoxy thermosets. J Therm Anal Calorim 133, 519–527 (2018). https://doi.org/10.1007/s10973-018-7158-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7158-2