Abstract
In the present work, we report the preparation and characterization of a new family of thermosets based on off-stoichiometric anhydride–epoxy formulations in the presence of an anionic initiator. Diglycidyl ether of bisphenol A (DGEBA) and hexahydro-4-methylphthalic anhydride (HHMPA) have been used as epoxy and anhydride comonomers, respectively, and 1-methylimidazole (1MI) has been used as anionic initiator. The isothermal curing kinetics and the thermal properties of the stoichiometric and the off-stoichiometric systems have been compared. The kinetics of the isothermal curing has been analyzed by differential scanning calorimetry (DSC) using an isoconversional method and the Šesták–Berggren equation to determine the activation energy, the frequency factor and the reaction orders. The materials obtained were characterized by DSC and dynamic mechanical analysis. Gelation during epoxy–anhydride condensation was determined by thermomechanical analysis. At the same curing temperature, the reaction is faster in the system with excess of epoxy groups. However, the glass transition temperatures of the partially cured stoichiometric system are greater. The gelation time of the off-stoichiometric system is shorter than that of the stoichiometric one. The results indicate that the dual-curing character of off-stoichiometric DGEBA/HHMPA thermosets with 1MI as anionic initiator makes them suitable for multistage curing processes with easy control of degree of cure and material properties in the intermediate stage and enhanced final material properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epoxy resins are widely used as adhesives, coatings, castings, electrical and electronic materials, encapsulation of semiconductor devices, matrix material for components, structural components [1,2,3,4,5,6,7,8,9,10,11,12], flame retardants [13, 14] and in cryogenic engineering [15,16,17] thanks to their mechanical and chemical properties, superior adhesion to various substrates, thermal stability and electrical characteristics. One of the most common curing agents for epoxy resins are anhydrides [4, 18, 19]. The reaction of epoxides with anhydrides can proceed through an anionic (catalyzed) or a non-catalyzed mechanism. Details of both mechanisms are provided in previous manuscripts [20, 21]. During the curing, there may also be a competition between two processes: The first and the most important one is the condensation reaction between the epoxy and anhydride groups [18]. The second one is epoxy homopolymerization that can only take place at higher temperatures [22, 23].
Dual curing is a methodology that can be applied in multi-step fabrication procedures, which have enormous advantages, such as flexibility in the fabrication and the possibility to create complex structures for advanced applications [24]. This dual-curing methodology consists in the combination of two different and independent curing reactions, each one for each curing stage. The first step of the dual-curing system and the characteristics of the intermediate material are controlled by the stoichiometry of the formulation. Thus, it is necessary that the curing reactions of the first step be completely controlled, allowing to select the intermediate material properties. The second step must only be initiated by increasing the temperature, by irradiation, etc., so that it does not overlap with the first curing stage. In previous studies of our group, click-type reactions were combined with homopolymerization processes of the monomer in excess. For example, a first thiol-epoxy reaction or a first epoxy–amine reaction was combined with a second anionic epoxy homopolymerization [24, 25]. The intermediate materials were stable for several times and processable, and the final thermosets obtained had good characteristics, which depend on the composition of the initial formulation.
Although the anhydride–epoxy reaction cannot be classified as click reaction, this process has been demonstrated to reach completion. According to that in the present work, we studied the possibility of obtaining a dual-curing system by adding an excess of the epoxy resin to epoxy–anhydride formulations in the presence of a tertiary amine, which catalyze both processes [24]. In the first step of this process, a stoichiometric epoxy–anhydride reaction takes place at low temperature. In the second step, at higher temperatures, the homopolymerization of the epoxy excess will occur. In this way, the intermediate properties of the system can be controlled to facilitate the preparation of easily handled and storage stable materials after the initial curing stage. Upon application of a second stimulus (heating), these materials attain their ultimate properties [24, 26].
In the present work, we report the preparation and characterization of a new family of thermosets based on off-stoichiometric anhydride–epoxy formulations in the presence of 1-methylimidazole. The kinetics of the isothermal curing stage have been analyzed by differential scanning calorimetry using an isoconversional method and the Šesták–Berggren equation to determine the activation energy, the frequency factor and the reaction orders [27]. The materials obtained were characterized by calorimetry and dynamic mechanical analysis. Gelation during epoxy–anhydride condensation was determined by thermomechanical analysis.
Experimental
Materials
Diglycidyl ether of bisphenol A (DGEBA, Epikote 828, Hexion Specialty Chemicals B. V.) and hexahydro-4-methylphthalic anhydride (HHMPA, Sigma-Aldrich) have been used as epoxy and anhydride comonomers, respectively. 1-methylimidazole (1MI, Sigma-Aldrich) was used as anionic initiator.
Preparation of curing formulations
Two systems have been studied: a stoichiometric DGEBA/HHMPA formulation with 2% by mass of 1MI (E828-HHMPA 100-1MI) and a DGEBA/HHMPA formulation with a 50% of excess of epoxy groups and 2% by mass of 1MI (E828-HHMPA 50-1MI). The different components have been mixed at room temperature and vigorously stirred.
Differential scanning calorimetry
The kinetics of the curing was evaluated by DSC analysis (Mettler DSC-822e calorimeter). First, the two systems have been cured isothermally at different temperatures (65, 70, 75, 80, 85 and 90 °C in the case of the system E828-HHMPA 100-1MI and 50, 55, 60, 65, 70, 75 and 80 °C in the case of the system E828-HHMPA 50-1MI). After that a dynamic scan has been performed from 0 to 250 °C at 10 °C min−1 to determine the glass transition temperature (Tg) and the residual heat (Δhres). In the case of the system E828-HHMPA 100-1MI, the use of ADSC (alternate differential scanning calorimetry) has been necessary for the determination of the Tg after isothermal curing in some cases, because of the overlapping between the relaxation and residual peaks. In the ADSC experiments, the samples were heated from 0 to 250 °C at a heating rate of 2 °C min−1, an amplitude of 0.5 °C and a modulation period of 1 min. The value of the Tg has been taken as the middle point of the heat capacity step in the reversing heat flow curve.
A dynamic scan from 0 to 250 °C at 10 °C min−1 was also performed on samples without isothermal treatment to determine the total heat of curing (Δhdyn) and the ultimate Tg (Tg∞).
Thermomechanical analysis
A Mettler thermomechanical analyzer SDTA840 was used to determine the gel point during epoxy–anhydride polycondensation (isothermal curing). A silanized glass fiber disk about 5 mm in diameter was impregnated with the liquid (uncured) formulation and sandwiched between two aluminum disks. The sample was placed at a determined temperature (65, 70 and 75 °C in the case of the system E828-HHMPA 100-1MI and 55, 65 and 80 °C in the case of the system E828-HHMPA 50-1MI) and subjected to an oscillatory force from 0.005 to 0.1 N with an oscillation frequency of 0.083 Hz. The gel time, tgel, was taken as the onset in the decrease of the oscillation amplitude measured by the probe. The conversion of epoxy groups at the gel point, αgel, was determined as the conversion reached in the DSC at the gel time.
Dynamic mechanical analysis
To prepare the specimens, the mixtures were cured in a rectangular mold, with different thermal treatments. The dimensions of the specimens were 20 × 10 × 2 mm3, approximately. The samples were tested in a DMA (dynamic mechanical analysis, TA instruments Q800) at 3 °C min−1 using the single cantilever mode, with an amplitude of 10 μm and a frequency of 1 Hz.
Theory
In the isothermal curing studied, the relative degree of conversion is defined as:
where Δht is the heat released up to a time t and Δhiso is the total reaction heat released during the isothermal cure. The kinetics of this curing has been analyzed by means of an integral isoconversional method, using the following equation [27]:
where R is the gas constant. At a determined relative degree of conversion, tα is the time attained, Aα is the frequency factor, Eα is the activation energy, and g(α) is the following integral obtained from a function of the relative degree of conversion f(α):
The activation energy at a given relative conversion can be obtained from the slope of the representation of ln tα versus the reciprocal of the temperature (see Eq. 2). Provided that the kinetic model is known, the natural logarithm of the frequency factor can be determined from the intercept at the origin. This analysis can be repeated at different relative degrees of conversion throughout the curing process.
Results and discussion
Differential scanning calorimetry
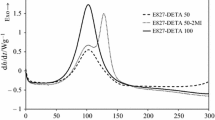
Figure 1 shows the isothermal curing curves at different temperatures of the system E827-HHMPA 50-1MI, corresponding to the first stage, epoxy–anhydride reaction, in the curing process. In the inset of this figure, the relative degree of conversion (Eq. 1) has been represented against reaction time. In this equation, the horizontal right baseline has been used to determine Δht. Figure 2 shows similar results for the stoichiometric E827-HHMPA 100-1MI system. As expected, reaction rate increases with temperature. The temperatures analyzed are different in both systems, due to their different reactivity, especially taking into consideration that in the case of the off-stoichiometric system, the homopolymerization of excess epoxy groups is activated at higher temperatures. Figures 1 and 2 also show two exothermic peaks that can be attributed to (1) the initiation of the reaction by nucleophilic attack of 1MI to the epoxy ring [28] followed by (2) the anionic alternating copolymerization of epoxy and anhydride groups. The mechanism of this reaction is shown in references [20, 21]. Epoxy homopolymerization should not be considered since the range of the temperature selected is too low. In previous manuscripts, it has been proved that in the presence of 1-MI, homopolymerization reaction does not start until 100–110 °C in off-stoichiometric amine–epoxy thermosets [26] and in off-stoichiometric thiol–epoxy thermosets [25]. Moreover, the vitrification of anhydride–epoxy system during isothermal curing can also prevent the activation of the polyetherification process and stabilize the intermediate material. Figure 3 compares the isothermal curing at 70 °C of both systems. In the inset of this Figure, the relative degree of conversion found using Eq. (1) has also been plotted versus time. In the off-stoichiometric formulation, the reaction is faster, due to the higher amount of hydroxyl groups coming from DGEBA that promotes the esterification of hydroxyl groups and the subsequent opening of the epoxy groups in an uncatalyzed mechanism [20, 21]. The higher amount of epoxy groups in the off-stoichiometric formulation also increases the reaction rate above that of the stoichiometric one, because the rate-limiting step in the anionic epoxy–anhydride copolymerization is the attack of the highly stable carboxylate anion to the epoxy ring [20, 21].
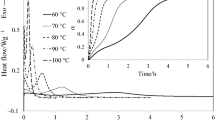
Figure 4 shows the dynamic scans performed after the isothermal curing at different temperatures of the off-stoichiometric formulation. In every dynamic scan, it can be observed that at temperatures higher than the Tg of the partially cured material, the materials devitrify, producing a shoulder and an exothermic peak associated with the first stage of the post-curing (ending of the epoxy–anhydride polycondensation) and with the homopolymerization of the excess of epoxy resin, respectively. It can also be observed, as previously stated, that the homopolymerization of the epoxy groups takes place at high temperatures. A suitable selection of the curing temperatures of both curing processes makes it possible to obtain a sequential curing. It is expected that off-stoichiometric intermediate materials, after the isothermal curing, will be stable and can be stored at room temperature (below Tg of intermediate materials) for a long time. The stability of these materials is established not only by the deactivation of the homopolymerization at low temperatures, but also by the vitrification of the intermediate formulations.
Table 1 shows the activation energy and the natural logarithm of the frequency factor of the isothermal epoxy–anhydride condensation of the two studied systems at different relative degrees of conversion, found using Eq. (2). The average regression coefficient on applying this equation is 0.996. The average error of the activation energy and the natural logarithm of the frequency factor values listed in Table 1 are 7.1 kJ mol−1 and 2.5, respectively, determined using a confidence interval of 95%. To determine the orders of the reaction, a model-fitting method and the Šesták–Berggren equation f(α) = αm(1 − α)n [27] were used with m + n = 2 [29]. With this condition, the following expression for g(α) is obtained:
The off-stoichiometric system always has activation energies and frequency factors smaller than the stoichiometric one. For the off-stoichiometric system, the activation energy always increases, although at the beginning of the process hardly changes. The frequency factor always increases, but at relative degrees of conversion between 0.1 and 0.3, it decreases slightly. Lower activation energies in the off-stoichiometric formulation agree with the higher reaction rate observed for this formulation (see Fig. 3 inset). The slight increase observed in activation energies values on increasing conversion can be explained by the competition between catalyzed and uncatalyzed mechanisms [21], which becomes more evident at high conversion.
The activation energy of the stoichiometric system slightly increases initially but the increase is rather high at the end of the process. The frequency factor slightly decreases at the beginning of the process, but it increases after a relative degree of conversion of 0.3. The high increase of activation energy at the end of the process could be related to diffusion phenomena, which does not exist in the first stage of the curing for off-stoichiometric formulations.
The values of the parameters m and n of both systems are shown in Table 2. They are similar indicating that the underlying reaction mechanism of the isothermal curing hardly changes. This could be expected if one assumes that for the off-stoichiometric E827-HHMPA 50-1MI formulation, only the epoxy–anhydride alternating copolymerization takes place during the isothermal experiments in the DSC in the range of temperatures selected.
Table 2 also shows the values of Tg∞ and the total heat of curing for both systems. The value of Tg∞ for both systems is the same. In previous studies of our group in dual-cured materials, an excess of epoxide leads to increase the Tg∞ of the material [25, 26], since the homopolymerization of epoxide always leads to a more compact network structure. Then, the higher the excess of epoxide, the higher the Tg∞ of the material. In the present case, this effect is not observed, since the values of Tg∞ of both systems are the same. The similarity between the total reaction heats and those reported for equivalent epoxy systems indicates that epoxides reacted almost completely [30, 31].
Tables 3 and 4 show the glass transition temperatures (Tg) of both systems after the isothermal curing. In the off-stoichiometric system (Table 3), they are similar to the corresponding curing temperature, indicating that at the end of the isothermal curing, the system indeed vitrifies and that the mobility of the system becomes severely restricted when the glass transition temperature approaches curing temperature. However, in the stoichiometric system (Table 4), the Tgs are always greater than the corresponding curing temperature, indicating that vitrification takes place before the end of the isothermal curing. This indicates that the reaction can continue so that the Tg can exceed the curing temperature [32, 33]. The presence of unreacted anhydride monomers in the stoichiometric system at the end of the isothermal curing may explain the higher mobility of the system near vitrification, and therefore, the higher Tg achieved at the end of the isothermal curing process.
The degree of conversion attained at the end of the isothermal curing (αiso) is defined as [33]:
Assuming that the addition of the residual heat and the heat of the isothermal cure is equal to the total heat of curing (Δhiso + Δhres = Δhdyn), Eq. (5) becomes [34]
In this work, αiso was calculated by using Eq. (6) instead of Eq. (5), since in some isothermal experiments, it was difficult to establish a baseline to determine the heat of reaction by integration of the calorimetric signal. Tables 3 and 4 show the values of αiso for the two studied systems. In both systems, αiso increases with temperature similar to Tg as it is shown in the same tables. The off-stoichiometric system (Table 3) presents smaller values of αiso than the stoichiometric one (Table 4), since within the experimental range of temperatures, the excess of epoxides remains unreacted. As it was shown, the homopolymerization of epoxy groups takes place at higher temperatures than the epoxy–anhydride polycondensation (see Fig. 4).
Thermomechanical analysis
Figure 5 shows the TMA and DSC analysis of the isothermal curing of the system E828-HHMPA 100-1MI at 70 °C. By the combined use of TMA and DSC, the gelation time (tgel) and the conversion at gel point (αgel), respectively, can be obtained. These parameters were determined for this system at different temperatures (Table 5) and for the system with an excess of epoxide (Table 6).
By increasing the curing temperature, the reaction rate is increased and the time to reach gelation becomes shorter. At 65 °C, the gelation time corresponding to E828-HHMPA 50-1MI is shorter than that of E828-HHMPA 100-1MI, in agreement with the higher reaction rate observed in off-stoichiometric formulations (see Fig. 3).
The values corresponding to αgel for the system E828-HHMPA 100-1MI (Table 5) are similar to the values found for other stoichiometric anhydride–epoxy thermosets [35]. The values of αgel for the system E828-HHMPA 50-1MI (Table 6) are smaller than those of E828-HHMPA 100-1MI. Probably, the differences observed in αgel values between both formulations can be due to the different hydroxyl contents and the effect of the excess of the DGEBA that inhibit the intramolecular cyclization responsible for the increase of αgel [36]. In any case, the values of αgel for the system E828-HHMPA 50-1MI (Table 6) are relatively close to those reported for other similar systems [35, 37].
Dynamic mechanical analysis
Figure 6 shows, for E828-HHMPA 100-1MI formulation, the DMA traces after isothermal curing at 65 °C with and without isothermal post-curing at 150 °C. In the specimen without isothermal post-curing it can be seen the α-relaxation (related with Tg) and the post-curing process. The post-curing results in an increase of the storage modulus and the apparition of the new maximum of tan δ (related with Tg) at higher temperatures. The completely cured sample (post-cured 1 h at 150 °C) has a higher temperature of α-relaxation than the partially one. The maximum of tan δ of the fully cured sample has a value comparable to that of the second maximum of the partially cured sample, indicating that the post-curing of this system in the DMA equipment produces a material with similar properties to that of the system cured in the oven following a different temperature schedule.
Figure 7 compares stoichiometric and off-stoichiometric fully cured formulations. E828-HHMPA 50-1MI has a lower maximum of tan δ temperature due to the less compact network structure. This result is in agreement with the Tg values obtained with DSC (Table 2). The DMA scan of the partially cured sample of E828-HHMPA 50-1MI could not be performed, because the specimen of this sample was too brittle for analysis.
Conclusions
A new dual-curing system based on off-stoichiometric anhydride–epoxy formulations was developed. A base-catalyzed anhydride–epoxy condensation takes place at low temperatures in the first curing stage, while the excess of epoxy mostly homopolymerizes in the second curing stage at higher temperatures.
The dual-curing process is quasi-sequential with stable intermediate materials that can be stored and further processed. This stability is ensured by the vitrification of the materials during the first curing stage and by deactivation of epoxy homopolymerization at low temperatures.
In the off-stoichiometric system, the isothermal reaction between anhydride and epoxy groups ends earlier than in the stoichiometric system. Although reaction kinetics is modified, the reaction mechanism has not changed since the values of the parameters of both model reactions are similar.
The catalytic effect of the hydroxyl groups present in the epoxy monomer on the anhydride–epoxy condensation was demonstrated by the decrease in the activation energy upon an increase in epoxy content.
Gelation time and conversion at gel point are influenced by the excess of epoxy groups. These results are in agreement with earlier studies of reaction kinetics using differential scanning calorimetry.
References
Riew CK, Siebert AR, Smith RW, Fernando M, Kinloch AJ. Toughened epoxy resins: performed particles as tougheners for adhesives and matrices. In: Riew CK, Kinloch AJ, editors. Toughened plastics II novel approaches in science and engineering, vol. 252., Advances in chemical seriesWashington: American Chemical Society; 1996. p. 33–44.
Saiki N, Yamazaki O, Ebe K. UV/heat dual-curable adhesive tapes for fabricating stacked packages of semiconductors. J Appl Polym Sci. 2008;108:1178–83.
Kang B-U. Interfacial fracture behavior of epoxy adhesives for electronic components. J Korea Acad Ind Cooper Soc. 2011;12:1479–87.
May CA, Tanaka GY. Epoxy resins. In: May CA, editor. Chemistry and technology, chap 1. New York: Marcel Dekker; 1988.
Petrie EM. Epoxy adhesive formulations. New York: McGraw-Hill; 2006.
Pascault JP, Williams RJJ. Epoxy polymers: new materials and innovations. Weinheim: Wiley-VCH; 2010.
Kinloch AJ, Shaw SJ, Tod DA, Hunston DL. Deformation and fracture behavior of a rubber-toughened epoxy: 1. Microstructure and fracture studies. Polymer. 1983;24:1341–54.
Ho T-H, Wang C-S. Toughening of epoxy resins by modification with dispersed acrylate rubber for electronic packaging. J Appl Polym Sci. 1993;50:477–83.
Mezzenga R, Boogh L, Månson JAE. A review of dendritic hyperbranched polymer as modifiers in epoxy composites. Compos Sci Technol. 2001;61:787–95.
Guo QP, Habrard A, Park Y, Halley PJ, Simon GP. Phase separation, porous structure, and cure kinetics in aliphatic epoxy resin containing hyperbranched polyester. J Polym Sci B. 2006;44:889–99.
Ratna D, Varley R, Simon GP. Toughening of trifunctional epoxy using an epoxy-functionalized hyperbranched polymer. J Appl Polym Sci. 2003;89:2339–45.
He S, Shi K, Bai J, Zhang Z, Li L, Du Z, Zhang B. Studies on the properties of epoxy resins modified with chain-extended ureas. Polymer. 2001;42:9641–7.
Zhou L, Zhang G, Li J, Jing Z, Qin J, Feng Y. The flame retardancy and thermal stability properties of flame-retarded epoxy resins based on α-hydroxyphosphonate cyclotriphosphazene. J Therm Anal Calorim. 2017;129:1667–78.
Mao W, Li S, Yang X, Cao S, Li M, Huang K, Xia J. Preparation of a flame-retardant epoxy curing agent based on castor oil and study on the curing reaction kinetics. J Therm Anal Calorim. 2017;130:2113–21.
Evans D, Canfer SJ. Radiation stable, low viscosity impregnating resin systems for cryogenic applications. Adv Cryog Eng. 2000;46:361–8.
Ueki T, Nishijima S, Izumi Y. Designing of epoxy resin systems for cryogenic use. Cryogenics. 2005;45:141–8.
Nishijima S, Honda Y, Okada T. Application of the positron annihilation method for evaluation of organic materials for cryogenic use. Cryogenics. 1995;35:779–81.
Pascault JP, Sautereau H, Verdu J, Williams RJJ. Thermosetting polymers. 1st ed. New York: Marcel Dekker, Inc.; 2002.
Thanki JD, Parsania PH. Dynamic DSC curing kinetics and thermogravimetric study of epoxy resin of 9,9′-bis-(4-hydroxyphenyl)anthrone-10. J Therm Anal Calorim. 2017;130:2145–56.
Foix D, Yu Y, Serra À, Ramis X, Salla JM. Study on the chemical modification of epoxy/anhydride thermosets using a hydroxyl terminated hyperbranched polymer. Eur Polym J. 2009;45:1454–66.
Rocks J, Rintoul L, Vohwinkel F, George G. The kinetics and mechanism of cure of an amino-glycidyl epoxy resin by a co-anhydride as studied by FT-Raman spectroscopy. Polymer. 2004;45:6799–811.
Fernández-Francos X, Santiago D, Ferrando F, Ramis X, Salla JM, Serra À, Sangermano M. Network structure and thermomechanical properties of hybrid DGEBA networks cured with 1-methylimidazole and hyperbranched poly(ethyleneimine)s. J Polym Sci Part B Polym Phys. 2012;50:1489–503.
Leena K, Soumyamol PB, Baby M, Suraj S, Rajeev R, Mohan DS. Non-isothermal cure and decomposition kinetics of epoxy–imidazole systems. J Therm Anal Calorim. 2017;130:1053–61.
Ramis X, Fernández-Francos X, De La Flor S, Ferrando F, Serra À. Click-based dual-curing thermosets and their applications. In: Guo Q, editor. Thermosets: structure, properties and applications, chapter 16. 2nd ed. Amsterdam: Elsevier; 2017.
Fernández-Francos X, Konuray AO, Belmonte A, De la Flor S, Serra À, Ramis X. Sequential curing of off-stoichiometric thiol-epoxy thermosets with a custom-tailored structure. Polym Chem. 2016;7:2280–90.
Konuray O, Areny N, Morancho JM, Fernández-Francos X, Serra À, Ramis X. Preparation and characterization of dual-curable off-stoichiometric amine-epoxy thermosets with latent reactivity. Polymer. 2018;146:42–52.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Heise MS, Martin GC. Curing mechanism and thermal properties of epoxy-imidazole systems. Macromolecules. 1989;22:99–104.
Flores M, Fernández-Francos X, Ramis X, Serra À. Novel epoxy-anhydride thermosets modified with a hyperbranched polyester as toughness enhancer. Thermochim Acta. 2012;544:17–26.
Ivin KJ. Polymer handbook. Brandrup J, Immergut EH, editors. New York: Wiley; 1975
Montserrat S, Flaqué C, Calafell M, Andreu G, Málek J. Influence of the accelerator concentration on the curing reaction of an epoxy-anhydride system. Thermochim Acta. 1995;269(270):213–29.
Santiago D, Fernández-Francos X, Ramis X, Salla JM, Sangermano M. Comparative curing kinetics and thermal-mechanical properties of DGEBA thermosets cured with a hyperbranched poly(ethylenimine) and an aliphatic triamine. Thermochim Acta. 2011;526:9–21.
Morancho JM, Ramis X, Fernández-Francos X, Salla JM, Konuray AO, Serra À. Curing of off-stoichiometric amine-epoxy thermosets. J Therm Anal Calorim. 2018;133:519–27.
Morancho JM, Salla JM. Influence of a carboxyl-terminated modifier (CTBN) on the cure of an epoxy resin. J Non Cryst Solids. 1994;172–174:656–60.
Fernàndez-Francos X, Ramis X, Serra À. From curing kinetics to network structure: a novel approach to the modeling of the network buildup of epoxy-anhydride thermosets. J Polym Sci Part A Polym Chem. 2014;52:61–75.
Tanaka Y, Stanford JL, Stepto R. Interpretation of gel points of an epoxy-amine system including ring formation and unequal reactivity: measurements of gel points and analyses on ring structures. Macromolecules. 2012;45:7197–205.
Mauri AN, Galego N, Riccardi CC, Williams RJJ. Kinetic model for gelation in the diepoxide–cyclic anhydride copolymerization initiated by tertiary amines. Macromolecules. 1997;30:1616–20.
Acknowledgements
The authors would like to thank MINECO (Ministerio de Economía y Competividad) and FEDER (Fondo Europeo de Desarrollo Regional) (MAT2017-82849-C2-1-R and MAT2017-82849-C2-2-R) and Generalitat de Catalunya (2017-SGR-77 and Serra Húnter program) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morancho, J.M., Ramis, X., Fernández-Francos, X. et al. Curing and thermomechanical properties of off-stoichiometric anhydride–epoxy thermosets. J Therm Anal Calorim 138, 2865–2872 (2019). https://doi.org/10.1007/s10973-019-08681-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08681-z