Abstract
The aim of this work was to study the effect of tris(3-nitrophenyl) phosphine (NPPh3), which showed a good thermal stability and carbon-forming ability, on the flame retardancy and thermal degradation mechanism of epoxy resins. A series of diglycidyl ether of bisphenol A (DGEBA) loaded with tris(3-nitrophenyl) phosphine (NPPh3) were prepared. It was found that NPPh3 can effectively improve the flame retardancy and thermal stability of the composites. When the loading amount of NPPh3 was 14%, the LOI value of the DGEBA composites was 29.2% (about 1.53 times the corresponding value of the original DGEBA resin). Thermal stability was studied by thermogravimetric analysis, and the results showed that the addition of NPPh3 can improve char formation of this system both in nitrogen and in air atmosphere. Specifically, its combustion residue at 800 °C in nitrogen atmosphere was about 4.26 times of the original resin. Differential scanning calorimetry indicated that NPPh3 slightly decreased the glass transition temperature of epoxy resins. Additionally, the gaseous degradation products were analyzed by thermogravimetric analysis/infrared spectrometry, providing insight into the thermal degradation mechanism. Scanning electron microscopy and Fourier transform infrared were brought together to evaluate the morphology and structure of the residual char obtained after combustion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epoxy resins (EP) have been extensively used as semiconductor encapsulation, structural adhesives, laminates, and coatings owing to its excellent mechanical properties, remarkable adhesion, outstanding corrosion resistance, high intensity, and good electrical performance [1,2,3,4]. However, the flammability is one of the major drawbacks of conventional epoxy resins, which restricts their further applications for safety consideration [5,6,7]. Therefore, for the sake of meeting application requirements, it is significant to improve the flame retardancy of epoxy resins [8]. Nowadays, adding flame retardant to the epoxy systems, preparing of flame-retardant curing agent, and building flame-retardant epoxy resins are the main ways to improve the flame retardancy of the epoxy resins [9].
Over the past decades, halogenated flame retardants were used in epoxy resins to obtain fire-retardant materials. However, they are subjected to strict regulations in many fields because of releasing toxic gases in the process of combustion [10]. Therefore, from the viewpoint of environmental protection, halogen-free epoxy resins have attracted increasing attention [11]. Among them, organic phosphorus compounds are important class of flame retardants with high-retardant efficiency due to its radical scavenger mechanism [12, 13]. It is an outstanding method to modify epoxy resins by using a flame-retardant additive with phosphorus [14].
Triphenyl phosphine (PPh3), a common phosphorus-containing compound, is the triphenyl substitution product of the phosphine, used on a large scale for pharmaceutical industry, organic synthesis, and dye process. From the structural point of view, its aromatic group and the phosphine element are beneficial to the flame retardant [15]. Previous studies also show that it has the advantages of low smoke, non-toxic, and halogen-free, suggesting that it is an excellent polymer flame retardant and in line with the current development direction of the flame retardant [16]. So, PPh3 and phosphorus flame retardant based on PPh3 have been developed rapidly.
Tris(3-nitrophenyl) phosphine (NPPh3), the derivative of PPh3, shows a better thermal stability and carbon-forming ability in TG analysis compared with PPh3. However, in the area of flame retardant, the researches and applications are rare. In this work, NPPh3 is used as flame retardant for the epoxy resins, and the effect of NPPh3 on the thermal and flame retardancy properties of epoxy resins is investigated.
Experimental
Materials
Diglycidyl ether of bisphenol A (DGEBA, epoxy value = 0.44) was purchased from Wuxi Bluestar Chemical Co., Ltd., China. Triphenyl phosphine (PPh3), diethyltoluenediamine (DETDA), sodium bicarbonate, sulfuric acid, and nitric acid were supplied by Chengdu Kelong Chemical Reagent Factory. Ethanol was obtained from Chengdu Changlian Chemical Reagent Co., Ltd., China.
Synthesis of tris(3-nitrophenyl) phosphine
NPPh3 was synthesized through the nitration reaction of PPh3, using already reported method [17]. PPh3 (2.62 g, 0.01 mol) and sulfuric acid (20 g, 0.2 mol) were added to a 100-mL three-necked round-bottom flask equipped with a thermometer, a stirrer, and a constant pressure dropping funnel. PPh3 was dissolved in sulfuric acid with stirring at room temperature. After that, the temperature of the mixed system was reduced to below 10 °C with an ice-salt bath and the mixed solution of sulfuric acid (30 g, 0.3 mol) and nitric acid (6 g, 0.06 mol) was slowly added dropwise over 30 min [18]. Then, the reaction mixture was warmed to room temperature and then stirred for 6 h. The product was precipitated by pouring the reaction mixture over an excess of ice-cold water, neutralizing with a saturated solution of sodium bicarbonate washing with water and filtering. Finally, 3.9 g light yellow product was obtained with the yield of 89%. The structure of NPPh3 is shown in Scheme 1.
Preparation of the cured epoxy resins
In order to obtain the epoxy resins with uniform dispersion, the NPPh3 was mixed with ethanol into a paste at first. Then, the mixture was put into DGEBA and dried to eliminate ethanol in rotary evaporators. Subsequently, the curing agent DETDA was added relative to the amount of DGTDA. After that, the blend was poured into the prepared mold for curing and postcuring at oven as the following procedure: 120–140–160 °C 2 h−1.
Characterization
Fourier transform infrared spectra
Fourier transform infrared spectra (FTIR) were obtained on a Nicolet Magna-FTIR 560 spectrometer (Nicolet Instrument Co, USA) with KBr pellets.
Nuclear magnetic resonance
1HNMR (600 MHz) spectrum was recorded on a FT-80A NMR using CDCl3 as the solvent and internal standard.
Differential scanning calorimetry
The Q-200 (TA Instrument) was used for differential scanning calorimetry (DSC) at 10 °C min−1; 8 mg of the sample was examined under nitrogen atmosphere.
Scanning electron microscopy
Scanning electron microscopy (SEM) analyses were used to study the morphologies of residues obtained from combustion by UL-94 using a FEI Quanta-250 SEM.
Thermogravimetric analysis
The test of thermogravimetric analysis (TG) was conducted on a Netzsch TG209 F1 with a heating rate of 10 °C min−1 from 30 to 800 °C under air and nitrogen atmosphere.
Limiting oxygen index
The limiting oxygen index (LOI) value was obtained on an oxygen index instrument (XYC-75) produced by Chengde Jinjian Analysis Instrument Factory according to ASTMD2863-08 standard with a specimen dimension of 130 × 6.5 × 3.5 mm3.
Vertical burning tests
Vertical burning tests (UL-94) were measured by a CZF-2 instrument (Jiangning Analysis Instrument Company, China) with sample dimension of 125 × 12.5 × 3 mm3 according to UL-94 ASTMD 635-77 standard.
Thermogravimetric analysis/infrared spectrometry
Thermogravimetric analysis/infrared spectrometry (TG-FTIR) was carried out on a Mettler Toledo TG/DSC 1 STARe System thermogravimeter couple with a Nicolet IS10 FTIR spectrophotometer at a linear heating rate of 20 °C min−1 under air within 50–800 °C.
Results and discussion
Characterization of NPPh3
In order to confirm the molecular structure of NPPh3, FTIR was carried out. Figure 1 shows the FTIR spectrum of NPPh3. For NPPh3, the absorption peaks at 3100–3000 cm−1 belonged to benzene ring. The absorptions at 1525 and 1346 cm−1 were assigned to NO2. The peaks at 1469 cm−1 were associated with the vibrations of P-Ph. The peaks at 879, 733, and 674 cm−1 indicated that the product was 1,3-substituted on the phenyl ring. The information above confirmed that the target product was synthesized successfully.
To further determine the structure of NPPh3, 1HNMR measurements were employed to characterize the product. Figure 2 shows the 1HNMR spectrum of NPPh3(ppm): 8.52–8.55 ppm (a), 8.46–8.52 ppm (b), 8.15–8.24 ppm (c), and 7.95–7.90 ppm (d). All these characteristics indeed matched the NPPh3 structure.
Flammability tests of the cured epoxy resins
LOI, an important parameter for evaluating the ease of extinguishment of polymeric materials in the same atmosphere, was defined as the lowest volume concentration of oxygen in an oxygen–nitrogen mixture [19]. The flame-retardant properties of the cured DGEBA/DETDA and DGEBA/NPPh3/DETDA systems were measured by LOI and UL-94. Data are presented in Table 1. It can be seen that the addition of NPPh3 was beneficial to improve the flame retardancy of DGEBA. As can be observed, the pure DGEBA was highly combustible. Its LOI value was only 19.0% and it was classed no rating (NR) in the vertical burning (UL-94) test. However, the LOI value of the cured DGEBA/NPPh3/DETDA was increased from 23 to 29.2% (about 1.5 times of the LOI value of the DGEBA) with the loading amount of NPPh3 increasing from 5 to 14%. When the loadings of NPPh3 were 10, 12, and 14%, it passed the UL94V-1 rating. The fact can be attributed to the prepared NPPh3 which promoted the formation of char layer in the condensed phase. The formed char layer can prevent oxygen and heat from propagation to the underlying polymer matrix during burning and protect the underlying matrix from further combustion.
Thermal properties of the cured epoxy resins
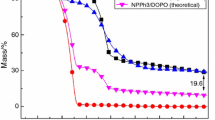
TG was widely utilized to study the thermal stability and thermal degradation behavior of polymers [11]. Figure 3 shows the TG and DTG curves of NPPh3, cured DGEBA/DETDA, and DGEBA/NPPh3/DETDA systems under nitrogen atmosphere. The relative detailed data are listed in Table 2. The T5% was defined as the temperature at 5% mass loss (the initial decomposition temperature), and T1max and T2max were defined as the temperature of the first maximum mass loss rate and the second maximum mass loss rate, respectively. Yc was defined as the char residue, Exp was the amount of residual carbon obtained from the experiment, and Cal was the theoretical value calculated by the formula:
wt% referred to the corresponding proportion of ingredients, Yexp was the actual amount of carbon residue.
From the DTG curves revealed in Fig. 3, it can be observed that the whole degradation process of NPPh3 had one stage and the maximum decomposition rate was about 9.75% °C−1 at 378.3 °C. The T5% of the synthesized NPPh3 flame-retardant additive was 291.4 °C, and the char residue at 800 °C was 28.16%, revealing greatly charring ability at high temperature due to the presence of abundant aromatic structure and nitro group in NPPh3 [20].
The DGEBA/DETDA started to decompose at 363.7 °C, and the char yield was only 4.13% at 800 °C. The thermal degradation course of the DGEBA/DETDA presented only one stage and the peak appeared at 391.7 °C with the loss mass rate of 18.01% °C−1 due to the char forming and the decomposition of the DGEBA/DETDA [21].
In Fig. 3, all cured resins had single-stage decomposition process, demonstrating that the original and modified DGEBA resins had similar degradation process. With the adding of NPPh3 content, the T5% of DGEBA/NPPh3/DETDA systems decreased. The value was decreased from 363.7 °C of pure epoxy resins to 300.4 °C of DGEBA/14%NPPh3/DETDA. This phenomenon originated from the decomposition of NPPh3 at an inferior temperature (291.4 °C) due to the low bond energy of P–C [22]. Meanwhile, the Rpeak was decreased from 18.01% °C−1 for the DGEBA/DETDA to 6.39% °C−1 when the contents of NPPh3 were 14%. This may be attributed to the previously formed char layer, protecting the underlying epoxy resins matrix from further degradation [23]. It was worth noting that the char yields at 800 °C remarkably increased from 4.13 to 17.60% with NPPh3 content increasing from 0 to 14%. And this phenomenon can be due to the formed char which had high thermal stability, resulting the increasing of the char residue at high temperature. Furthermore, as listed in Table 2, the theoretical char residue was lower than the experimental value at 800 °C. And the more the amount of NPPh3 added, the larger the difference was. This also showed that the addition of NPPh3 increased the mass of residual carbon content significantly.
TG and DTG curves of NPPh3, cured DGEBA/DETDA, and DGEBA/NPPh3/DETDA systems in the temperature range of 30–800 °C under an air atmosphere are shown in Fig. 4, and the detailed data are summarized in Table 3.
As can be seen, the degradation of NPPh3 in atmosphere was observed in three stages which occurred at the temperature ranges of 240–460 °C, 460–600 °C, and 600–800 °C, and the peak of maximum thermal decomposition rate appears at 384.9, 538.6, and 708.5 °C with the loss rate of 7.39, 0.68, and 1.14% °C−1. Initial decomposition occurred when the temperature reached 319.5 °C, and the char yield was 36.48 and 13.62% when the temperature was 500 and 800 °C.
Compared with only one decomposition stage taken place in nitrogen atmosphere, the DGEBA/DETDA and DGEBA/NPPh3/DETDA systems had two decomposition stages existed in air atmosphere. The first stage was the thermal degradation of the polymer network, and the second stage was due to the char formed in the early degradation that behaved unstable thermal stability and reoxidized [24]. Figure 4 shows that in the presence of increasing amount of NPPh3, the values of T5%, T1max, and R1peak were decreased. This trend was the same as that obtained in the nitrogen atmosphere, suggesting that the incorporation of flame retardants affected the decomposition process of epoxy resins matrix. In addition, the value of T2max increased with the addition of NPPh3 content and R2peak except for DGEBA/14%NPPh3/DETDA was decreased. This may be due to the physical barrier of the oxygen diffusion caused by the accumulation of NPPh3 moieties on the surface of the residue, which reduced the amount of oxygen available for the oxidation of underlying char and thus delayed the oxidation process as a whole. As shown in Fig. 4 and Table 3, char yields at 500 °C of the flame-retardant thermosets increased with the increasing content of NPPh3. When the amount of NPPh3 increased from 0 to 14%, the char residue increased from 18.14 to 32.54%, and the experimental residual carbon content at 500 °C was higher than the theoretical value. However, the residual carbon content at 800 °C was decreased with the addition of NPPh3. Meanwhile, the experimental value obtained at this time was lower than the theoretical value. This phenomenon suggested that the addition of NPPh3 did promote the formation of the carbon layer before 500 °C. With the continuous increasing of temperature, the addition of NPPh3 had a certain influence on the decomposition of carbon layer [9, 25].
Figure 5 gives DSC curves of DGEBA/DETDA and DGEBA/14%NPPh3/DETDA. They exhibited single glass transition temperature (T g ). It was well known that an absolutely miscible blend presents only one Tg peak. This single Tg indicated a homogeneous morphology of these systems. Moreover, DGEBA/14%NPPh3/DETDA composites exhibited lower Tg value than DGEBA/DETDA. This phenomenon can be partially explained by two possible factors, which were the crosslink density of epoxy resins decreased due to the addition of NPPh3 and the presence of NPPh3 particles in epoxy resins loosened the cohesive forces between the epoxy molecules.
Pyrolysis products analysis of DGEBA/DETDA and DGEBA/14%NPPh3/DETDA
In order to further investigate the thermal degradation mechanism, the pyrolysis products during thermal decomposition were analyzed by TG-FTIR in an air atmosphere. FTIR spectra of the volatilized products at maximum decomposition rate and 3D TG-FTIR spectra of the pyrolysis products of DGEBA/DETDA and DGEBA/14%NPPh3/DETDA are shown in Figs. 6 and 7. As shown in Fig. 6, the addition of NPPh3 did not affect the type of volatile products. The DGEBA/14%NPPh3/DETDA released the similar decomposition products to that of DGEBA/DETDA. The major pyrolytic gases were water (3650 cm−1), aromatic components (2969, 1604, 1509, and 699 cm−1) [26], CO2 (2356 and 2310 cm−1), CO (2181 and 2107 cm−1) [27], and ester/ether components (1747, 1258, and 1177 cm−1).
From Fig. 7, it can be seen that the pyrolysis products from DGEBA/14%NPPh3/DETDA released earlier than DGEBA/DETDA, which had no pyrolysis product below 300 °C. This can be interpreted that NPPh3 catalyzed the thermal decomposition of epoxy resins [2]. In the temperature range of 300–500 °C, for DGEBA/DETDA, its peaks strength of CO2 and aromatic components firstly increased with increasing temperature. Then, the strength decreased gradually after the maximum degradation rate. The intensity of this area was stronger than that of DGEBA/14%NPPh3/DETDA, which meant that the rate of chain scission of DGEBA/DETDA in 300–500 °C was higher.
Compared with DGEBA/DETDA, the peaks strength of CO2 and aromatic components of DGEBA/14%NPPh3/DETDA were higher in the temperature ranged from 500 to 800 °C, especially in the temperature after 650 °C. This stage was assumed as the oxidation process of the char moiety [28]. It was clear that the addition of NPPh3 made the oxidation of the carbon char intensified. It was worth noting that there was no characteristic peak of phosphorus-containing fragment in the FTIR spectra of volatilized products of DGEBA/14%NPPh3/DETDA volatiles, which meant that the interaction between NPPh3 and epoxy resins occurred in the condensed phase in the time of thermal decomposition, and a large amount of phosphorus compound stayed in condensed phase [19].
Investigation of the char formation
Morphologies of the residual chars
Figure 8 presents SEM micrographs of char residues of the cured DGEBA/DETDA and DGEBA/14%NPPh3/DETDA collected from vertical burning tests. It was obvious that for the cured DGEBA/DETDA, there were many small crevasses and holes on the surface due to insufficient char formation during the burning process. This char cannot effectively protect DGEBA from heat and flammable volatiles. However, with the incorporation of NPPh3, the char residues of DGEBA/14%NPPh3/DETDA exhibited a continuous, compact, and strong structure. The above phenomenon suggested that NPPh3 can catalyze the formation of a dense char layer, which contributed to the improvement in the flame-retardant properties of the epoxy resins.
FTIR study of the residual chars
In order to further explore the formation of condensed phase, the solid products of cured DGEBA/DETDA and DGEBA/14%NPPh3/DETDA collected after UL-94 test were measured by FTIR, and the spectra are shown in Fig. 9. The absorbance peaks at 3444, 2800–3000, 1627, and 878 cm−1 appeared simultaneously in both spectrograms. The broad peaks around 3444 cm−1 were N–H and O–H bonds, which is attributed to the ammonium compounds and the pyrolysis products of the hydroxyl compounds [29]. The absorption peaks appearing at 2800–3000, 1627, and 878 cm−1 can be assigned to aromatic compounds [30]. Besides, new absorbance peaks at 1508, 1300, 1238, 1176, and 1030 cm−1 appeared in the FTIR spectrum of DGEBA/14%NPPh3/DETDA. The absorbance peaks at 1508 and 1300 cm−1 indicated the existence of N–O bond in the char residue, which meant the nitro group did not completely decompose after burning. It was worth noting that the evident absorbance peaks of P=O and P–O can be discerned at 1238 cm−1 and 1176 and 1030 cm−1, suggesting that the char layer structure contained polyphosphoric acid and also proved the flame-retarded effect of NPPh3 in condensed phase. The FTIR results coincided with the TG-FTIR tests discussed above.
Conclusions
In this paper, NPPh3 was added as a flame retardant to the epoxy resin. A series of DGEBA/NPPh3/DETDA containing 0, 5, 10, 12, and 14% content of NPPh3 were prepared. When the NPPh3 concentration was 14%, the cured DGEBA/14%NPPh3/DETDA passed UL-94V-1 rating and the value reached 29.2%, which was about 1.5 times that of the corresponding value of DGEBA/DETDA. The results of TG showed that NPPh3 improved the char formed acts of epoxy resins and enhanced the thermal stability of the polymer. Moreover, the TG-FTIR was employed to identify the degradation mechanism. The residues were analyzed by SEM and FTIR, and the result indicated that the addition of NPPh3 helped epoxy resins form a tight char layer which contained aromatic group, nitro group, and phosphate groups. In summary, NPPh3 can effectively improve the flame retardancy of epoxy resin.
References
Hu J, Shan J, Zhao J, Tong Z. Isothermal curing kinetics of a flame retardant epoxy resin containing DOPO investigated by DSC and rheology. Thermochim Acta. 2016;632:56–63.
Zhao W, Liu J, Peng H, Liao J, Wang X. Synthesis of a novel PEPA-substituted polyphosphoramide with high char residues and its performance as an intumescent flame retardant for epoxy resins. Polym Degrad Stab. 2015;118:120–9.
Yang S, Wang J, Huo S, Wang J, Tang Y. Synthesis of a phosphorus/nitrogen-containing compound based on maleimide and cyclotriphosphazene and its flame-retardant mechanism on epoxy resin. Polym Degrad Stab. 2016;126:9–16.
Zhang T, Liu W, Wang M, Liu P, Pan Y, Liu D. Synergistic effect of an aromatic boronic acid derivative and magnesium hydroxide on the flame retardancy of epoxy resin. Polym Degrad Stab. 2016;130:257–63.
Zhang W, Fina A, Cuttica F, Camino G, Yang R. Blowing-out effect in flame retarding epoxy resins: insight by temperature measurements during forced combustion. Polym Degrad Stab. 2016;131:82–90.
Liu S, Fang Z, Yan H, Chevali VS, Wang H. Synergistic flame retardancy effect of graphene nanosheets and traditional retardants on epoxy resin. Compos A Appl Sci Manuf. 2016;89:26–32.
Huo S, Wang J, Yang S, Wang J, Zhang B, Zhang B, Chen X, Tang Y. Synthesis of a novel phosphorus-nitrogen type flame retardant composed of maleimide, triazine-trione, and phosphaphenanthrene and its flame retardant effect on epoxy resin. Polym Degrad Stab. 2016;131:106–13.
Tan Y, Shao ZB, Yu LX, Xu YJ, Rao WH, Chen L, Wang YZ. Polyethyleneimine modified ammonium polyphosphate toward polyamine-hardener for epoxy resin: thermal stability, flame retardance and smoke suppression. Polym Degrad Stab. 2016;131:62–70.
Wang S, Xin F, Chen Y, Qian L, Chen Y. Phosphorus-nitrogen containing polymer wrapped carbon nanotubes and their flame-retardant effect on epoxy resin. Polym Degrad Stab. 2016;129:133–41.
Zhang M, Luo Z, Zhang J, Chen S, Zhou Y. Effects of a novel phosphorus–nitrogen flame retardant on rosin-based rigid polyurethane foams. Polym Degrad Stab. 2015;120:427–34.
Xu GR, Xu MJ, Li B. Synthesis and characterization of a novel epoxy resin based on cyclotriphosphazene and its thermal degradation and flammability performance. Polym Degrad Stab. 2014;109(109):240–8.
Ding J, Tao Z, Fan L, Yang S. Preparation and characterization of flame retardant epoxy resins based on phosphorus-containing biphenyl-type phenolic resin. e-Polymers. 2010;10(1):1372–84.
Sun S, He Y, Wang X, Wu D. Flammability characteristics and performance of halogen-free flame-retarded polyoxymethylene based on phosphorus–nitrogen synergistic effects. J Appl Polym Sci. 2010;118(1):611–22.
Xiao L, Sun D, Niu T, Yao Y. Syntheses and characterization of two novel 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-based flame retardants for epoxy resin. Appl Phys A. 2014;118(4):1365–70.
Zhang X, Zhong Y, Mao ZP. The flame retardancy and thermal stability properties of poly (ethylene terephthalate)/hexakis (4-nitrophenoxy) cyclotriphosphazene systems. Polym Degrad Stab. 2012;97(8):1504–10.
Wang SB, Wang LS. Synthesis and application of triphenylphosphine and its derivates. Mod Chem Ind. 2006;26(6):66–9.
Agrawal S, Narula AK. Synthesis and characterization of phosphorus containing aromatic poly(amide-imide)s copolymers for high temperature applications. Polym Bull. 2013;70(12):3241–60.
Bai CY, Tang XD, Chen XT, Zhang JH. Synthesis and characterization of bis(3-aminophenyl)phenyl phosphine oxide. Chem Reag. 2010;32(5):470–2.
Chen X, Ma C, Jiao C. Enhancement of flame-retardant performance of thermoplastic polyurethane with the incorporation of aluminum hypophosphite and iron-graphene. Polym Degrad Stab. 2016;129:275–85.
Yang Y, Liu J, Cai X. Antagonistic flame retardancy between hexakis(4-nitrophenoxy) cyclotriphosphazene and potassium diphenylsulfone sulfonate in the PC system. J Therm Anal Calorim. 2016;126(2):571–83.
Xu MJ, Ma Y, Hou MJ, Li B. Synthesis of a cross-linked triazine phosphine polymer and its effect on fire retardancy, thermal degradation and moisture resistance of epoxy resins. Polym Degrad Stab. 2015;119:14–22.
Bao X, Cai X. Synergistic effect of methyl phenyl silicone resin and DOPO on the flame retardancy of epoxy resins. J Therm Anal Calorim. 2014;118(1):369–75.
Xu MJ, Xu GR, Leng Y, Li B. Synthesis of a novel flame retardant based on cyclotriphosphazene and DOPO groups and its application in epoxy resins. Polym Degrad Stab. 2016;123:105–14.
Zhang L, Wang Y, Liu Q, Cai X. Synergistic effects between silicon-containing flame retardant and DOPO on flame retardancy of epoxy resins. J Therm Anal Calorim. 2016;123(2):1–8.
Yang S, Wang J, Huo S, Cheng L, Wang M. Preparation and flame retardancy of an intumescent flame-retardant epoxy resin system constructed by multiple flame-retardant compositions containing phosphorus and nitrogen heterocycle. Polym Degrad Stab. 2015;119:251–9.
Ding H, Wang J, Wang C, Chu F. Synthesis of a novel phosphorus and nitrogen-containing bio-based polyols and its application in flame retardant polyurethane sealant. Polym Degrad Stab. 2016;124:43–50.
Zhang T, Liu W, Wang M, Liu P, Pan Y, Liu D. Synthesis of a boron/nitrogen-containing compound based on triazine and boronic acid and its flame retardant effect on epoxy resin. High Perform Polym. 2016;29(5):513–23.
Zhang L, Wang Y, Cai X. Effect of a novel polysiloxane-containing nitrogen on the thermal stability and flame retardancy of epoxy resins. J Therm Anal Calorim. 2016;124(2):791–8.
Xu W, Wirasaputra A, Liu S, Yuan Y, Zhao J. Highly effective flame retarded epoxy resin cured by DOPO-based co-curing agent. Polym Degrad Stab. 2015;122:44–51.
Wang P, Yang F, Li L, Cai Z. Flame retardancy and mechanical properties of epoxy thermosets modified with a novel DOPO-based oligomer. Polym Degrad Stab. 2016;129:156–67.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, H., Yang, Y., Cao, X. et al. Thermal degradation mechanism and flame retardancy of epoxy systems containing tris(3-nitrophenyl) phosphine. J Therm Anal Calorim 132, 1629–1637 (2018). https://doi.org/10.1007/s10973-018-7081-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7081-6