Abstract
Aluminum-pillared montmorillonites are useful materials for their application as catalysts, adsorbents and ceramic composites. The precursor is a pillared montmorillonite that is not thermally stabilized. The precursor preparation methods, textural properties and catalytic activity have been extensively investigated, but comparatively, studies concerning their thermal transformations at high temperature are limited. In this work, precursors were prepared using two types of montmorillonites, Cheto (Ch) and Wyoming (W), and using two different OH–Al polymer sources: hydrolyzed (H) and commercial (C) solutions. Structural and thermal transformations of the precursors with heating up to 1200 °C were determined by X-ray diffraction and thermogravimetric analysis. Thermal analysis of these precursors below 600 °C revealed the influence of OH–Al polymers from the two solutions. The major phases developed at 1200 °C from the original montmorillonites were mullite for W and cordierite for Ch. The content of these phases depended on the aluminum in the octahedral sheet of the pristine montmorillonites. Amorphous phase, cristobalite, spinel, sapphirine and others phases were also found. The intercalation of OH–Al polymers in montmorillonites caused an increase in amorphous content after treatment at 1030 °C; however, it favored mullite development above 1100 °C. Although total aluminum content of both W and Ch precursors was similar, the transformation to mullite was directly related to the octahedral aluminum/magnesium ratio. The phase composition of the products at 1200 °C was not dependent on the type of intercalated OH–Al polymers. The increase in mullite content of the thermally treated precursors contributes to its possible application as advanced ceramic products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pillared interlayer clays (PILCs) are usually prepared by calcination of precursors made of clay minerals intercalated with inorganic polyhydroxylated species of Al, Zr, Ti, Fe, etc. [1, 2]. Thermal treatment of the precursor is performed in the range of 350–750 °C depending on both the type of clay and/or intercalated species. These products are used in catalysis [3] and as adsorbents [1, 4].
The composition of the pillared clay becomes enriched in the metal of the intercalated species and thus modifies the physical properties and phases that are developed by calcination. In particular, the effectiveness of the intercalation process, physicochemical and textural characterization of the intercalated clay has been extensively investigated by different techniques [5]. Structural characterization of the precursor and PILCs is usually performed using X-ray diffraction (XRD) [3], Fourier transform infrared spectroscopy [6] and Mossbauer analysis [7], including other specific techniques being applied such as nuclear magnetic resonance [8], scanning electron microscopy [9] and energy-dispersive X-ray spectroscopy [9]. The thermal behavior of the precursors is commonly examined by differential thermal analysis (DTA), thermogravimetry (TG) and/or differential scanning calorimetry (DSC) up to 750 °C [10] where the transformation of intercalated metal hydroxylated species to metal oxide occurs, which were caused by the interaction between species and structural clay layer and the thermal treatment. These reactions stabilize the linking between the species and the clay structure [3]. When further heating at high temperatures (1000–1300 °C) is conducted, a series of transformations occur within the clay involving phases that disappear and simultaneously others are formed [11].
The importance of the phase and microstructure evolution in clays with temperature has been reported in many studies [12–17]. The nature of the clay is the most important factor controlling the transformations on heating. Moreover, due to the natural origin of clays, several factors such as the chemical and mineralogical composition of the clay mineral, the presence of impurities (quartz, feldspar, etc.) and structural defects influence the formation of crystalline and liquid phases at high temperature [15, 16].
Montmorillonite, a clay mineral belonging to the smectite group, is commonly used as starting material for the syntheses of pillared clays [3]. Smectite is a hydrated aluminum silicate having a layered structure of the 2:1 type which is constituted by one aluminum octahedral sheet between two tetrahedral silica sheets (T–O–T). In both tetrahedral and octahedral sheets, the isomorphic replacement of Si+4 and Al+3 cations, respectively, can take place generating negative charges in the layer. These charges are compensated by exchangeable cations such as Na+, K+, Ca2+, Mg2+, etc. in the interlayer spacing.
In general, transformation to mullite from aluminosilicates has been specially considered because mullite is a ceramic component of traditional and advanced ceramic products with important characteristics including high mechanical properties [12]. For smectites, mullite transformation is directly related to the aluminum content of the structure [11].
The aim of this work was to study the thermal and structural behavior of precursors prepared from montmorillonites and pillaring solutions after heating up to 1200 °C. We focused on the influence of clay mineral composition and type of solution on the thermal transformations and crystalline phases developed by thermal treatment in a range of temperature of 1000–1200 °C. Specifically, the precursors were prepared from two different montmorillonites intercalated with two types of aluminum polymeric species solutions. We examined their thermal transformations by DTA–TG and the crystalline phases developed with thermal treatment by XRD. The results obtained were compared with those of the pristine montmorillonite.
Experimental
Two montmorillonitic clays, namely a Wyoming (W) and a Cheto (Ch) types, were used as starting materials for the preparation of intercalated clays. As described in a previous work, both clays contain montmorillonite as principal clay mineral, with low amounts of quartz and feldspars. These clays have different aluminum content, i.e., 17.6 and 14.6 mass% Al2O3 in W and Ch, respectively. The structural formulae of the montmorillonites are: \(\left[ {\left( {{\text{Si}}_{3.94} {\text{Al}}^{\text{IV}}_{0.06} } \right)\left( {{\text{Al}}^{\text{VI}}_{1.36} {\text{Fe}}_{0.06} {\text{Mg}}_{0.60} } \right){\text{O}}_{10} \left( {\text{OH}} \right)_{2} } \right]\) and \(\left[ {\left( {{\text{Si}}_{3.91} {\text{Al}}^{\text{IV}}_{0.09} } \right)\left( {{\text{Al}}^{\text{VI}}_{1.61} {\text{Fe}}_{0.13} {\text{Mg}}_{0.26} } \right){\text{O}}_{10} \left( {\text{OH}} \right)_{2} } \right]\) for the Ch and W, respectively [18]. The first term in parenthesis of the formula indicates the chemical composition of the tetrahedral sheet, while the second parenthesis corresponds to the octahedral sheet of montmorillonite.
Two OH–Al solutions with different amounts and types of OH–Al polymers were used for intercalating the montmorillonites. Solution H was prepared by partial neutralization of a 0.1 M AlCl3 solution with 0.1 M Na(OH) and aged at room temperature for 4 days [19]. This solution consists mainly of OH–Al polyhydroxycations with Al +713 structure and a low proportion (<5%) of Al ions as monomers. The solution C was obtained by dilution with distilled water of a commercial concentrate solution (6.0 M; Tort Valls S.A., Argentina) up to 0.1 M Al and aged for 7 days at room temperature. This solution contains a large proportion of slow-reacting polymers that have a molecular structure similar to gibbsite [18–20].
The OH–Al–montmorillonite was synthesized by an ion exchange reaction. The clay was dispersed in water (2 mass%), and subsequently, the polymeric OH–Al solution was slowly added to this suspension while maintaining a constant stirring. The added Al amount was 3.25 mmolAl g−1 clay. After 24 h in contact, the solid was separated by centrifugation and washed several times to remove the electrolyte excess. The precursors obtained this way were named according to the type of aluminum source (i.e., H or C polymeric solutions) and clay (i.e., W or Ch), as shown in Table 1.
Gravimetric and differential thermal analyses (TG–DTA) up to 1000 °C were performed with a Netzsch 409 equipment using α-Al2O3 as reference at a heating rate of 10 °C min−1. The thermal treatment of the pristine clays and precursors was carried out in an electric furnace at a heating rate of 5 °C min−1 up to 1030, 1100 and 1200 °C for 2 h. The identification of crystalline phases and the structural characterization of the pristine clays, precursors and heated products were carried out by XRD using a diffractometer Philips with goniometer 3020 and PW3710 controller with radiation of Cu-Kα and Ni filter in the range of 3°–70°(2θ). Al2O3 content in mullite was calculated for heated products at 1200 °C, according to Ban and Okada method by evaluating the 220 and 111 reflections ratio (as integrated area) [21].
Results and discussion
Characterization and thermal analysis up to 1000 °C of pristine and precursor samples
Previous studies have demonstrated that the cation exchange reaction in natural clay mineral consists in the replacement of the natural interlayer cations by the treated cations [22]. Consequently, the interlayer spacing of the clay can be modified. Figure 1 shows that the interlayer spacing of Ch montmorillonite shifted from 13.9 to 19.6 Å for Chah and to 21.0 Å for the Chac material after treating with H and C polymeric OH–Al solutions, respectively. Similarly, W montmorillonite interlayer spacing shifted from 15.6 to 19.4 Å for Wah and to 20.5 Å for Wac.
The increase in the interlayer spacing indicated the successful intercalation of the OH–Al complexes in the clay minerals and the different values between treatment using H and C solutions were attributed to distinctive OH–Al species present in the solutions [23]. According to Aceman et al. [24], in the first stage of the adsorption of the Keggin ion species into the clay mineral layer, these ions can dissociate into smaller units and monomeric aluminum. This monomeric aluminum is preferentially adsorbed.
The chemical analysis results indicated that initial aluminum content (Ali) of pristine Ch and W was 2.90 and 3.45 mmol g−1, respectively, and increased after the exchange reaction with Al. Each montmorillonite retained different amounts of aluminum (Alr): 2.39 and 2.03 mmolAl g−1 after treating with H solution and 2.34 and 2.26 mmolAl g−1 using the C solution, respectively (Table 2). The higher values of Alr in Ch montmorillonite (2.39 and 2.34 mmolAl g−1) respect to those of W montmorillonite (2.03 and 2.26 mmolAl g−1) were attributed to the higher initial exchangeable cation capacity of this sample (i.e., Ch 110 meq/100 g and W 97 meq/100 g) [18]. An interesting feature was that the total aluminum after OH–Al treatment (Alt) was similar for both clays, in a range of 5.24–5.71 mmol g−1. Taking into account that magnesium is present in the octahedral sheet, Table 2 also includes the calculated Al–Mg molar ratio of pristine samples. As expected, these ratios increased after OH–Al treatments from 2.3 for Ch and 5.94 for W to ~4.16–4.2 for Al-treated Ch and to ~9.45–9.85 for Al-treated W.
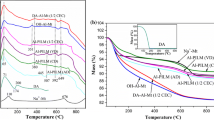
DTA curves of precursors are shown in Fig. 2. Temperature of the endothermic and exothermic peaks of the curves for precursors and pristine montmorillonites is given in Table 3. The temperature of the first endothermic peak corresponding to the loss of interlayer water was 174 °C for natural Ch and 142 °C for the W clay. The difference between them is related to the type of predominant cation present in the interlayer spacing [18]. The second endothermic peak was centered at 653 °C for Ch and 712 °C for W and represents the dehydroxylation of the structure of the clay minerals. The corresponding third endothermic peak was observed at 881 and 916 °C and resulted from the structural collapse (breakdown) of clay structure [25]. The exothermic peaks near 900 and 950 °C are attributed to the formation of new phases.
DTA curves in Fig. 2 show that the dehydration temperature was displaced after treatment using H solution to 136 °C for Ch and to 152 °C for W montmorillonites (Table 3) and those temperatures for precursors obtained from solution C appeared at 166 and 129 °C. These changes were caused by the presence of higher amounts of aluminum. Table 3 also shows a significant reduction in dehydroxylation temperature for both precursors. Probably, chemical bonds between Al pillars and montmorillonite layer can be formed via thermal treatment up to 500 or 600 °C as it has been studied by Volzone and Garrido [26] and Aceman et al. [27, 28], respectively.
Dehydroxylation temperature in DTA diagrams markedly differed between precursors obtained from H and C (Fig. 2, Table 3). In the first case, an endothermic broad band centered at 440 °C is also observed. As mentioned above, intercalated Al species produced a shift to lower temperature in the structural OH loss of the precursors compared with the pristine samples (Table 3). The reduction in the dehydroxylation temperature is coincident with that reported in previous studies [2] by transforming a pillared saponite to enstatite and silica.
T–O–T layer charge of the dioctahedral smectites coming from tetrahedral substitution of Si by Al and/or octahedral substitution of Al by Mg and/or Fe affects the clay mineral surface properties. Aceman et al. [24, 27, 28] have studied the mechanism of Al pillaring of different smectites types (montmorillonite, beidellite, saponite, hectorite and laponite) up to 600 °C. They found that Al tetrahedral substitution in smectites (such as beidellite) increases the strength of hydrogen bonds between either water or interlayer polyhydroxy cation and the clay mineral surface.
On the other hand, smectites with octahedral charge (montmorillonite) exhibit more extensive interlayer hydrolysis than those with mainly tetrahedral charge. In this work, both montmorillonites have low tetrahedral charge contribution (1.5% for Ch and 2.2% for W) and important contributions of octahedral charge (32.0% for Ch and 19.5% for W). Thus, we consider that the influence of the octahedral substitutions on the clay minerals studied in these work is more important than the basicity associated with the surface of their tetrahedral layers.
Table 4 shows the mass losses observed in TG curves (not shown) for all samples. For precursors prepared with solution C, a continuous mass loss up to 600 °C was associated with the dehydroxylation as it was observed in DTA diagram. Mass loss due to dehydroxylation of precursors obtained using solution H occurred in two stages. This revealed the presence of distinctive Al species from those contained in the intercalated clays prepared from solution C [18] and their specific interaction with the structure of each montmorillonite. This could suggest that water loss from Al species intercalated using commercial solution possibly had interactions with a variation greater in associated energy. According to an earlier study [23], the types of intercalated Al species do not interact uniformly with the clay surface. The retention of Al species on the clay surface and between clay layers is controlled by the nature and basicity of the intercalating species in the solution as well as the heterogeneity of the surface sites of clays. Previous results about deintercalation of Al polymers in clays by an ion exchange reaction with NaCl indicated that the Al species have different affinity with the montmorillonite and differences exist in their trend to form gibbsite during aging [18]. A similar situation is found for the low temperature reactions of montmorillonites, at which water molecules held with interlayer cations are released on heating. As water molecules are strongly bonded due to interactions with exchangeable cations, a higher dehydration temperature is necessary for their removal. The total mass loss of the precursors prepared with solution C was higher for both types of montmorillonite. This increase was attributed to a higher proportion of OH–Al of such species, and result was consistent with the presence of gibbsite in the samples (see peak gibbsite in DTA at 300–330 °C). No observable mass changes after 800 °C were found.
Phase evolution of pristine montmorillonite and precursors with heating temperature up to 1200 °C
Phase development in pristine montmorillonites
Table 5a, b and Figs. 3–5 show the phases developed from pristine and precursors thermally treated at 1030, 1100 and 1200 °C.
As mentioned above, pristine Ch clay is constituted by montmorillonite as main phase and low quartz and feldspar as impurities [29]. At 1030 °C, the heated product mainly consisted of a new aluminum magnesium silicate phase, Al2MgO12Si4; scarce cristobalite, SiO2; residual feldspar (anorthite, CaAl2Si2O); and quartz, SiO2. Reflections of Al–Mg silicate disappeared at 1100 °C as cordierite, 2MgO·2Al2O3·5SiO2; and cristobalite developed. These phases were the major constituents of the samples calcined at 1200 °C, together with scarce anorthite and quartz as minor components.
For W heated at 1030 °C (W1030), high amorphous content (evidenced a broad band centered at ~22°(2θ)), as well as spinel, scarce mullite and cristobalite formed, accompanied by residual quartz (in a higher amount than that of Ch1030) and feldspars (orthoclase, KAlSi3O8 and albite, NaAlSi3O8). At 1100 °C, intensity of orthorhombic mullite (i.e., orthorhombic crystal structure, 3Al2O3·2SiO2) and cristobalite reflections significantly increased, whereas those of a spinel-type phase reduced and little quartz and traces of feldspar (orthoclase) remained. Sample calcined at 1200 °C (W1200) mainly consisted of mullite and cristobalite, with additional phases such as cordierite and sapphirine (stoichiometric 4MgO·5Al2O3·2SiO2) while spinel disappeared, and quartz reduced in comparison with W1100.
XRD patterns (Fig. 3a, b) confirmed the structure breakdown of montmorillonite in samples treated at 1030 °C in agreement with results obtained by DTA–TG (Table 3).
Significant differences in structural transformation of pristine clays were found. For Ch clay (Figs. 3a–5a), Al–Mg silicate formed at 1030 °C probably as intermediate (not detected by XRD at higher temperatures) for transformation to cordierite and cristobalite, whose contents gradually increased by heating to 1200 °C.
For W clay (Figs. 3b–5b), a spinel-type phase initially appeared at 1030 °C, accompanied with cristobalite. At 1100 °C, the reflections of those phases grew and mullite was formed. Heating up to 1200 °C caused the disappearance of the spinel phase, while mullite and cristobalite contents continued to grow.
These results may be explained by the difference in chemical and structural composition of montmorillonites as evidenced from their structural formulae (mentioned above). Thus, the sequence of phase transformations at high temperature determined in the present work is consistent with that reported previously for smectites [11, 13, 14, 30] involving destruction of the clay structure and recrystallization to new phases. This would occur by the formation of a liquid phase rich in Si (involving constituents of the tetrahedral layer) and subsequent reorganization of the octahedral layer of the structure of dehydroxylated clay. Therefore, the constituents of the tetrahedral sheet of montmorillonite as well as alkalis, iron, etc. would form a silica-rich liquid phase from which cristobalite would grow at temperatures above 1030 °C. Additionally, Mg, Al and Fe associated with the octahedral layer take part in the spinel formation. However, phase transformation sequence has not been well established because it is dependent on the structure and composition of each specific clay and, also, dependent on other factors such as the impurities present [17].
Phase development in Al-treated montmorillonites
The Chah1030 and Chac1030 samples (Fig. 3a) were mainly composed of non-crystalline phase (XRD amorphous) coexisting with crystalline phases, such as spinel, Al–Mg silicate and cristobalite. At 1100 °C (Fig. 4a), abundant cristobalite and orthorhombic mullite appeared for Chah1100; scarce sapphirine and cordierite were also present. At this temperature, Chac1100 contained tetragonal mullite (i.e., high alumina mullite having a tetragonal structure, 2Al2O3·SiO2) with fewer amounts of cristobalite and spinel. Since the Alt content was almost the same, this result could be attributed to the presence of different OH–Al species intercalated in both precursors. Considering that chemical composition of mullite obtained by thermally treated gels has been related with local heterogeneities in composition [31], the tetragonal mullite formed in Chac1100 would indicate that a large heterogeneity in polymers arrangements occurred in this sample. At 1200 °C (Fig. 5a), the content of crystalline phases such as mullite, cordierite and cristobalite increased, and sapphirine began to develop. Quartz remained even at 1200 °C. Thus, development of mullite, cristobalite and cordierite began at a lower temperature for Chah than that of Chac due to different OH–Al polymers present in the two pillaring solutions studied. The amorphous phase, spinel and Al–Mg silicate gradually disappeared with thermal treatment at higher temperatures. The nature and relative content of minor components present in the heated Ch products also depended on the pillaring solutions used.
Precursors prepared from W heated at 1030 °C (i.e., Wah1030 and Wac1030 samples) also contained relevant amorphous phase, but formation of spinel and quartz in a higher proportion regarding to that of cristobalite was observed (Fig. 3b). For this montmorillonite, the first development of orthorhombic mullite occurred at temperatures as low as 1030 °C for both precursors obtained from H and C solutions. The intercalation of OH–Al polymers originated the formation of low amounts of Al–Mg silicate in Ch clay and promoted spinel phase in W sample after heating at 1030 °C. This indicated a reaction between the intercalated Al compounds and the montmorillonite structure.
Figures 3 and 4 show significant structural modifications from amorphous to more crystalline structure (well-defined XRD peaks) for all precursors treated at 1100 °C in comparison with those treated at 1030 °C. Wah1100 and Wac1100 (Fig. 4b) exhibited similar phase compositions (cristobalite, mullite and low amount of spinel and quartz), and mullite achieved a comparatively higher content with that of the pristine clay (W1100). Moreover, significant differences in phase composition between Al-treated Ch and Al-treated W clays existed (Fig. 4), where cordierite and sapphirine were present in Chah1100 but not detected in Wah1100, in which mullite and cristobalite predominated. This result is attributed to the high magnesium content in octahedral sheet in Ch respect to W montmorillonite (see structural formulae). On the other hand, Al–Mg silicate, a delay in cordierite growth, and formation of new phases such as sapphirine, spinel and mullite were observed in OH–Al-treated and heated product of Ch, respect to pristine Ch.
Figure 5 shows that, in all cases, Al species intercalated on clays favored the transformation to mullite at 1200 °C.
In the precursors obtained from the Ch clay (Fig. 5a), this increase in mullite content was accompanied with a slight increase in cordierite content. Nevertheless, the amount of cordierite developed at this temperature was smaller than in pristine Ch. Transformation to mullite from the W precursors was significantly enhanced with thermal treatment, at this temperature Cristobalite content also increased, and, in minor amounts, sapphirine and cordierite were formed (Fig. 5b).
Since mullite can crystallize with a variable chemical composition, the Al2O3 content of the mullite obtained from all precursors heated at 1200 °C was determined. The contents calculated were 62% for Chah1200 and 53% for Wah1200, and the values were 63 and 55% for Chac and Wac samples, respectively. This indicated the slight influence of different Al polymers intercalated on mullite composition. For W samples, the anomalous Al2O3 < 60% (present in nominal mullite 3Al2O3·2SiO2) suggested that octahedral Fe (as indicated in the W montmorillonite structural formula) formed a solid solution with mullite.
Conclusions
Thermal and structural transformations up to 1200 °C of two Aluminum-pillared montmorillonite precursors prepared from Ch and W montmorillonite types were analyzed. The influence of different OH–Al polymer solutions as Al sources on phase evolution with temperature of precursor samples was determined and compared with their corresponding natural samples.
Cordierite or mullite accompanied with cristobalite as major phases was developed at high temperature in pristine montmorillonites, depending on amount of Al or Mg in structural composition. Similarly, Al–Mg silicate or spinel types were intermediate phases that gradually disappeared at 1200 °C.
Intercalation of aluminum species in these montmorillonites caused an increase in amorphous content after heating at 1030 °C, respect to the non-treated montmorillonites. However, transformation to mullite from precursors began approximately at 1030 °C with a relevant increase in mullite content at 1200 °C. These results yield promising features for the potential use of these precursors as an alternative source for the obtention of ceramic materials with high mullite content.
The Ch precursor with Al-to-Mg molar ratio of 4.2 developed mullite with cordierite and sapphirine as minor compounds at 1200 °C. These phases were not present in the heated pristine Ch sample. Heated precursor derived from commercial pillaring solution first formed tetrahedral mullite (rich in alumina), but further heating at 1200 °C transformed it to an orthorhombic phase. For W precursor with Al-to-Mg molar ratio of 9.5, higher amounts of orthorhombic mullite with scarce cordierite were found in comparison with the pristine W montmorillonite.
Although the aluminum total contents of both W and Ch precursors were similar, the transformation to mullite was significantly enhanced in W precursor. This result indicated that the reaction to mullite was mainly affected by the composition of the octahedral layer of montmorillonite, being the role of the total aluminum content less relevant.
Phase composition at 1200 °C was independent on the type Al-pillaring solution used for each montmorillonite. However, the influence of intercalated OH–Al polymers on dehydration and dehydroxylation processes at low temperature was more relevant than compositional and structural effects.
References
Vaughan DEW, Lussier RJ. Preparation of molecular sieves based on pillared interlayered clays (PILC). In: Rees LVC, editor. Proceedings of 5th international conference on zeolites. London: Heyden Press; 1980. p. 94–101.
Vicente M, Bañares-Muñoz M, Gandía L, Gil A. On the structural changes of a saponite intercalated with various polycations upon thermal treatments. Appl Catal A. 2001;217(1):191–204.
Kloprogge JT. Synthesis of smectites and porous pillared clay catalysts: a review. J Porous Mater. 1998;5(1):5–41.
Gil A, Assis FCC, Albeniz S, Korili SA. Removal of dyes from wastewaters by adsorption on pillared clays. Chem Eng J. 2011;168(3):1032–40.
Gil A, Korili SA, Trujillano R, Vicente MA. A review on characterization of pillared clays by specific techniques. Appl Clay Sci. 2011;53(2):97–105.
Kloprogge JT, Evans R, Hickey L, Frost RL. Characterisation and Al-pillaring of smectites from Miles, Queensland (Australia). Appl Clay Sci. 2002;20(4):157–63.
Aouad A, Anastácio AS, Bergaya F, Stucki JWA. Mössbauer spectroscopic study of aluminum- and iron-pillared clay minerals. Clays Clay Miner. 2010;58(2):164–73.
Fripiat J. High resolution solid state NMR study of pillared clays. Catal Today. 1988;2(2):281–95.
Tomul F, Balci S. Characterization of Al, Cr-pillared clays and CO oxidation. Appl Clay Sci. 2009;43(1):13–20.
Elkhalifah AE, Bustam MA, Murugesan T. Thermal properties of different transition metal forms of montmorillonite intercalated with mono-, di-, and triethanolammonium compounds. J Therm Anal Calorim. 2013;112(2):929–35.
Volzone C, Garrido LB. High temperature structural modifications of intercalated montmorillonite clay mineral with OH–Al polymers. Proced Mater Sci. 2012;1:164–71.
Santana L, Gomes J, Neves G, Lira H, Menezes R, Segadães A. Mullite formation from bentonites containing kaolinite: effect of composition and synthesis parameters. Appl Clay Sci. 2014;87:28–33.
Önal M, Sarıkaya Y. Thermal behavior of a bentonite. J Therm Anal Calorim. 2007;90(1):167–72.
Bayram H, Önal M, Yılmaz H, Sarıkaya Y. Thermal analysis of a white calcium bentonite. J Therm Anal Calorim. 2010;101(3):873–9.
Zhang Y, Liu Q, Wu Z, Zhang Y. Thermal behavior analysis of two bentonite samples selected from China. J Therm Anal Calorim. 2015;121(3):1287–95.
McConville CJ, Lee WE. Microstructural development on firing illite and smectite clays compared with that in kaolinite. J Am Ceram Soc. 2005;88(8):2267–76.
Lee W, Souza G, McConville C, Tarvornpanich T, Iqbal Y. Mullite formation in clays and clay-derived vitreous ceramics. J Eur Ceram Soc. 2008;28(2):465–71.
Volzone C, Garrido L. Retention of OH–Al complexes by dioctahedral smectites. Clay Miner. 2001;36(1):115–23.
Hsu PH. Aluminum hydroxides and oxyhydroxides. In: Dixon JB, Weed SW, editors. Minerals in soil environments. 2nd ed. Madison: Soil Science Society of America; 1989.
Volzone C, Garrido LB. Retention of chromium by modified Al-bentonite. Cerâmica. 2002;48:153–6.
Ban T, Okada K. Structure refinement of mullite by the rietveld method and a new method for estimation of chemical composition. J Am Ceram Soc. 1992;75(1):227–30.
Bergaya F, Lagaly G, Vayer M. Chapter 12.10 cation and anion exchange. In: Faïza Bergaya BKGT, Gerhard L, editors. Developments in clay science. Amsterdam: Elsevier; 2006. p. 979–1001.
Hsu PH. Reaction of OH–Al polymers with smectites and vermiculites. Clays Clay Miner. 1992;40(3):300–5.
Aceman S, Lahav N, Yariv S. A thermo-XRD study of Al-pillared smectites differing in source of charge, obtained in dialyzed, non-dialyzed and washed systems. Appl Clay Sci. 2000;17(3–4):99–126.
Mackenzie RC, Caillere S. Data handbook for clay materials and other non-metallic minerals. Oxford: Pergamon Press; 1979.
Ma L, Zhou Q, Li T, Tao Q, Zhu J, Yuan P, et al. Investigation of structure and thermal stability of surfactant-modified Al-pillared montmorillonite. J Therm Anal Calorim. 2014;115(1):219–25.
Aceman S, Lahav N, Yariv S. XRD study of the dehydration and rehydration behaviour of Al-pillared smectites differing in source of charge. J Therm Anal. 1997;50(1):241–56.
Aceman S, Lahav N, Yariv S. A thermo-FTIR-spectroscopy analysis of Al-pillared smectites differing in source of charge, in KBr disks. Thermochim Acta. 1999;340–341:349–66.
Volzone C. OH–Cr(III) in dioctahedral and trioctahedral smectites: texture and structure changes. Mater Chem Phys. 1997;47(1):13–6.
Grim RE, Kulbicki G. Montmorillonite: high temperature reactions and classification. Am Mineral. 1961;46:1329–69.
Okada K, ŌTslka N. Change in chemical composition of mullite formed from 2SiO2·3Al2O3 xerogel during the formation process. J Am Ceram Soc. 1987; 70(10):245–247.
Acknowledgements
The authors thank to CONICET and MINCYT for financial supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martinez, J.M., Volzone, C. & Garrido, L.B. Thermal transformations up to 1200 °C of Al-pillared montmorillonite precursors prepared by different OH–Al polymers. J Therm Anal Calorim 128, 61–69 (2017). https://doi.org/10.1007/s10973-016-5938-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5938-0