Abstract
The process of the hydrothermal crystallization of montmorillonite Na2x(Al2(1 – x),Mg2x)-Si4O10(OH)2 · nH2O (0 < x ≤ 1) is studied in a weakly acidic medium with pH 4–4.5 at 220°С using HF as a mineralizer. The results of the study showed that montmorillonite samples in the acidic medium can be obtained both with the use of the HF mineralizer and without it. Under any conditions of carrying out the synthesis, there is an insignificant amount of corundum in the final product. It is established that a weakly acidic medium favors the formation of a dioctahedral structure of smectites with insignificant isomorphic substitutions (0.5 ≤ x ≤ 1). At a higher aluminum content, the acidic medium does not contribute to the formation of two-dimensional silicon-oxygen layers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Layered silicates with a montmorillonite (MMT) structure have great potential for the development of innovative materials such as medical materials (drug carriers, enterosorbents, materials for application sorption), catalyst carriers, matrices for stabilizing metallic nanoparticles and clusters, sorbents for purification of industrial and sewage water, and fillers of polymeric nanocomposites [1–6].
The directed hydrothermal synthesis makes it possible to prepare aluminosilicates of a given structure, morphology, and a certain phase and chemical composition. This makes it possible to clearly regulate the sorption, porous-textural, surface, and other characteristics of the materials being developed.
Earlier, the authors presented the results of studying the effect of the synthesis conditions on the hydrothermal crystallization process and the porous-textural characteristics of aluminum-magnesium MMT Na2x(Al2(1 – x),Mg2x)Si4O10(OH)2 · nH2O (0 < x ≤ 1) prepared by the hydrothermal treatment of dried gels of the appropriate formulations in an aqueous medium [7, 8]. The synthesis was carried out at temperatures from 200 to 350°С, autogenous pressure of 20 to 70 MPa, and the synthesis duration was 5–288 h. It was found that the temperature and duration of the synthesis have the greatest effect on the MMT’s crystallization in a neutral medium. The optimal temperature for obtaining single-phase well-crystallized MMT samples is 350°С. The particle size in the plane perpendicular to the с axis is 20 ± 3 nm and does not depend on the synthesis conditions and the chemical composition of the samples.
In the literature, there are several works describing the production of MMT in a weakly acidic medium [9, 10] using HF as a mineralizer. This method would have good prospects for the development in the case of its successful implementation, since according to the data of [10], it makes it possible to reduce the synthesis temperature to 220°C.
In this paper, we present the results of studying the feasibility of synthesizing aluminum-magnesium MMT using the procedure proposed in [10] with the addition of an HF mineralizer, as well as using a modified procedure without a mineralizer.
EXPERIMENTAL
Synthesis of MMT. The synthesis was carried out using the hydrothermal treatment of hydrogels prepared according to the formula of the final product having the following form: Na2x(Al2(1 – x),Mg2x)-Si4O10(OH)2 · nH2O. The depletion of the surface charge x varied from 0 to 1, changing the aluminum content of the original gel. The starting gels were prepared using deionized water, sodium acetate (NaCOOCH3, 99%, Fluka), magnesium acetate (Mg(COOCH3)2 · 4H2O, 99%, Fluka), alumina (Al2O3, 95%, NevaReaktiv), silica gel (SiO2, NevaReaktiv), and hydrofluoric acid (HF, 45%, NevaReaktiv). The required amounts of the starting reagents were mixed and the pH value of the supernatant was 4–4.5. The resulting hydrogels were subjected to the hydrothermal treatment in steel autoclaves with teflon crucibles at the temperature of 220°С for 24–120 h and the fill factor was 0.8. The crystallization products were washed with distilled water and dried at 80°С for 12 h.
Experimental methods. The X-ray phase analysis of the synthesized samples was carried out using a D8-Advance powder diffractometer (Bruker), Cu Kα-radiation, operation mode of the tube 40 kW/40 mA, a Vantec-1 position-sensitive counter, θ-θ geometry, the 2θ range: 5°–110° (step 2θ = 0.0224°), and the maximum peak intensity was ~283 000 pulses.
The chemical analysis of the samples for the Si, Mg, and Al content was carried out by the gravimetric method using quinolate of the silicon-molybdenum complex and by complexometric titration. The sodium content of the samples was determined by the flame photometry method using an iCE3000 atomic absorption spectrometer.
RESULTS AND DISCUSSION
The results of the study of the obtained samples showed that in a weakly acidic medium under hydrothermal conditions, MMT can be synthesized either with the use of the HF mineralizer or without it. The range of MMT compositions with the isomorphous substitution of magnesium for aluminum is somewhat narrower than for the previously described synthesis method in the neutral medium [7, 8], and it is limited by the region 0.5 ≤ x ≤ 1.
Table 1 shows the results of the chemical analysis of the samples and their designations. The designations are introduced in accordance with the amount of aluminum in the chemical formula of the sample by synthesis, i.e., a sample of the chemical composition Na1.8Al0.2Mg1.8Si4O10(OH)2 · H2O prepared in an acidic medium with the addition of a mineralizer, Al0.2-ac-HF, and without a mineralizer, Al0.2-ac.
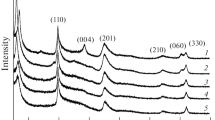
Figure 1 shows the X-ray diffraction patterns of MMT samples prepared at 220°С for 72 h with the addition of HF. All samples (with the exception of magnesium silicate compositions) contain small impurities of corundum according to the X-ray diffraction analysis (Figs. 1b, 1c).
According to the chemical analysis (Table 1), the impurity of corundum is present in magnesium-silicate samples of composition Al0.
Without the HF samples of MMT formulations for 0.5 ≤ x ≤ 1 can be prepared under the same conditions of synthesis (Fig. 2); however, insignificant impurities of corundum are contained in these and other samples.
The presence of the peak in the region 2θ = 60.8 (060) in the diffractograms indicates the dioctahedral structure of the samples and the presence of vacancies in the octahedral layers [8–11].
The analysis of the results of the chemical analysis shows that the samples contain significantly less Na2O than is necessary to compensate the positive charge of the octahedral layers of MMT, and what is contained in the MMT samples obtained from gels in the neutral medium [7], which should affect their cation-exchange properties.
The average particle size of the synthesized MMT determined from the X-ray diffraction data using the Scherrer formula is 20 ± 6 nm, the same as for samples obtained by the hydrothermal treatment of gels in the neutral medium.
The X-ray diffraction patterns of samples obtained in a weakly acid medium show a tendency opposite to the one observed for samples obtained during the hydrothermal treatment of gels in a neutral medium. Samples with a low aluminum content (0.5 ≤ x ≤ 1) are characterized by a high degree of crystallinity and ordering of the layers, which is expressed in narrow sharp peaks in the diffractogram and a high intensity of the reflection in the range of angles 2θ = 7°–8° (d001), characterizing the ordering of the distribution of the layers (packets) along the [001] direction. As the aluminum content increases, the intensity of the basal reflection is significantly reduced, while for the samples obtained in a neutral medium, reflection d001 on the diffractograms of samples with a low aluminum content was absent and manifested itself only in samples with a dioctahedral structure.
In [11], it was noted that it is impossible to obtain MMT in a weakly acidic medium without the use of a mineralizer. Our results disprove this conclusion for a number of compounds. At the same time, for compositions with a high aluminum content in the original gel (Al1.8-ac), it was not possible to obtain single-phase samples using this technique. In the case of using a mineralizer, MMT crystallizes (Fig.3) but contains significant impurities of magnesium fluoride and corundum. The appearance of the reflection (330) at 2θ = 62.3 instead of (060) at 2θ = 60.8 typical for other samples indicates the trioctahedral structure of the sample [8–11]. Without using the mineralizer, a sample of the Al1.8 composition does not crystallize under these conditions.
The increase in the duration of the synthesis from 24 to 96 h (Fig. 4) leads to the formation of a packet structure ordered along the [001] direction, which is manifested in the appearance of reflection d001. In this case, the particle size in the plane perpendicular to the c axis determined by the Scherrer formula remains unchanged and is 20 ± 3 nm. Apparently, the weakly acidic medium favors the formation of the dioctahedral structure of smectites with insignificant isomorphic substitutions (up to х = 0.5). At a higher aluminum content, the acidic medium does not contribute to the formation of two-dimensional silicon-oxygen layers. It is well known that brucite layers play a major role in the formation of the two-dimensional orientation of SiO4 tetrahedra and in the formation of clay minerals. It can be assumed that the magnesium content in the reaction medium for compositions with x = 0–0.5 suffices for the formation of brucite layers, which are not formed with the further increase in the aluminum content at slightly acidic pH values. As the aluminum content in the system increases, the sodium content decreases, which was introduced according to stoichiometry. The acidity of the reaction medium for compositions with the high aluminum content will be higher than for compositions with x < 0.5. The polycondensation of silicic acid in the acidic medium proceeds through the donor-acceptor mechanism to form a transition complex in which the Si atom temporarily acquires the coordination number of 6 (instead of 4) [12, 13]. All these factors do not allow the formation of the MMT crystal structure, which is consistent with the fact that in nature MMTs are formed during the weathering of alkaline rocks of volcanic origin, and the most important factor is the rock composition, namely, the content of magnesium and alkali in it [14, 15].
We should not forget the possible effect of the salts of acetic acid present in the reaction medium on the course of crystallization. Acetic acid is a weak single-basic carboxylic acid; thus, its presence in the reaction medium can be considered as synthesis using organic modifiers.
CONCLUSIONS
Under hydrothermal conditions, samples of magnesium montmorillonite Na2x(Al2(1 – x),Mg2x)-Si4O10(OH)2 · nH2O (0 < x ≤ 1) are obtained using precursors of the corresponding metals in the presence of the HF mineralizer at pH 4–4.5. The studies showed that, despite the synthesis temperature of MMT in the acidic medium decreasing from 350 to 220°С in comparison with synthesis methods in neutral and alkaline media, this method narrows the range of the crystallization of MMT compositions with the isomorphous substitution of magnesium for aluminum to the region 0.5 ≤ x ≤ 1. In addition, the results of the chemical analysis of the samples suggests a decrease in the cation exchange capacity of such samples and changes in other physicochemical characteristics due to the presence of acetic acid salts in the reaction medium, which requires further study.
REFERENCES
Dong, Y. and Feng, S.-S., Poly(d,l-lactide-co-glycolide)/montmorillonite nanoparticles for oral delivery of anticancer drugs, Biomaterials, 2005, vol. 26, no. 30, pp. 6068–6076.
Joshi, G.V., Patel, H.A., Kevadiya, B.D., and Bajaj, H.C., Montmorillonite intercalated with vitamin B1 as drug carrier, Appl. Clay Sci., 2009, vol. 45, no. 4, pp. 248–253.
Kollar, T., Palinko, I., Konya, Z., and Kiricsi, I., Intercalating amino acid guests into montmorillonite host, J. Mol. Struct., 2003, vols. 651–653, pp. 335–340.
Lee, Y.-H., Kuo, T.-F., Chen, B.-Y., Feng, Y.-K., Wen, Y.-R., Lin, W.-C., and Lin, F.H., Toxicity assessment of montmorillonite as a drug carrier for pharmaceutical applications: Yeast and rats model, Biomed. Eng.: Appl., Basis Commun., 2005, vol. 17, pp. 72–78.
Bukhanov, V.D., Vezentsev, A.I., Ponomareva, N.F., Kozubova, L.A., Korol’kova, S.V., Volovicheva, N.A., and Peristyi, V.A., Antibacterial properties of montmorillonite containing sorbents, Nauch. Vedom., Ser.: Estestv. Nauki, 2011, vol. 111, no. 17, pp. 57–63.
Alexandre, M. and Dubois, Ph., Polymer layered silicate nanocomposites: properties and uses of a new class of materials, Mater. Sci. Eng., 2000, vol. 28, pp. 1–63.
Golubeva, O.Yu., Effect of synthesis conditions on hydrothermal crystallization, textural characteristics and morphology of aluminum-magnesium montmorillonite, Microporous Mesoporous Mater., 2016, vol. 224, pp. 271–276.
Golubeva, O.Yu., Ul’yanova, N.Yu., Kostyreva, T.G., Drozdova, I.A., and Mokeev, M.V., Synthetic nanoclays with the structure of montmorillonite: preparation, structure, and physico-chemical properties, Glass Phys. Chem., 2013, vol. 39, no. 5, pp. 535–539.
Reinholdt, M., Miehe-Brendle, J., Delmotte, L., Tuolier, M.-H., Dred, R.-E., Cortes, R., and Flank, A.-M., Fluorine route synthesis of montmorillonite containing Mg or Zn and characterization by XRD, thermal analysis, MAS NMR, and EXAFS spectroscopy, Eur. J. Inorg. Chem., 2001, pp. 2831–2841.
Reinholdt, M., Miehe-Brendle, J., Delmotte, L., Le Dred, R., and Tuilier, M.H., Synthesis and characterization of montmorillonite-type phyllosilicates in a fluoride medium, Clay Miner., 2005, vol. 40, pp. 177–190.
Jaber, M. and Miehe-Brendle, J., Synthesis, characterization and applications of 2 : 1 phyllosilicates: contribution of fluoride to study the octahedral sheet, Microporous Mesoporous Mater., 2008, vol. 107, pp. 121–127.
Iler, R.K., Colloid Chemistry of Silica and Silicates, Ithaca: Cornell Univ. Press, 1955.
Iler, R.K., Chemistry of Silica. Solubility, Polymerization, Colloid and Surface Properties and Biochemistry of Silica, Chichester: Wiley, 1979.
Kukovskii, E.G., Osobennosti stroeniya i fiziko-khimicheskie svoistva glinistykh mineralov (Features of Structure and Physicochemical Properties of Clay Minerals), Kiev: Naukova Dumka, 1996.
Komarov, V.S., Adsorbtsionno-strukturnye, fiziko-khimicheskie i kataliticheskie svoistva glin Belorussii (Adsorption-Structural, Physicochemical and Catalytic Properties of Clays of Belarus), Minsk: Nauka Tekhnika, 1970.
ACKNOWLEDGMENTS
I am grateful to the employees of the analytical group of the Laboratory of Physical Chemistry of Glass, Grebenshchikov Institute of Silicate Chemistry, Russian Academy of Sciences, T.G. Kostyreva, L.N. Kurilenko, and L.A. Doronina, for the chemical analysis of the samples.
This work was supported by the Russian Foundation for Basic Research (project no. 18-03-00156).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Mosina
Rights and permissions
About this article
Cite this article
Golubeva, O.Y. Features of the Hydrothermal Synthesis of Montmorillonite in an Acidic Medium. Glass Phys Chem 44, 616–619 (2018). https://doi.org/10.1134/S1087659618060081
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1087659618060081