Abstract
For combining the properties of organoclays and pillared clays, inorganic–organic clays have attracted much attention in recent years. In this study, Al Keggin cation pillared montmorillonites (Al-Mts) were first prepared and parts of Al-Mts were calcined at different temperatures (C-Al-Mts). The inorganic–organic montmorillonites were synthesized by intercalating Al-Mts and C-Al-Mts with the cationic surfactant, hexadecyltrimethyl ammonium bromide (HDTMAB). The products were characterized by X-ray diffraction, X-ray fluorescence, and simultaneous thermogravimetric analysis. For HDTMAB-modified uncalcined Al Keggin cation pillared montmorillonites (H-Al-Mts), the basal spacing increased with the increment of surfactant loading level, but the Al content of H-Al-Mts decreased simultaneously, indicating that the intercalated surfactant replaced some Al Keggin cations in the interlayer space. However, in the case of C-Al-Mts, the interlayer spaces could not be further expanded after surfactant modification, implying that the neighboring montmorillonite layers were “locked” by the aluminum pillars which were formed by dehydroxylation of Al Keggin cation pillars during thermal treatment. The thermal stability of HDTMAB-modified C-Al-Mts (H-C-Al-Mts) was much better than that of H-Al-Mts. The major mass loss of H-C-Al-Mts occurred at ca. 410 °C, corresponding to decomposition of intercalated surfactant cations. In contrast, H-Al-Mts displayed two mass loss temperatures at ca. 270 and 410 °C, corresponding to the evaporation of surfactant molecules and the decomposition of surfactant cations in the interlayer space, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Montmorillonite which consists of an aluminum octahedral sheet sandwiched between two opposing siloxane tetrahedral sheets is one of the most common swelling clay minerals. The montmorillonite layers are usually negatively charged due to the substitution of Mg2+ or Fe2+ for Al3+ in octahedral sheets and substitution of Al3+ for Si4+ in tetrahedral sheets. This induces occupancy of the interlayer space by exchangeable cations. Thus, the physical and chemical properties of natural montmorillonites can be modified via cation exchange reaction, and products with different interlayer structure and surface properties can be obtained, e.g., pillared interlayered clays (PILCs) [1, 2] and organo-montmorillonites (OMts) [3, 4].
PILCs can be prepared by replacing the original inorganic cations in montmorillonite interlayer space with metal complex cations and then calcining the products under certain temperature and atmosphere. Because of their large specific surface area, high pore volume, micro-/meso-porosity, and strong surface acidity, PILCs have significant applications in diverse areas [5–9]. For example, they can be used as efficient adsorbents for oxyanionic contaminants [10–12] and heavy metals [13, 14], and as attractive cracking catalysts for heavy petroleum [1, 15, 16]. However, PILCs do not efficiently remove the hydrophobic organic compounds from water because of the hydrophilic surface. In contrast, OMts show excellent hydrophobicity and can be used as efficient adsorbents for organic pollutants [17–20] and appropriate fillers in synthesis of clay-based nanocomposites [21–23].
However, in the environment, wastewater may contain both organic and inorganic contaminants. So to reduce wastewater treatment cost, it is desirable to develop adsorbents that can simultaneously remove both organic and inorganic contaminants. Previous studies used both metal complex cations and organic cations as intercalators to modify the montmorillonites, and obtained inorganic–organic montmorillonites (IOMts) [24, 25]. The IOMts are of the adsorption sites/domains for both organic and inorganic contaminants, and used as an effective adsorbent for the both organic and inorganic pollutants from wastewater [11, 25–27]. In addition, IOMts have also been used as novel adsorbents for volatile organic compounds [28], and can remove organic compounds through an adsorption combined with catalytic degradation process [29].
Despite of the excellent properties and wide applications of IOMts, very limited attentions have been paid on their interlayer microstructure. Information of IOMt microstructure and thermal stability is of great importance not only for well understanding the interaction mechanism between the IOMts and pollutants but also for exploring novel industrial applications. On the other hand, previous studies have shown that the property of montmorillonite (e.g., CEC), configuration and used dosage of the intercalators, and pretreatment conditions (e.g., heating temperature) had significant influences on the microstructure of the resulting OMts and PILCs [4, 6, 30–32]. How these factors affect the interlayer structure and properties of IOMts is also very important for synthesis and application of this family of materials. Hence, in this study, a series of IOMts were prepared under different conditions using hydroxy-aluminum ([AlIVAl VI12 O4(OH)24(H2O)12]7+, Al Keggin cation) and hexadecyltrimethyl ammonium bromide (HDTMAB) as the inorganic and organic intercalator, respectively. The basal spacings, chemical compositions, and thermal stabilities of resulting products were investigated by X-ray diffraction (XRD), X-ray fluorescence (XRF), and thermogravimetric analysis (TG). The influences of thermal pretreatment of pillared clays and the used dosage of surfactant on the interlayer structure and thermal stability of the obtained IOMts were investigated. Meanwhile, the structure models of the IOMts in this study were speculated. These new insights are very important for synthesis and application of IOMts.
Experimental
Materials
Montmorillonite (Ca-Mt) used in this study was taken from Inner Mongolia China, with a purity >95 % and used without further purification. The chemical compositions of the montmorillonite determined by X-ray fluorescence (XRF) are SiO2 58.16 %, Al2O3 16.95 %, Fe2O3 5.26 %, CaO 2.29 %, MgO 3.57 %, K2O 0.15 %, Na2O 0.19 %, MnO 0.027 %, TiO2 0.2 %, P2O5 0.08 %, and the ignition loss is 13.23 %. The cation exchange capacity (CEC), measured by adsorption of [Co(NH3)6]3+ [33], is 110.5 meq/100 g. The surfactant, HDTMAB, was purchased from Nanjing Robiot Co. Ltd., China, with a purity of 99 %.
Synthesis of Al-pillared montmorillonite
To synthesize hydroxyl-aluminum solution containing Al Keggin cations, 0.5 M Na2CO3 solution was slowly added to 1.0 M AlCl3 solution with vigorous stirring under water bath at 60 °C, and kept the final ratio of OH−/Al3+ = 2.4. After that, stirring was kept for 12 h, and then the solution was aged for 24 h at 60 °C [24].
Montmorillonite was added into the hydroxyl-aluminum solution, and the Al/clay ratio was set to 10 mmol g−1. The mixture was stirred for 24 h and then aged for 24 h at 60 °C. The products were collected by centrifugation and washed eight times with distilled water, then were freeze dried for 48 h. The Al Keggin cation pillared montmorillonite was denoted as Al-Mt. Its caclined derivatives (C-Al-Mts) were prepared by heating Al-Mt samples in an oven for 8 h at 300, 400, and 500 °C and the obtained products were denoted as C300-Al-Mt, C400-Al-Mt, and C500-Al-Mt, respectively.
Preparation of inorganic–organic montmorillonites (IOMts)
IOMts were obtained by the following method: a desired amount of surfactant was dispersed in distilled water by stirring at 60 °C for 0.5 h, and then 10 g Al-Mt or C-Al-Mts were added to the surfactant solution. The added amount of surfactants was equivalent to 1.0 or 2.0 times of the montmorillonite’s CEC. The mass ratio of water/clay was 20:1. The mixture was stirred for 12 h at 60 °C, and then the products were washed eight times with distilled water and freeze dried for 48 h. The product prepared using Al-Mt and 1.0 CEC of surfactant was marked as H1.0-Al-Mt and the others were marked in the same way.
Characterization methods
Powder XRD patterns were collected between 1° and 20° (2θ) at a scanning speed of 1° (2θ) min−1 on a Bruker D8 Advance diffractometer with Ni-filtered Cu Kα radiation (λ = 0.154 nm, 40 kV, and 40 mA).
Elemental analysis was conducted on a Rigaku RIX 2000 X-ray fluorescence spectrometer (XRF). Calibration lines used in quantification were produced by bivariate regression of data from 36 reference materials encompassing a wide range of silicate compositions, and analytical uncertainties were mostly between 1 and 5 %.
TG was performed on a Netzsch STA 409PC instrument. The samples were heated at a rate of 10 °C min−1 under a flow of high pure nitrogen (60 mL min−1) from 30 to 1,000 °C. The differential thermogravimetric curve was derived from the TG curve automatically. The amount of loaded surfactant on the modified montmorillonites was determined from TG curves.
Results and discussion
Microstructure of IOMts
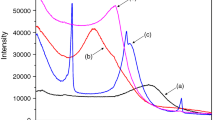
The XRD patterns of the Al-pillared montmorillonites and their surfactant-modified counterparts are shown in Fig. 1. The basal spacing (d 001) of Al-Mt is 1.88 nm (Fig. 1a) and the gallery height of Al-Mt (1.88 − 0.96 = 0.92 nm) consists with the size of Al Keggin cation (0.9 nm), indicating a successful intercalation of Al Keggin cation [9]. After heating Al-Mt at 300, 400, and 500 °C, the values of d 001 decrease to 1.82, 1.80, and 1.77 nm, respectively. The XRD patterns show that the C-Al-Mts retain ordered layered structure. The slight decrease of basal spacing is attributed to the dehydroxylation of intercalated Al Keggin cations, and the difference of basal spacing values of the three C-Al-Mts indicates different extent of dehydroxylation. [34].
The basal spacings of IOMts are different from those of Al-Mt and C-Al-Mts (Fig. 1b, c). The basal spacing of H1.0-Al-Mt increases to 2.17 nm. The interlayer galleries are further expanded when increasing the amount of added surfactant, and the basal spacing reaches up to 3.78 nm when the amount of added surfactant increases to 2.0 CEC (Fig. 1c). The basal spacings of H1.0-Al-Mt and H2.0-Al-Mt are similar to those of the corresponding OMts [4]. However, the basal spacing values of HDTMAB-modified C-Al-Mts (H-C-Al-Mts) remain relatively constant (ca. 1.71 nm) in the whole surfactant addition range. This suggests that the Al pillars have “locked” the neighboring layers of montmorillonites after calcination and the interlayer space is unable to be further expanded by HDTMAB.
Table 1 presents the chemical analysis results and the surfactant content of Al-Mt and IOMts. The Al/Si ratio of Al-Mt is 0.49, larger than that of Ca-Mt (0.29), indicating the successful intercalation of Al Keggin cations into the interlayer space. This is in agreement with the XRD results. For H-Al-Mts, after surfactant modification, HDTMAB content increases from 26.2 % for H1.0-Al-Mt to 40.5 % for H2.0-Al-Mt, but Al/Si ratio decreases from 0.49 for Al-Mt to 0.32 for H2.0-Al-Mt. This suggests that some pre-intercalated Al Keggin cations have been replaced by the intercalated HDTMAB since the Al atoms in montmorillonite structure cannot be moved away. In the other words, when Al Keggin cations are intercalated into interlayer spaces of montmorillonites, they link with the adjacent montmorillonite layers via weak electrostatic force [24, 35]. But when HDTMAB are intercalated into interlamellar spaces, some Al Keggin cations are driven away and replaced by HDTMAB.
Al/Si ratios in H-C-Al-Mts are almost unchanged after surfactant modification and slightly smaller than that of Al-Mt. This indicates that the most intercalated Al Keggin cations have transformed into Al pillars via dehydroxylation during thermal treatment [36]. Under calcination at 500 °C, the structure of the supporting pillars were approximated by blocks of 13 Al atoms with a size of 0.98 nm × 1.09 nm × 0.84 nm [30]. In the C-Al-Mts, the pillars are linked with the siloxane surface of montmorillonites by Si–O–Alpillar bonds [30, 37]. These observations are in agreement with above-mentioned XRD results.
Thermal analysis
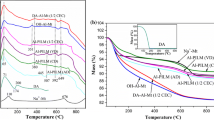
Figure 2 gives the TG-DTG curves of all samples. Two major mass losses are clearly resolved in the TG curve of Ca-Mt: (1) the removal of water molecules from the surface and interlayer space in the temperature range of 30–200 °C, and (2) the dehydroxylation at 650–700 °C [38]. Similar to Ca-Mt, both Al-Mt and C-Al-Mts are of major mass losses at ca. 105 °C which are attributed to the evaporation of adsorbed water, and the mass losses in the temperature range of 300–700 °C are attributed to dehydroxylation of montmorillonite and intercalated Al Keggin cations [9]. Mass losses of C-Al-Mts in this temperature range decrease with the increase of thermal treatment temperature. After calcination at 500 °C, mass loss of C500-Al-Mt appearing at ca. 620 °C is close to the dehydroxylation temperature of Ca-Mt, indicating that the dehydroxylation of Al Keggin cations is almost completed.
Compared with Al-Mt and C-Al-Mts, the TG-DTG curves of IOMts show a prominent decrease of the mass loss corresponding to the absorbed water. This should be attributed to the surface affinity change of the clay minerals (from hydrophilicity to hydrophobicity) [38]. The mass losses of H1.0-Al-Mt and H2.0-Al-Mt in the range of 200–500 °C are 27.98 and 41.13 %, respectively, much more than that of Al-Mt in this temperature range. Two prominent mass losses for H-Al-Mts were recorded at ca. 270 and 412 °C. According to previous studies of OMts, mass loss at relatively low temperature (ca. 270 °C) corresponds to evaporation of the surfactant molecules (ionic pairs) located within the interlayer spaces and interparticle pores, while that at high temperature (ca. 412 °C) corresponds to decomposition of the intercalated surfactant cations [38, 39]. Therefore, the mass loss of H-Al-Mts at ca. 412 °C corresponds to decomposition of the intercalated surfactant cations and dehydroxylation of Al Keggin cations, and the mass loss at ca. 270 °C mainly corresponds to evaporation of surfactant molecules (ionic pairs) in the interlayer spaces and increases with the increment of surfactant loading level.
However, as shown in the TG-DTG curves of H-C-Al-Mts (Fig. 2b, d), only one prominent mass loss occurred at ca. 415 °C in the temperature range of 200–500 °C, corresponding to decomposition of surfactant cations and dehydroxylation of Al pillars in the interlayer spaces. This suggests that surfactants in the interlayer space mainly exist as cations but not molecules. Meanwhile, mass losses of H-C-Al-Mts decrease as the calcining temperatures increase, and the amount of added surfactant only has little influence on the mass loss. C-Al-Mts have a permanent porosity in the interlayer space after heating [36], and intercalated surfactants in H-C-Al-Mts occupy the interlayer pore. Hence, no more surfactant molecules will be adsorbed because of the limited space. The thermal stability of H-C-Al-Mts is dramatically improved when compared with H-Al-Mts.
The microstructure model
According to the results of XRD, XRF, and TG analysis, the microstructure model of the IOMts is proposed and shown in Fig. 3. After the intercalation of Al Keggin cations into the montmorillonite galleries, the Al Keggin cations link with the negatively charged layers through electrostatic attraction. In the surfactant modification process, surfactant cations replace some Al Keggin cations in the interlayer space. Then, more and more surfactants are intercalated into the galleries via Van der Waals force (physical adsorption) between surfactant molecules, and the interlayer spaces are gradually occupied by HDTMAB. However, the thermal stability decreases due to the physically adsorbed surfactant molecules (ion pairs) [39]. Hence, the microstructure of H-Al-Mt may be similar to that of OMts, i.e., the intercalated surfactants adopt an arrangement model of paraffin-type layer when the amount of added surfactant reaches 2.0 CEC [4]. However, since the intercalated Al Keggin cations co-exist with surfactants in the interlayer space, the microstructure of H-Al-Mt is much more complicated than OMts.
On the other hand, chemical bonds between Al pillars and montmorillonite platelet can be formed via thermal treatment. Hence, the neighboring layers are “locked” by Al pillars and permanent pores are formed in the interlayer spaces after calcination. HDTMAB can still be intercalated into the “locked” galleries and most of them exist as cations. As previous studies showed [38, 39], the intercalated surfactant cations displayed better thermal stability than surfactant molecules. This can help us to well understand why H-C-Al-Mts show relatively high thermal stability. These materials with higher thermal stability can be used in a variety of fields, such as recyclable adsorbent of volatile organic compounds which demands high thermal stability for thermal desorption.
Conclusions
In this study, series of IOMts were synthesized using Al Keggin cations and surfactant (HDTMAB). In the case of H-Al-Mts, surfactant loading causes a further expansion of interlayer spaces and desorption of pre-intercalated Al Keggin cations. The intercalated surfactants within the interlayer space of H-Al-Mts exist as both cations and molecules. However, for H-C-Al-Mts, the interlayer spaces are “locked” by Al pillars formed during calcination. Thus the galleries cannot be further expanded by surfactant intercalation, and only surfactant cations are intercalated into the interlayer pores. The amount of loaded surfactant on H-C-Al-Mts is mainly affected by thermal treatment temperature. The interlayer microstructure is the key factor resulting in different thermal stability between H-Al-Mts and H-C-Al-Mts. These new findings are of high importance for synthesis and application of IOMts.
References
Pinnavaia TJ. Intercalated clay catalysts. Science. 1983;220:365–71.
Lahav N, Shani U, Shabtai J. Cross-linked smectites. 1. Synthesis and properties of hydroxy-aluminum-montmorillonite. Clays Clay Miner. 1978;26:107–15.
Lagaly G. Characterization of clays by organic-compounds. Clay Miner. 1981;16:1–21.
He HP, Ma YH, Zhu JX, Yuan P, Qing YH. Organoclays prepared from montmorillonites with different cation exchange capacity and surfactant configuration. Appl Clay Sci. 2010;48:67–72.
Kloprogge JT. Synthesis of smectites and porous pillared clay catalysts: a review. J Porous Mater. 1998;5:5–41.
Occelli ML, Bertrand JA, Gould SAC, Dominguez JM. Physicochemical characterization of a Texas montmorillonite pillared with polyoxocations of aluminum. Part I: the microporous structure. Microporous Mesoporous Mater. 2000;34:195–206.
Ding Z, Kloprogge JT, Frost RL, Lu GQ, Zhu HY. Porous clays and pillared clays-based catalysts. Part 2: a review of the catalytic and molecular sieve applications. J Porous Mater. 2001;8:273–93.
Yuan P, He HP, Bergaya F, Wu DQ, Zhou Q, Zhu JX. Synthesis and characterization of delaminated iron-pillared clay with meso-microporous structure. Microporous Mesoporous Mater. 2006;88:8–15.
Qin ZH, Yuan P, Zhu JX, He HP, Liu D, Yang SQ. Influences of thermal pretreatment temperature and solvent on the organosilane modification of Al13-intercalated/Al-pillared montmorillonite. Appl Clay Sci. 2010;50:546–53.
Kasama T, Watanabe Y, Yamada H, Murakami T. Sorption of phosphates on Al-pillared smectites and mica at acidic to neutral pH. Appl Clay Sci. 2004;25:167–77.
Zhu RL, Zhu LZ, Zhu JX, Ge F, Wang T. Sorption of naphthalene and phosphate to the CTMAB-Al13 intercalated bentonites. J Hazard Mater. 2009;168:1590–4.
Lenoble V, Bouras O, Deluchat V, Serpaud B, Bollinger JC. Arsenic adsorption onto pillared clays and iron oxides. J Colloid Interface Sci. 2002;255:52–8.
Bhattacharyya KG, Gupta SS. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Colloid Interface Sci. 2008;140:114–31.
Karamanis D, Assimakopoulos PA. Efficiency of aluminum-pillared montmorillonite on the removal of cesium and copper from aqueous solutions. Water Res. 2007;41:1897–906.
Gyftopoulou ME, Millan M, Bridgwater AV, Dugwell D, Kandiyoti R, Hriljac JA. Pillared clays as catalysts for hydrocracking of heavy liquid fuels. Appl Catal A Gen. 2005;282:205–14.
Martinez-Ortiz MJ, Fetter G, Dominguez JM, Melo-Banda JA, Ramos-Gomez R. Catalytic hydrotreating of heavy vacuum gas oil on Al- and Ti-pillared clays prepared by conventional and microwave irradiation methods. Microporous Mesoporous Mater. 2003;58:73–80.
Zhou Q, Frost RL, He HP, Xi YF, Zbik M. TEM, XRD, and thermal stability of adsorbed paranitrophenol on DDOAB organoclay. J Colloid Interface Sci. 2007;311:24–37.
Zhu LZ, Chen BL. Sorption behavior of p-nitrophenol on the interface between anion-cation organobentonite and water. Environ Sci Technol. 2000;34:2997–3002.
Yilmaz N, Yapar S. Adsorption properties of tetradecyl- and hexadecyl trimethylammonium bentonites. Appl Clay Sci. 2004;27:223–8.
Park Y, Frost RL, Ayoko GA, Morgan DL. Adsorption of p-nitrophenol on organoclays. J Therm Anal Calorim. 2013;111:41–7.
Okamoto K, Ray SS, Okamoto M. New poly(butylene succinate)/layered silicate nanocomposites. II. Effect of organically modified layered silicates on structure, properties, melt rheology, and biodegradability. J Polym Sci B Polym Phys. 2003;41:3160–72.
Okada A, Usuki A. Twenty years of polymer-clay nanocomposites. Macromol Mater Eng. 2006;291:1449–76.
Gao Z, Xie W, Hwu JM, Wells L, Pan WP. The characterization of organic modified montmorillonite and its filled PMMA nanocomposite. J Therm Anal Calorim. 2001;64:467–75.
Zhu RL, Wang T, Ge F, Chen WX, You ZM. Intercalation of both CTMAB and Al13 into montmorillonite. J Colloid Interface Sci. 2009;335:77–83.
Ouellet-Plamondon C, Lynch RJ, Al-Tabbaa A. Comparison between granular pillared, organo- and inorgano–organo-bentonites for hydrocarbon and metal ion adsorption. Appl Clay Sci. 2012;67–68:91–8.
Zhu LZ, Zhu RL. Simultaneous sorption of organic compounds and phosphate to inorganic–organic bentonites from water. Sep Purif Technol. 2007;54:71–6.
Li SZ, Wu PX. Characterization of sodium dodecyl sulfate modified iron pillared montmorillonite and its application for the removal of aqueous Cu(II) and Co(II). J Hazard Mater. 2010;173:62–70.
Zhu LZ, Tian SL, Zhu JX, Shi Y. Silylated pillared clay (SPILC): a novel bentonite-based inorgano–organo composite sorbent synthesized by integration of pillaring and silylation. J Colloid Interface Sci. 2007;315:191–9.
An TC, Chen JX, Li GY, Ding XJ, Sheng GY, Fu JM, et al. Characterization and the photocatalytic activity of TiO2 immobilized hydrophobic montmorillonite photocatalysts degradation of decabromodiphenyl ether (BDE 209). Catal Today. 2008;139:69–76.
Occelli ML, Auroux A, Ray GJ. Physicochemical characterization of a Texas montmorillonite pillared with polyoxocations of aluminum. II. NMR and microcalorimetry results. Microporous Mesoporous Mater. 2000;39:43–56.
Thomas SM, Occelli ML. Effects of synthesis conditions on the thermal stability of a Texas montmorillonite expanded with [Al13O4(OH)24(H2O)12]7+ cations. Clays Clay Miner. 2000;48:304–8.
Khalaf H, Bouras O, Perrichon V. Synthesis and characterization of Al-pillared and cationic surfactant modified Al-pillared Algerian bentonite. Microporous Mater. 1997;8:141–50.
Zhu LZ, Zhu RL, Xu LH, Ruan XX. Influence of clay charge densities and surfactant loading amount on the microstructure of CTMA-montmorillonite hybrids. Colloids Surf A. 2007;304:41–8.
Kloprogge JT, Geus JW, Jansen JBH, Seykens D. Thermal-stability of basic aluminum sulfate. Thermochim Acta. 1992;209:265–76.
Pusch R, Yong RN. Microstructure of smectite clays and engineering performance. 1st ed. London: Taylor & Francis; 2006.
Bergaya F, Aouad A, Mandalia T. Pillared clays and clay minerals. In: Bergaya F, Theng BKG, Lagaly G, editors. Handbook of clay science. Amsterdam: Elsevier Science; 2006. p. 393–421.
Acemana S, Lahav N, Yariv S. A thermo-FTIR-spectroscopy analysis of Al-pillared smectites differing in source of charge, in KBr disks. Thermochim Acta. 1999;340–341:349–66.
Zhu JX, Shen W, Ma YH, Ma LY, Zhou Q, Yuan P, et al. The influence of alkyl chain length on surfactant distribution within organo-montmorillonites and their thermal stability. J Therm Anal Calorim. 2012;109:301–9.
He HP, Ding Z, Zhu JX, Yuan P, Xi YF, Yang D, et al. Thermal characterization of surfactant-modified montmorillonites. Clays Clay Miner. 2005;53:287–93.
Acknowledgements
We gratefully acknowledge financial support from the Knowledge Innovation Program of the Chinese Academy of Sciences (Grant No. KZCX2-EW-QN101), the “Strategic Priority Research Program” of the Chinese Academy of Sciences (Grant No. XDB05050200), and the National Natural Science Foundation of China (Grant No. U0933003, 41272060). This is contribution No. IS-1657 from GIGCAS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, L., Zhou, Q., Li, T. et al. Investigation of structure and thermal stability of surfactant-modified Al-pillared montmorillonite. J Therm Anal Calorim 115, 219–225 (2014). https://doi.org/10.1007/s10973-013-3190-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3190-4