Abstract
In this study, a novel spirocyclic pentaerythritol bisphosphorate disphosphoryl-di-prop-2-en-1-amine (SPSA) compound containing a functional double bond (C=C) was synthesized and characterized by Fourier transform infrared spectroscopy and 1H nuclear magnetic resonance spectroscopy (1H NMR). In this paper, a new intumescent flame retardant (IFR) was designed by combining ammonium polyphosphate (APP) and SPSA (mass ratio of APP and SPSA was 2:1) and this IFR was mixed with polypropylene (PP) via twin-screw extruder to prepare a series of flame retardant PP composites. The thermal stability and fire behaviors of these flame retardant PP composites were investigated by thermogravimetric analysis, limiting oxygen index (LOI), vertical burning test (UL-94) and cone calorimeter test. Furthermore, the morphology of the char residues of flame retardant PP composites after cone calorimeter test was studied by scanning electron microscopy with energy dispersive X-ray analysis (EDS). Aiming to further improve the thermal stability of the composites, electron beam irradiation has been used to treat these composites. The results showed that LOI value of flame retardant PP composite containing 30 % IFR was 24.0 and passed UL 94 V-0. In the cone calorimeter test, both peak heat release rate and total heat release results of PP/IFR system were lower than those of PP/APP system. In addition, this IFR provided relatively good quality of char residue in the combustion. Moreover, electron beam treatment of the composites improved the thermal stability of these designed flame retardant PP composites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polypropylene (PP) has excellent mechanical and physicochemical properties. Thus, it is used in a wide range of applications, but its inherent combustibility limits its broader application [1–3]. Thus, the flame retardancy of PP has to be improved. The use of intumescent flame retardants (IFR) is established in polymeric materials since it is regarded as a safe and friendly flame retardant method [4]. IFR is generally made of three constituents: acid source (e.g., ammonium polyphosphate, etc.), carbon source (e.g., pentaerythritol, sorbitol, etc.) and foaming agent (e.g., melamine, etc.).
Many researches have shown that synergistic agents can effectively increase the strength and stability of char layer as well as promote catalyzing the reactions among IFR components in IFR-PP systems. Wang et al. [5] proposed to use the catalytic action of phosphotungstic acid in the synthesis of melamine salts of pentaerythritol phosphate in order to solve the problems of conventional preparation methods. Nie et al. [6] suggested the synthesis of a char-forming agent and microencapsulated ammonium polyphosphate was incorporated in PP via an intumescence mechanism. Wang reported [7, 8] the synthesis of a novel char former for intumescent system based on a charring agent and ammonium polyphosphate. Bourbigot et al. [9] investigated charring polyamide-6 clay nanocomposite hybrid (PA-6-nano) as a carbonization agent. Some phosphorus and nitrogen-containing intumescent agents were synthesized, such as spiro and caged bicyclic phosphate (SBCPO) [10], spirocyclic pentaerythritol bisphosphorate disphosphoryl melamine (SPDPM) [11], poly(4,4-diaminodiphenyl methane spirocyclic pentaerythritol bisphosphonate) (PDSPB) [12] and poly(2-hydroxy propylene spirocyclic pentaerythritol bisphosphonate) (PPPBP) [13]. Recently, Wang et al. [14, 15] found that P–O–C and P–N–C structures could greatly improve the residues layer during combustion. Since traditional IFR is non-reactive type compounds to polymers, it may result in the low thermal stability of flame retardant polymer due to the initial decomposition of the components in IFR.
It is well known that electron beam (EB) technology is an environment-friendly method [16–18]. It can be used to chemically modify polymer [19]. Its main advantage is the spatial and temporal precise generation of free radicals in accordance with the requirement of the chemical reaction without any use of chemicals as well as without any dependence on the temperature and the state of aggregation [20]. Formed the cross-linked structure may be used to improve the thermal stability of polymers.
In this work, a novel halogen-free intumescent flame retardant containing a functional double bond (C=C) that may form cross-linking structure with PP in the electron beam treatment, spirocyclic pentaerythritol bisphosphorate disphosphoryl-di-prop-2-en-1-amine (SPSA), was developed and mixed in the PP system via melt compounding. The thermal stability and fire behavior of these flame retardant PP composites were investigated by thermogravimetric analysis (TG), limiting oxygen index (LOI), vertical burning test (UL-94) and cone calorimeter test. Furthermore, the morphology of the char residues of flame retardant PP composites after cone calorimeter test was studied by scanning electron microscopy (SEM) with energy dispersive X-ray analysis (EDS). In the end, the thermal stability of non-irradiated and electron beam irradiated flame retardant PP composites was investigated.
Experimental
Materials
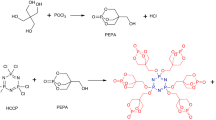
Pentaerythritol, phosphoryl chloride, triethylamine, prop-2-en-1-amine, trichloromethane, sodium hydroxide and tetrahydrofuran were supplied by Sigma-Aldrich Chemical Reagent Co., Ltd. and used without further purification. Polypropylene (PP, homopolymer, HD 120MO) was supplied by Borealis, Porvoo, Finland. Ammonium polyphosphate (APP) was supplied by Budenheim Company. Spirocyclic pentaerythritol bisphosphorate disphosphoryl-di-prop-2-en-1-amine (SPSA) was prepared from pentaerythritol and phosphoryl chloride, leading to spirocyclic pentaerythritol bisphosphorate disphosphoryl chloride, which was then reacted with prop-2-en-1-amine, as shown in Fig. 1.
Synthesis of spirocyclic pentaerythritol bisphosphorate disphosphoryl chloride [21]
In a 500-mL glass flask equipped with a magnetic stirrer, a thermometer, a heating bath and circumference condenser, pentaerythritol (68 g, 0.5 mol) and phosphoryl chloride (618 g, 4.0 mol) were mixed. The mixture was stirred about 100 °C until HCl evolution subsided. Then, the mixture was gradually heated and refluxed until no HCl gas was emitted. The raw product obtained was filtered and purified using a filter with chloroform sequentially. The product was dried to constant mass at 55 °C in vacuum. Thus, spirocyclic pentaerythritol bisphosphorate disphosphoryl chloride, a white solid powder, was obtained (108 g, yield: 73 %).
Characterization: 1H NMR (500 MHz, d 6 -DMSO) (ppm): 4.23–4.20 (d, -CCH2O-PO-, 8H). FTIR (cm−1): 1305 (vs, P=O), 1025 (vs, P–O–C).
Synthesis of new phosphorus-containing flame retardant (SPSA)
A mixture of prop-2-en-1-amine (28.5 g, 0.5 mol) and triethylamine (70 mL, 0.5 mol) was added dropwise to a stirred solution of spirocyclic pentaerythritol bisphosphorate disphosphoryl chloride (60 g, 0.2 mol) in anhydrous tetrahydrofuran (100 mL) under nitrogen gas at 0–5 °C. The resulting mixture was allowed to warm to room temperature and left stirring for 12 h. Then, the mixture was filtered off. Finally, the filtrate was concentrated at reduced pressure. Thus, the product SPSA (57 g, yield: 85 %) was distilled under reduced pressure to provide the desired compounds. The reactions are shown in Fig. 1.
Preparation of flame retardant PP composites
APP and SPSA were dried in a vacuum oven at 80 °C for 12 h. Then, PP and IFR (mass ratio of APP and SPSA at 2:1) were compounded by a twin-screw extruder (KETSE 20/40 EC, Brabender) (compositions see Table 1) with a rotational speed of 150 rpm at a fixed temperature protocol for the feed zone to the die: 175, 180, 190, 185, 180 and 170 °C. Finally, the specimens were applied for LOI, UL-94, and cone calorimeter test was carried out by injection molding (Arburg 320 C) at a processing temperature around 200 °C.
Electron beam irradiation
Flame retardant PP composites were irradiated at room temperature in nitrogen atmosphere with an average dose 32 kGy using an ELV-2 electron accelerator (BINP, Novosibirsk, Russia). The electron energy was 1.5 MeV, and the electron current amounted to 4 mA.
Experimental devices description
The Fourier transform infrared (FTIR) spectrum of SPSA sample was recorded using the Bruker Vertex 80 V spectrometer in the wave number range of 600–4000 cm−1.
1H nuclear magnetic resonance (1H NMR) was obtained on a Bruker spectrometer using CDCl3 as a solvent.
The thermogravimetric analysis (TG) was carried out using a TA Instruments TGA Q 5000 in the range between room temperature and 700 °C. The experiments were done in nitrogen atmosphere at heating rate of 10 K min−1, and the flow rate of the nitrogen was 60 mL min−1.
The UL-94 test was performed using a vertical burning instrument (Fire Testing Technology, UK), and the specimens in this work for testing were of dimensions 130 mm × 13 mm × 3.2 mm.
Limiting oxygen index (LOI) was measured on sheets (120 mm × 6.5 mm × 3.2 mm) according to the standard DIN EN ISO 4589-1 and 4589-2. It describes the minimum concentration of oxygen in nitrogen that supports the combustion of a polymer. The unit of concentration is percentage.
The cone calorimeter tests (CC) were carried out on a cone calorimeter (FTT, UK). The squared specimens (100 mm × 100 mm × 4 mm) were wrapped with aluminum foil and placed in a frame without grid. The specimens were irradiated at a heat flux of 50 kW m−2. Each sample was tested three times.
Scanning electron microscopy (SEM) (microscope model: Ultra Plus, Carl Zeiss SMT) with energy dispersive X-ray analysis (EDS) was used to study morphological features of the powdered samples. The samples were placed on a sample holder using conducting copper tape and then coated with a thin layer of platinum (layer thickness 3 nm) using a sputter coater (BAL-TEC SCD 500 sputter coater).
Results and discussion
Structure characterization of SPSA
FTIR spectroscopy
In order to identify the structure of SPSA, firstly, FTIR spectroscopy was used to characterize SPSA, as shown in Fig. 2. The characteristic absorption peak at 3311 cm−1 was attributed to the N–H bond segments in structure of SPSA. Also, the characteristic absorption peaks for carbon–carbon double bonds (1635 cm−1) were clearly observed. The peak at 987 cm−1 was assigned to P–O–C bond on the structure of SPSA segments.
1H NMR spectroscopy
Further structural conformation for SPSA was made by means of 1H NMR spectroscopy. As shown in Fig. 3, the peak groups at 5.80–5.11 ppm could be assigned to the protons on the carbon–carbon double bond on the structure of SPSA segments (corresponding to a, b in Fig. 3). The peak groups at 4.51–3.00 ppm could be assigned to the protons on methylene groups of the structure of SPSA segments (corresponding to d, e in Fig. 3).
Fire behaviors
In the following, polypropylene (PP)/intumescent flame retardant (IFR) composites have been prepared in different compositions as given in Table 2 and the composites have been compared with pure PP in the fire behavior. Ammonium polyphosphate (APP) alone and together with SPSA in a 2:1 ratio were used as IFR.
LOI and UL94
Aiming to evaluate flammability of PP and flame retardant PP composites, usually, LOI and UL-94 tests are used [22–24]. In this study, both LOI and UL94 were used to investigate the flammability of the composites and the results are given in Table 2. Obviously, LOI value of virgin PP was only 17.6 %, showing high flammability. At a load of 30 mass% of APP, the LOI value of PP/APP composite increased to 20.4 %, but there was no rating in the UL 94 test. It meant that APP alone was not an efficient flame retardant to PP. In comparison, the LOI values of PP/IFR composites containing 20, 25 and 30 mass% IFR were 21.0, 22.5 and 24.0, respectively. More importantly, PP + 30 % IFR passed UL 94 V-0, indicating that this IFR was more efficient than APP alone.
Cone calorimeter test
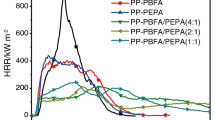
While the LOI and UL 94 tests are widely used to evaluate the flame retardation of materials, especially for screening flame retarded formulations of polymers, the cone calorimeter provides a wealth of information on the combustion behavior [25]. Some cone calorimeter results correlate well with those obtained from large-scale fire tests and can be used to predict the behavior of materials in real fires [26]. The heat release rate (HRR) has come to be recognized as one of the most important fire properties of a material [27], and it is a measure of the heat release per unit of surface area of burning materials. Figure 4 shows the HRR curves of the pure PP and flame retardant PP composites. It was noted that the pure PP resin burned very fast after ignition and a sharp HRR peak appears with a peak PHRR of 842 kW m−2. In the case of PP with 30 mass% of APP, the PHRR decreased to 489 kW m−2. However, the PHRR of PP/IFR containing 20, 25, and 30 mass% of IFR was 473, 349 and 430 kW m−2, respectively. It confirmed that this IFR was a more efficient flame retardant to PP compared to APP.
The total heat release (THR) is another important parameter for fire hazard evaluation. THR value of pure PP, PP/APP and PP/IFR composites are shown in Fig. 5. In comparison with pure PP, all the flame retardant PP composites (PP1, PP2, PP3 and PP4, see Table 1) showed lower THR within the first 350 s. Consequently, these flame retardant PP composites showed a lower heat release during the combustion compared to that of pure PP. Further, the difference in the THR between PP3 and PP4 indicates that SPSA acts as an excellent flame retardant synergist to traditional APP since both composites contain 30 mass% flame retardant additives.
Morphology and structure of the char residues
From the digital photographs (as shown in Fig. 6) of the specimens after cone calorimeter test, there was almost no residue for pure PP. In comparison, after adding the flame retardant to PP, more char residues remained in the sample holder. In particular, by introducing IFR into PP, the quantity was increased and quality of formed char was improved. These improved char layers will act as good barrier to prevent the transmission of fuel and oxygen.
In order to further understand the relationship between the microstructure of intumescent chars and flame retardancy of PP composites, SEM micrographs of the char residues of the outer surface (Fig. 7a) and inner surface (Fig. 7b) of PP/30 % IFR were imaged. It is noted that the char morphology on the outer surface of PP/30 % IFR showed the formation of a continuous and compact char layer, which would act as an insulating barrier to heat and would prevent the access of oxygen during combustion. Aiming to further understand elements distribution on the char residues, energy dispersive spectrometry (EDS) was employed to characterize the residue. The results are shown in Fig. 7c. It is noted that in the elemental composition of the char residue of the PP/30 % IFR, the content of C, O, N, P were 34.37, 7.97, 36.78, 20.88 mass%, respectively. This means that rich P and N-based compounds are formed on the surface via varied decomposition and reconstruction approaches.
Thermal stability of flame retardant PP composites
The effect of IFR on the thermal stability of PP composites was revealed through TG test. TG and DTG curves of PP and its composites are shown in Fig. 8. The corresponding data are given in Table 3. From Fig. 8, it was observed that all samples studied showed only one degradation step within the experimental temperature range. It was clear from Fig. 8 and Table 3 that the addition of IFR had an obvious effect on the thermal stability of PP. The onset degradation temperature of pure PP, T onset, defined as the temperature at which 5 % mass loss occurred, was 374 °C. In the case of PP with 30 mass% of APP, the onset degradation temperature decreased to 265 °C. In contrast, the T onset of flame retardant PP composites containing 20, 25 and 30 % IFR were 315, 314 and 309 °C, respectively. This indicates that the addition of IFR decreased the thermal stability of PP-based composites. However, as given in Table 3, T max of PP amounts to 427 °C, while the maximal decomposition temperature T max of PP with 20, 25 and 30 % IFR was 425, 414 and 430 °C. Consequently, there is only a small impact on the T max of PP after adding IFR.
Effect of electron beam irradiation on the thermal stability of flame retardant PP composites
Figure 9 presents the TG and DTG curves of non-irradiated and irradiated flame retardant PP composites, and the corresponding values are listed in Table 3.
Obviously, the thermal stability of flame retardant PP composites was remarkably improved via electron beam (EB) treatment. For example, T onset of PP + 20 % IFR was increased from 315 to 340 °C after EB treatment. For the other flame retardant PP composites, a similar trend was shown. Moreover, the maximal decomposition temperatures (T max) of flame retardant PP composites were also increased via EB treatment. Herein, a possible grafting and/or cross-linking mechanism between the novel flame retardant PP was proposed, as shown in Fig. 10. Firstly, electron beam treatment resulted in the generation of PP macroradicals. Then, the macroradical reacts with the C=C group presented in the SPSA to generate the first grafting reaction. The same reaction can be induced by the macroradicals at the second C=C group of SPSA. Finally, a cross-linked structure was generated in the flame retardant PP composites.
Conclusions
In order to develop high-performance flame retardant PP composites, in this article, a novel flame retardant, spirocyclic pentaerythritol bisphosphorate disphosphoryl-di-prop-2-en-1-amine (SPSA), containing two functional double bond (C=C) that may form cross-linked structure with PP in the electron beam treatment was synthesized and characterized successfully. Then, an intumescent flame retardant (IFR) based on aluminum polyphosphate (APP) and SPSA (mass ratio of APP and SPSA at 2:1) was developed and used in PP system to prepare fire retardant PP composites. LOI value of flame retardant PP composite containing 30 % IFR was 24.0, while the LOI value of PP + 30 % APP was only 20.4. More importantly, PP + 30 % IFR passed UL 94 V-0, indicating that this IFR was more efficient than APP alone. In the cone calorimeter test, both PHRR and THR results of PP/IFR system were lower than those of PP/APP system. It proved that this IFR was a more efficient flame retardant to PP compared to APP. In addition, the analysis of char residues proved that this IFR provided relatively good quality of char residue in the combustion that acted as good barrier to prevent the transmission of fuel and oxygen. Moreover, it could be shown that electron beam treatment of the composites is an efficient approach to improve the thermal stability of these designed flame retardant PP composites. Further investigations on the optimized dose of electron beam irradiation will be reported in the future. This irradiated flame retardant PP system might be a promising formulation for the industrial application such as insulated material of wire, cable, etc.
References
Zanetti M, Camino G, Canavese D, Morgan AB, Lamelas FJ, Wilkie CA. Fire retardant halogen-antimony-clay synergism in polypropylene layered silicate nanocomposites. Chem Mater. 2002;14:189–93.
Borysiak S. The thermo-oxidative stability and flammability of wood/polypropylene composites. J Therm Anal Calorim. 2015;119:1955–62.
Nie S, Zhou C, Peng C, Liu L, Zhang C, Dong X, Wang DY. Thermal oxidative degradation kinetics of novel intumescent flame-retardant polypropylene composites. J Therm Anal Calorim. 2015;120:1183–91.
Alongi J, Han ZD, Bourbigot S. Intumescence: tradition versus novelty. A comprehensive review. Prog Polym Sci. 2015;51:28–73.
Liu Y, Wang Q. Catalytic action of phospho-tungstic acid in the synthesis of melamine salts of pentaerythritol phosphate and their synergistic effects in flame retarded polypropylene. Polym Deg Stab. 2006;91:2513–9.
Nie S, Song L, Guo Y, Wu K, Xing W, Lu H, Hu Y. Intumescent flame retardation of starch containing polypropylene semibiocomposites: flame retardancy and thermal degradation. Ind Eng Chem Res. 2009;48:10751–8.
Ke CH, Li J, Fang KY, Zhu QL, Zhu J, Yan Q, Wang YZ. Synergistic effect between a novel hyperbranched charring agent and ammonium polyphosphate on the flame retardant and anti-dripping properties of polylactide. Polym Deg Stab. 2010;95:763–70.
Liu Y, Wang DY, Wang JS, Song YP, Wang YZ. A novel intumescent flame-retardant LDPE system and its thermo-oxidative degradation and flame-retardant mechanisms. Polym Adv Technol. 2008;19:1566–75.
Bourbigot S, Bras ML, Duquesne S, Rochery M. Recent advances for intumescent polymers. Macromol Mater Eng. 2004;289:499–511.
Tian N, Wen X, Jiang Z, Gong J, Wang Y, Xue J. Tang T Synergistic effect between a novel char forming agent and ammonium polyphosphate on flame retardancy and thermal properties of polypropylene. Ind Eng Chem Res. 2013;52:10905–15.
Song YP, Wang DY, Wang XL, Lin L, Wang YZ. A method for simultaneously improving the flame retardancy and toughness of PLA. Polym Adv Technol. 2011;22:2295–301.
Ma H, Tong L, Xu Z, Fang Z, Jin Y, Lu F. A novel intumescent flame retardant: Synthesis and application in ABS copolymer. Polym Deg Stab. 2007;92:720–6.
Chen DQ, Wang YZ, Hu XP, Wang DY, Qu MH, Yang B. Flame-retardant and anti-dripping effects of a novel char-forming flame retardant for the treatment of poly(ethylene terephthalate) fabrics. Polym Deg Stab. 2005;88:349–56.
Shao ZB, Deng C, Tan Y, Chen MJ, Chen L, Wang YZ. An efficient mono-component polymeric intumescent flame retardant for polypropylene: preparation and application. ACS Appl Mater Interfaces. 2014;6:7363–70.
Shao ZB, Deng C, Tan Y, Yu L, Chen MJ, Chen L, Wang YZ. Ammonium polyphosphate chemically-modified with ethanolamine as an efficient intumescent flame retardant for polypropylene. J Mater Chem A. 2014;2:13955–65.
Wang B, Tai Q, Nie S, Zhou K, Tang Q, Hu Y, Song L. Electron beam irradiation cross linking of halogen-free flame-retardant ethylene vinyl acetate (EVA) copolymer by silica gel microencapsulated ammonium polyphosphate and char-forming agent. Ind Eng Chem Res. 2011;50:5596–605.
Thakur V, Leuteritz A, Gohs U, Kretzschmar B, Wagenknecht U, Bhowmick AK, Heinrich G. Montmorillonite nanocomposites with electron-beam modified atactic polypropylene. Appl Clay Sci. 2010;49:200–4.
Thakur V, Gohs U, Wagenknecht U, Heinrich G. Electron-induced reactive processing of thermoplastic vulcanizate based on polypropylene and ethylene propylene diene terpolymer rubber. Polym J. 2012;44:439–48.
Gohs U, Leuteritz A, Naskar K, Volke S, Wiessner S, Heinrich G. Reactive EB processing of polymer compounds. Macromol Symp. 2010;296:589–95.
Mondal M, Gohs U, Wagenknecht U, Heinrich G. Additive free thermoplastic vulcanizates based on natural rubber. Mater Chem Phys. 2013;143:360–6.
Wang DY, Ge XG, Wang YZ, Wang C, Qu MH, Zhou Q. A novel phosphorus-containing poly(ethylene terephthalate) nanocomposite with both flame retardancy and anti-Dripping effects. Macromol Mater Eng. 2006;291:638–45.
Barbosa R, Alves TS, Araújo EM, Mélo TJA, Camino G, Fina A, Ito EN. Flammability and morphology of HDPE/clay nanocomposites. J Therm Anal Calorim. 2014;115:627–34.
Kalali EN, Juan SD, Wang X, Nie SB, Wang R, Wang DY. Comparative study on synergistic effect of LDH and zirconium phosphate with aluminum trihydroxide on flame retardancy of EVA composites. J Therm Anal Calorim. 2015;121:619–26.
Yang D, Hu Y, Li H, Song L, Xu H, Li B. Synergistic flame retardant effect of α-zirconium phosphate in low-density polyethylene/ethylene–vinyl acetate/aluminum hydroxide hybrids. J Therm Anal Calorim. 2015;119:619–24.
Lu SY, Hamerton I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog Polym Sci. 2002;27:1661–712.
Zhang S, Horrocks AR. A review of flame retardant polypropylene fibres. Prog Polym Sci. 2003;28:1517–38.
Babrauskas V, Peacock RD. Heat release rate: the single most important variable in fire hazard. Fire Saf. 1992;18:255–61.
Acknowledgements
One of the authors ((Mr. Dan Xiao) would like to thank the financial support from the China Scholarship Council (Ph.D. Program No. 201308530045) and STSM support from COST MP1105 (MP1105-090315-053686). This research is also partly supported by Spanish Ministry of Economy and Competitiveness (MINECO) under Ramón y Cajal fellowship (RYC-2012-10737) and COMETAD project (MAT2014-60435-C2-2-R).
Funding
Mr. Dan Xiao would like to thank the financial support from the China Scholarship Council (Ph.D. Program No. 201308530045) and the STSM support from COST MP1105 (MP1105-090315-053686). Dr. De-Yi Wang acknowledges this research is partly supported by Ramón y Cajal fellowship (RYC-2012-10737) and COMETAD project (MAT2014-60435-C2-2-R).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Xiao, D., Li, Z., De Juan, S. et al. Preparation, fire behavior and thermal stability of a novel flame retardant polypropylene system. J Therm Anal Calorim 125, 321–329 (2016). https://doi.org/10.1007/s10973-016-5352-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5352-7