Abstract
High-density polyethylene/modified bentonite clay/polar compatibilizer nanocomposites were prepared through the melt intercalation process. The clay was organophilizated using different percentages of quaternary ammonium salt 100, 125, and 150 % based cation exchange capacity of the clay. The nanocomposites were prepared in a counter-rotating twin-screw extruder and then specimens were injection molded. For the evaluation of flammability of the test system was used for burning in the horizontal position according to the norm (Underwriters Laboratories, UL94HB) and to the method of cone calorimeter. The thermal behavior of nanocomposites was evaluated by thermogravimetry and X-ray diffraction techniques, and transmission electron microscopy were used to characterize the morphology and analyze the degree of expansion of the clays prepared and the degree of exfoliation of nanocomposites. It was observed that the percentage of ammonium salt and the compatibilizer polar influence on the final properties of the systems and consequently improving the thermal stability and reducing the flammability of the matrix.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
“Fire” is an important topic, not just from a scientific viewpoint, but also from a daily living perspective. As polymers form the core of the modern world, understanding this science to fine-tune the materials for achieving highest fire safety standards is extremely important. But considering eco-friendliness, ultimate mechanical/physical properties, and processing difficulties, unsatisfactory fire performance of polymers and their composites is a major obstacle. The incorporation of nanoscale fillers (like clays, nanotubes, POSS, etc.) in polymers, though, showed a positive potential toward flame retardancy (reductions in heat release/mass loss rates and delayed burning) [1].
High-density polyethylene (HDPE) (0.941 < density < 0.965) is a thermoplastic material composed of carbon and hydrogen atoms joined together forming high molar mass products. HDPE resin has a greater proportion of crystalline regions than low-density PE. PE has the simplest structure of polymers on the market, which gives it great versatility with the variety of processes and applications, resulting in characteristics of density, molar mass, and molar mass distribution. In the PE matrix, particles of montmorillonite clay and bentonite clay will be introduced, giving the polymer characteristics of a nanocomposite [2, 3].
The main factors that control the properties of clays are the mineralogical composition of clay minerals and, particle size distributions, electrolyte content of exchangeable cations and soluble salts, the nature and content of organic and textural characteristics of the clay. The clay may have a lattice structure of fibrous or layered (lamellar). Therefore, the most falls in the second case and is called phyllosilicates. Each layer is composed of one or more leaves of silica tetrahedra and octahedra of aluminum hydroxide (or other metal) and the amount of leaves for the clay layer divides into two groups: diform (1:1 layers, i.e., each layer of clay is composed of one tetrahedral leaf linked to a leaf of octahedra) or triform (2:1 layers, i.e., two leaves of tetrahedra surrounding an octahedron leaf) [4].
Bentonite can be defined as a rock that consists mainly of a mineral clay montmorillonite (smectite), formed by devitrification and subsequent chemical alteration of a glassy material of igneous origin, usually a tuff or volcanic ash. The term bentonite was derived from the first commercial deposit of a plastic clay in the USA. This clay has the property to increase several times its initial volume in the presence of moisture [5]. In 1897, Knight reported that since 1888 William Taylor marketed a unique clay found in Fort Benton, Wyoming, USA, and proposed the name Taylorite, suggesting then “bentonite” as the first name was already used [6]. Organophilic bentonites are clays that can be prepared from sodium bentonite, which is highly hydrophilic and with the addition of quaternary ammonium salts (with at least one chain containing 12 or more carbon atoms) in an aqueous dispersion of sodium smectite clays acquire organophilic characteristics. In aqueous dispersion of sodium bentonite, the organic cation salt substitutes the cations of sodium bentonite, passing it from hydrophilic to organophilic [7].
Nanocomposites are materials in which the dispersed phase presents at least one of the dimensions in the nanometer scale. In polymer/clay nanocomposites, the dispersed phase (clay) is present in the form of flakes about 1.0 nm thick and hundreds of nanometers wide and long and has a high aspect ratio. Moreover, the concentration of clay in nanocomposites is less than 5 % by mass [8].
The preparation of polymer–clay nanocomposites can be achieved in several ways, including: in situ polymerization, solution and melt intercalation. The latter is the most viable alternative technology because it is not required to use solvents and the equipments used for this (extruders, etc.) are those that are already available in industrial production lines [9].
The process of preparation of nanocomposites via intercalation in the molten state was first reported by several researchers [10]. Some structures can be formed after the synthesis of polymer–clay composite, according to the nature of the used components (clay, coupling agent, and polymer matrix) and the preparation method such as: microcomposite, intercalated or exfoliated nanocomposites [11].
Polymers have many applications in the construction and transportation industries, areas where the behavior of materials under fire conditions is crucial for the personnel security and material properties. Many polymeric materials used in such applications have flame resistance, by adding a chemical to interfere in one or more of the three requirements for combustion: heat, fuel, and oxygen [11, 12].
Like most organic products, polymers are greater or lesser degree, flammable. This is because during the heating small molecules are released, which act as fuel in the presence of fire. In some applications, it is essential to prevent burning, which have encouraged the development of formulations of flame retardant, thus reducing the likelihood of combustion during the initiation of fire and the speed of flame propagation. The increasing demands of safety, in some applications, flammability is one of the barriers to use of some polymers [13].
For obtaining nanocomposite, it is necessary that formation interactions between the clay and the polymer, which is very difficult for nonpolar PE (HDPE). The solution to enhance this interaction, and thus, to improve the properties of the nanocomposites were prepared using a polar compatibilizer based on PE and maleic anhydride (PE-g-MA), as an alternative to the production of PE–organoclay nanocomposites. The objective of this research is to analyze the morphology, the thermal stability, and flammability of HDPE/clay nanocomposites.
Experimental
Materials
The clay turned sodium was the 1346 supplied by the Industry Northeast Bentonit (BUN), located in the city of Campina Grande—PB. It was used as the Praepagen WB® (stearyldimethylammonium chloride) salt, provided in the gel state with the amount of active matter consists of about 75 %, manufactured by Clariant, Recife/Brazil. The matrix used in this study was the HDPE commercial (JV-060U), provided by the Braskem (Camaçari/BA—Brazil). It has a good processability, high stiffness, dimensional stability and mechanical strength, and good impact strength at low temperatures. The polar compatibilizing used was Polybond 3009 (PE-g-MA), MFI = 5 g 10−1 min, with 1 % of MA, supplied by Crompton—olefin additives and styrene—São Paulo/Brazil.
Clay preparation
Initially, dispersions containing 768 mL of distilled water and 32 g of clay were prepared. The clay was added slowly with mechanical stirring and with the addition of all the clay, the agitation was maintained for 20 min. Then, it was added a solution of distilled water and the quaternary ammonium salt. The agitation was continued for another 20 min. Thus, the containers were sealed and kept at room temperature for 24 h. After that time, the obtained material was filtered to remove the excess salt. The washing was performed with 2,000 mL of distilled water, using Buchner funnel with Kitassato coupled to a vacuum pump with a pressure of 635 mmHg. The obtained agglomerates were dried in an oven at 60 ± 5 °C for a period of 48 h. Finally, the dried agglomerates were broken with the aid of mortar to obtain materials in powder form, which were spent in mesh sieve no. 200 (D = 0.074 mm) to be subsequently added in the polymer matrix.
Nanocomposites preparation
The nanocomposites were prepared in the extruder. To promote better dispersion, a concentrate was produced (polar compatibilizer/clay) in an internal mixer coupled to a Torque Rheometer Haake Blüchler. The concentrate was grounded and was incorporated into HDPE in a counter-rotating twin-screw extruder, coupled to a Torque Rheometer Haake Blüchler. The processing conditions in extruder were: 170 °C in the first zone and 200 °C in other zones, at 60 rpm. The extruded material was granulated and was injection molded at 200 °C in an Injector, model FLUIDMEC. The used concentration was the proportion of 91:6:3 (PE:PEG:MMT). The systems were named by PE/PE-g-MA/without clay (PE 3009 NA), PE/PE-g-MA/organoclay with 100 % of the salt (PE 3009 100), PE/PE-g-MA/organoclay with 125 % of the salt (PE 3009 125), and PE/PE-g-MA/organoclay with 150 % of the salt (PE 3009 150).
Nanocomposites characterization
The X-ray diffraction (XRD) analysis was conducted in apparatus XRD-6000 Shimadzu, using Cu Kα radiation, voltage 40 kV, 30 mA current, scanning 2θ of 1.5–30°, and scan rate of 2° min−1. The transmission electron microscope (TEM) used was a Philips CM 120, operating with an acceleration voltage of 120 kV. Samples were taken from the center of the specimens and prepared for impact by reducing the area by use of the “trimming” in trapezoid shape with an area of approximately 0.5 mm2. The sections of the samples were performed in an ultramicrotome RMC brand, model MT-7000, using a diamond knife brand Diatom Cryohisto type 45 with a temperature of 80 °C in the sample and a diamond knife on a cryogenic conditions, with cutting speed of 0.1 mm s−1 and thickness between 25 and 50 nm.
Analysis of thermogravimetry (TG) and differential thermal analysis were made in equipment simultaneous of Shimadzu, using a flow rate of 50 mL min−1 of air, of the room temperature up to 900 °C, using a heating rate of 10 °C min−1. It used about 5 mg of sample.
The Underwriters Laboratories (UL94) test is applied to materials that continue to burn and propagate flame after removal of the initial call. It is used to classify as the HB polymer material as a burning rate below a specified minimum value (40 mm min−1). The implementation is given by specimens injected to make the normalization of UL94. The dimensions of the specimens are as follows: 125 ± 5 mm in length, 13 ± 0.5 mm in width, and thickness of 3.0 ± 0.2 mm. On average five specimens were tested.
The cone calorimeter tests were performed according to the criteria of ISO 5660/ASTM 1354. The rate of heat release (HRR), time to ignition (TTI), the relationship between both (TTI/HRR) and the average value for the rate of heat released were measured. The sample sizes were 100 mm × 100 mm and maximum thickness was 50 mm. The heat flux used was 50 kW m−2 and the samples were tested in a horizontal position. On average, three specimens were tested.
Results and discussion
XRD of the clays and nanocomposites
The Fig. 1a shows the XRD curves of the clay after organophilization with different percentages of organic salts. Diffractograms of Fig. 1b illustrate the HDPE nanocomposites/organoclay with different levels of salt/PE-g-MA (PE 3009 100, -125 and -150). It can be noted that the system of nanocomposites with variations in the percentage of salt increases the basal interplanar distance d 001, which corresponds to the intercalation of molecules of PE between the layers of organoclay and the presence of two shoulders, probably to distance baseline d 002 and d 003, respectively, which can indicate that a small portions of the clay layers were intercalated by polymer molecules. These structures were also observed by researchers [14]. The peaks of second and third order were also reported by Goettler and coworkers [15]. According to Souza Santos in crystallography X-ray is not necessary to consider the reflection plane (002) and second order (001), since the plans (002) lattice are uniquely identified as any other plane (hkl) . However, it is common to find references to the reflection of the plans (002) and (003) as being respectively second and third orders of the plan (001) [16–20]. These same observations were seen for both organoclays as for the nanocomposite systems.
Maged et al. [21] evaluated the influence of different organic modifiers to the organophilization of montmorillonites and a variety of clays with varied cation exchange capacity. The authors observed values of interplanar basal distances between 18 and 40 Å nanocomposites for HDPE/organoclay. The results of XRD and TEM indicated that exfoliated structure was not found. And in order to improve the interfacial tension between the matrix and the organoclay, a third component, such as functionalized polymers were used as compatibilizer agents to facilitate the mechanism of intercalation. It is understood that the process of dispersion of silicate happens when the functionalized polymers are first intercalated into the organoclay during the melt intercalation, thus increasing the basal interplanar distance [16–18]. Then, when the structure undergoes disorganization through the process of mixing and shear, the layered silicates are finally exfoliated within the polymer matrix.
It can be observed in the Table 1 that the increase of interlayer spacing d 001 for all nanocomposites, indicating the formation of intercalated structures. There is also the system with 100 % of the salt content with more variation of d 001 of about 6.07 Å, probably indicating that the ability to merge has a certain limit, does not require the use of surfactant over an optimal concentration. Through these results, it is evident the expansion of the layers of clay and therefore it will influence the morphology of the systems and the final properties of the resulting nanocomposites.
System PE 3009 125 showed a small variation in basal interplanar distance d 001 of organoclay, corresponding to a difference of 0.47 Å. This behavior should be attributed to influence during processing, the type of quaternary ammonium salt and the polar compatibilizer were kept constant for the clay.
The most promising strategy currently is the addition of small amounts of polyolefins functionalized with MA nanocomposite systems. It is believed that the characteristic of polar anhydride has a greater affinity with the clay, i.e., acting as a compatibilizer between the matrix and the load [22–25].
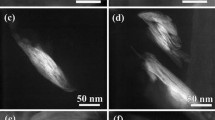
TEM of the nanocomposites
Figures 2, 3, and 4 present the photomicrographs of the systems of HDPE/PE-g-MA/clay modified with different percentages of solutions of ammonium salt (100, 125, and 150 %).
It can be observed that increasing the salt content and the presence of polar compatibilizer has favored the dispersion of clay in the polymer matrix in the presence of structure intercalated regions, as also it observed by XRD. This behavior is corroborated by [24, 25], since the presence of PE-g-MA have a tendency promoted the organoclay exfoliation when compared to the system without compatibilizer agent. The strong interaction between the PE-g-MA and the silicate layers can have caused a better dispersion of clay in PE matrix. These results were also observed by Minkova et al. [26] and revealed the importance of studying the morphology by XRD and TEM.
The results of TEM show that the clay was well-dispersed in the matrix and the presence of a hybrid structure intercalated and partially exfoliated can contribute to the general properties of the nanocomposites.
Nanocomposites TG measurements
The nanoparticles can inhibit or retard the degradation of the polymer matrix, since the greater the intensity of the intercalation of polymer chains between the layers of clay, the greater the thermal stability of the material. Another factor that must be considered is that the clay has the ability to adsorb small molecules on their surface, such as antioxidants and molecules that make the matrix more susceptible to degradation [27–30].
Figure 5 shows the TG curves of the HDPE and PE/clay without modification or organoclay systems with different amounts of salt in organophilization (HDPE, PE 3009 NA, -100, -125, and -150).
The addition of compatibilizer polar agents subtly improved the process of matrix degradation of PE in some systems, i.e., the compatibilizer increased the polarity improving adherence of the clay/polymer nanocomposite and then making the systems more thermally stable. A polymer matrix is more susceptible to the action of oxygen with respect to degradation as compared to materials containing clay.
Table 2 presents the values obtained by TG temperatures to 10 and 50 % degradation (T 10 % and T 50 %).
In principle, it can be noted that the presence of clay and compatibilizers helped to improve the thermal stability of materials. It appears that all nanocomposites showed higher initial thermal stability (T 10 %) than PE. In T 50 %, they behaved near the polymer matrix, and all systems, except those with 125 % of the salt content showed a more fast decomposition. This is probably due to degradation of the ammonium salt present in the layers of clay.
Marco and Luigi [31] found that the displacement of the temperature of initiation of degradation of composites is probably due to physical adsorption and chemical degradation products on the surface of silicates and the effect of the labyrinth of flakes dispersed in silicate nanocomposites. The combination of these effects slows the volatilization of the products generated by thermal degradation of the matrix. However, these effects act only at the beginning of thermal degradation, since the loss of 50 % (T 50 %) of the mass is practically all materials.
Nanocomposites UL94HB measurements
In this type of test it is very difficult to obtain absolute data for all materials in all applications, since the actual conditions vary widely. Each institution security or testing of materials has registered a large number of methods, for example, minimum ignition temperature, rate of flame spread, smoke density, and mass loss. The study was conducted following the specifications of the UL94HB.
The firing rates of the array of HDPE and PE 3009 systems are shown in Fig. 6. Table 3 presents the values of burning rate for all systems studied.
It is observed that most systems present the results of burning rate are higher than the polymer matrix. And the increase was practically gradual when it increased the amount of ammonium salt to the organophilization. These results corroborate the results of TG that can be attributed to the greater number of units of carbonic modifier of clay, which degrade at lower temperatures than the matrix. Therefore, not causing any delay in the process of specimen burning and the clay did not show performance as an insulator volatile product of degradation.
For this analysis, these systems showed the behavior observed by Zhongfu et al. [32] in studies of PE/clay composites, concluding that the catalytic effect of clay can lead to degradation of the polymer matrix, decreasing the thermal stability.
Nanocomposites cone calorimeter measurements
The tests were performed according to the criteria of ISO 5660/ASTM 1354. It measured the HRR, peak HRR (pkHRR), and TTI for all systems studied.
The Fig. 7 shows the curves of the heat loss rate as a function of time obtained by the test of cone calorimeter for HDPE, the system PE–clay without modification/PE-g-MA (PE 3009 NA), the system PE/clay content variation in the ammonium salt/PE-g-MA (PE 3009 100, -125, and -150).
It appears that the system PE 3009 showed a decrease in the heat loss rate in relation to the polymer matrix except system containing clay without modification (PE 3009 NA). The system with clay without modification has TTI of 28 and 29 s, respectively, therefore, entering ignition ahead of other systems (Table 4). This fact is related to studies by Rongjun et al. [33], where they studied the flammability properties of polypropylene/clay organophilic nanocomposites and without modification and they have concluded that only the incorporation of organoclays creates a carbonaceous protective layer. This difference is attributed to the fact that the acid sites are formed only on the surface of organoclay.
It was also observed that with the increase in the percentage of ammonium salt to the organophilization, the values of the peak burning heat rate (pkHRR) decreased gradually. The protective layer and the behavior of the curves obtained by cone calorimeter depend on the content of ammonium salts; the largest catalytic effect was seen with increasing the amount of organic modifier, and hence, the intensive training of the protective layer [34].
By XRD and TEM, it was verified that these systems merged the morphologies partially exfoliated. It has been usually seen in the literature that the formation of intercalated or exfoliated nanocomposites provides good properties of flame retardance. However, some other studies have investigated the importance of exfoliated structure as indicative of improvements in flammability. Also it has been found that simple intercalated structures can have similar or even higher than exfoliated nanocomposites [35].
Studies of degradation in nanocomposites has shown that the clay layers can act as a barrier decreasing the rate of diffusion of the products of polymer degradation as well as they assist in the diffusion of oxygen to support combustion. Moreover, many authors have shown that, in general, nanocomposites show a considerable reduction in peak heat loss rate (pkHRR), changes in the final structure of charred materials with increased formation of ash and a decrease in the rate of mass loss during combustion tests in the cone calorimeter [36–40].
Conclusions
The study of the characteristic of the XRD, the thermal stability, the TEM, and flammability of uncompatibilized and compatibilized HDPE/clay nanocomposites led to the following conclusions: the results of diffraction of X-rays indicated the intercalation of ions of ammonium salt studied within the silicate layers with the expansion of basal spacing d 001. It was observed by TEM that the increase in salt content and the presence of polar compatibilizer favored the dispersion of clay in the polymer matrix in the presence of merged regions and partially exfoliated. The presence of clay and compatibilizers helped to improve the thermal stability of materials. While, on the surface, the clay layers act as a barrier to combustion, increasing the protection of the matrix and reducing the peak heat loss rate (pkHRR).
References
Alfonso G, Aravind D, Berta H, Emeric P, Julio S, Antonio E, Szu-Hui L. Fire retardancy behavior of PLA based nanocomposites. Polym Degrad Stab. 2012;97:248–56.
John AB. Plastics materials. 4th ed. London: Butterwork Scientific; 1982.
Norbert B, Charles GO, Georg M. Encyclopedia of polymer science and engineering. New York: Wiley, Nova Iorque; 1986.
Lagaly G, Ogawa M, Dérkány I. In: Bergaya F, Theng BKG, Lagaly G, editors. Handbook of clay science. Amsterdam: Elsevier; 2006. p. 309–78.
Henry CHD, George RG. Composition and properties of drilling and completion fluids. 5th ed. Houston: Gulf Publishing Company; 1988.
Gerhard L. Layer charge heterogeneity in vermiculite. Clays Clay Miner. 1982;30:215–22.
Jordan JW. Organophilic bentonites. I: Swelling in organic liquids. J Phys Colloid Chem. 1949;53:294–306.
Luciano FB. Nanocompósitos de Poli (tereftalato de etileno) e argila. 89 páginas. Dissertação (Mestrado Em Ciência e Engenharia de Materiais)- UFSCar, São Carlos, 2001.
Michael A, Philippe D. Polymer layered silicate nanocomposites: preparation, properties and uses of a new class of materials. Mater Sci Eng. 2000;28:1–63.
Richarde AV, Hope I, Emmanuel PG. Synthesis and properties of two-dimensional nanostructures by direct intercalation of polymer melts in layered silicates. Chem Mater. 1993;5:1694–6.
Donald RP, Lloyd MR. Polymer nanotechnology: nanocomposites. Polymer. 2008;49:3187–204.
Holmes M. Flame retardants: world markets. Plast Addit Compd. 2000;2:18–9.
Marcelo R. Aditivação de Polímeros. São Paulo: Artliber Editora Ltda.; 2000.
Lucilene BP, Ana RM, Thiago RG. Propriedades Mecânicas de Nanocompósitos de Polipropileno e Montmorilonita Organofílica. Polím Ciênc Tecnol. 2006;16:136–40.
Keun YL, Lloyd AG. Structure–property relationships in polymer blend nanocomposites. Polym Eng Sci. 2004;44:1103–11.
Júnior ARO. Obtenção e Caracterização de Nanocompósitos Polipropileno-Argila. In: NANOTEC EXPO, ITM Expo, São Paulo; 2005.
Giulio M, Silvia R, Nahal L, Aldo P, Nadka TD, Francesco PLM. Intercalation effects in LDPE/o-montmorillonites nanocomposites. Eur Polym J. 2007;43:328–35.
Manias E. Origins of the materials properties enhancements in polymer/clay nanocomposites. 2001. http://raman.plmsc.psu.edu/manias/pdfs/nano2001b.pdf. Accessed 20 Sep 2012.
Pérsio SS. Ciência e Tecnologia de Argilas, vol. 1. São Paulo: Ed. Edgard Blucher Ltda.; 1989.
Hotta S, Donald RP. Nanocomposites formed from linear low density polyethylene and organoclays. Polymer. 2004;45:7639–54.
Maged AO, Rupp JEP, Suter UW. Gas permeation properties of polyethylene-layered silicate nanocomposites. J Mater Chem. 2005;15:1298–304.
Hongdian L, Yuan H, Junfeng X, Qinghong K, Zuyao C, Weicheng F. The influence of irradiation on morphology evolution and flammability properties of maleated polyethylene/clay nanocomposite. Mater Lett. 2005;59(6):648–51.
Morawiec J, Pawlak A, Slouf M, Galeski A, Piorkowska E, Rasnikowa N. Preparation and properties of compatibilized LDPE/organo-modified montmorillonite nanocomposites. Eur Polym J. 2005;41:1115–22.
Hongbo Z, Weibing X, Hanyang G, Zhengfa Z, Shijun S, Qiusheng S. Preparation and characterization of PE and PE-g-MAH/montmorillonite nanocomposites. Eur Polym J. 2004;40:2539–45.
Ki HW, Min HC, Chong MK, Yeong SC, In JC. Synthesis and characterization of maleated polyethylene/clays nanocomposites. Polymer. 2001;42:9819–26.
Minkova L, Peneva Y, Tashev E, Filippi S, Pracella M, Magagnini P. Thermal properties and microhardness of HDPE/clay nanocomposites compatibilized by different functionalized polyethylenes. Polym Test. 2009;28:528–33.
Pulickel MA, Linda SS, Paul VB. Nanocomposites science and technology. Weinheim: Wiley-VCH Verlag; 2003.
Andrzej N, Grażyna P, Ewa K, Krzysztof M, Zbigniew Z. Radiation-induced modification of montmorillonite used as a filler in PP composite. Radiat Phys Chem. 2007;76:893–900.
Araújo EM, Barbosa R, Morais CRS, Soledade LEB, Souza AG, Vieira MQ. Effects of organoclays on the thermal processing of PE/clay nanocomposites. J Therm Anal Calorim. 2007;90:841–8.
Araújo EM, Barbosa R, Oliveira AD, Morais CRS, Souza AG, de Mélo TJA. Thermal and mechanical properties of PE/organoclay nanocomposites. J Therm Anal Calorim. 2007;87:811–4.
Marco Z, Luigi C. Preparation and combustion behaviour of polymer/layered silicate nanocomposites based upon PE and EVA. Polymer. 2004;45:4367–73.
Zhongfu Z, Tao T, Yongxin Q, Baotong H. Effects of surfactant loadings on the dispersion of clays in maleated polypropylene. Langmuir. 2003;19:7157–9.
Rongjun S, Zhe W, Xiaoyu M, Baoyan Z, Tao T. Influences of catalysis and dispersion of organically modified montmorillonite on flame retardancy of polypropylene nanocomposites. J Appl Polym Sci. 2007;106:3488–94.
Bourbigot S, Duquesne S, Jama C. Polymer nanocomposites: how to reach low flammability? Macromol Symp. 2006;233:180–90.
Jin Z, Charles AW. Thermal and fire studies on polystyrene–clay nanocomposites. Polym Int. 2000;49:1158–63.
Jeffrey WG, Catheryn LJ, Alexander BM, Richard HH Jr. Flammability properties of polymer-layered-silicate nanocomposites, polypropylene and polystyrene nanocomposites. Chem Mater. 2000;12:1866–73.
Shaofeng W, Yuan H, Zhenlong L, Zhengzhou W, Yonglong Z, Zuyao C, Weicheng F. Flammability and phase-transition studies of nylon 6/montmorillonite nanocomposites. Colloids Polym Sci. 2003;281:951–6.
Mariachiara Z, Takashi K, Falqui L, Giovanni C. Cone calorimeter combustion and gasification studies of polymer layered silicate nanocomposites. Chem Mater. 2002;14:881–7.
Qilong T, Richard KKY, Lei S, Yuan H. A novel polymeric flame retardant and exfoliated clay nanocomposites: preparation and properties. Chem Eng J. 2012;183:542–9.
Przemysław R, Grazyna J, Małgorzata J, Agnieszka P. Thermal properties and flammability of nanocomposites based on diene rubbers and naturally occurring and activated halloysite nanotubes. J Therm Anal Calorim. 2012;107:1243–9.
Acknowledgements
The authors thank to PhD Elias Hage Júnior from—DEMa/UFSCar/Brazil for performing the TEM measurements, Braskem for donating the PE, Bentonit União Nordeste for donating of the clay, Clariant for donating of the salts, RENAMI, MCT/CNPq and CAPES/PROCAD-NF for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbosa, R., Alves, T.S., Araújo, E.M. et al. Flammability and morphology of HDPE/clay nanocomposites. J Therm Anal Calorim 115, 627–634 (2014). https://doi.org/10.1007/s10973-013-3310-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3310-1